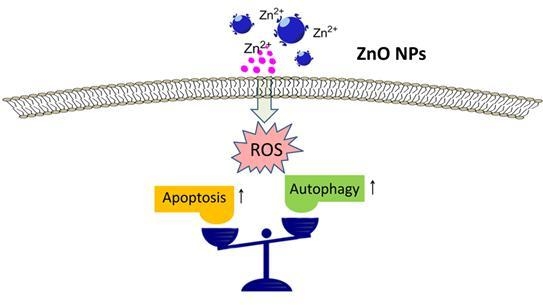
Role of Autophagy in Zinc Oxide Nanoparticles-Induced Apoptosis of Mouse LEYDIG Cells
Abstract
1. Introduction
2. Results
2.1. Characteristics and Morphology of ZnO NPs
2.2. ZnO NPs Cause Testis Damage to Male Mice
2.3. ZnO NPs Induce Apoptosis of Mouse Leydig TM3 Cells
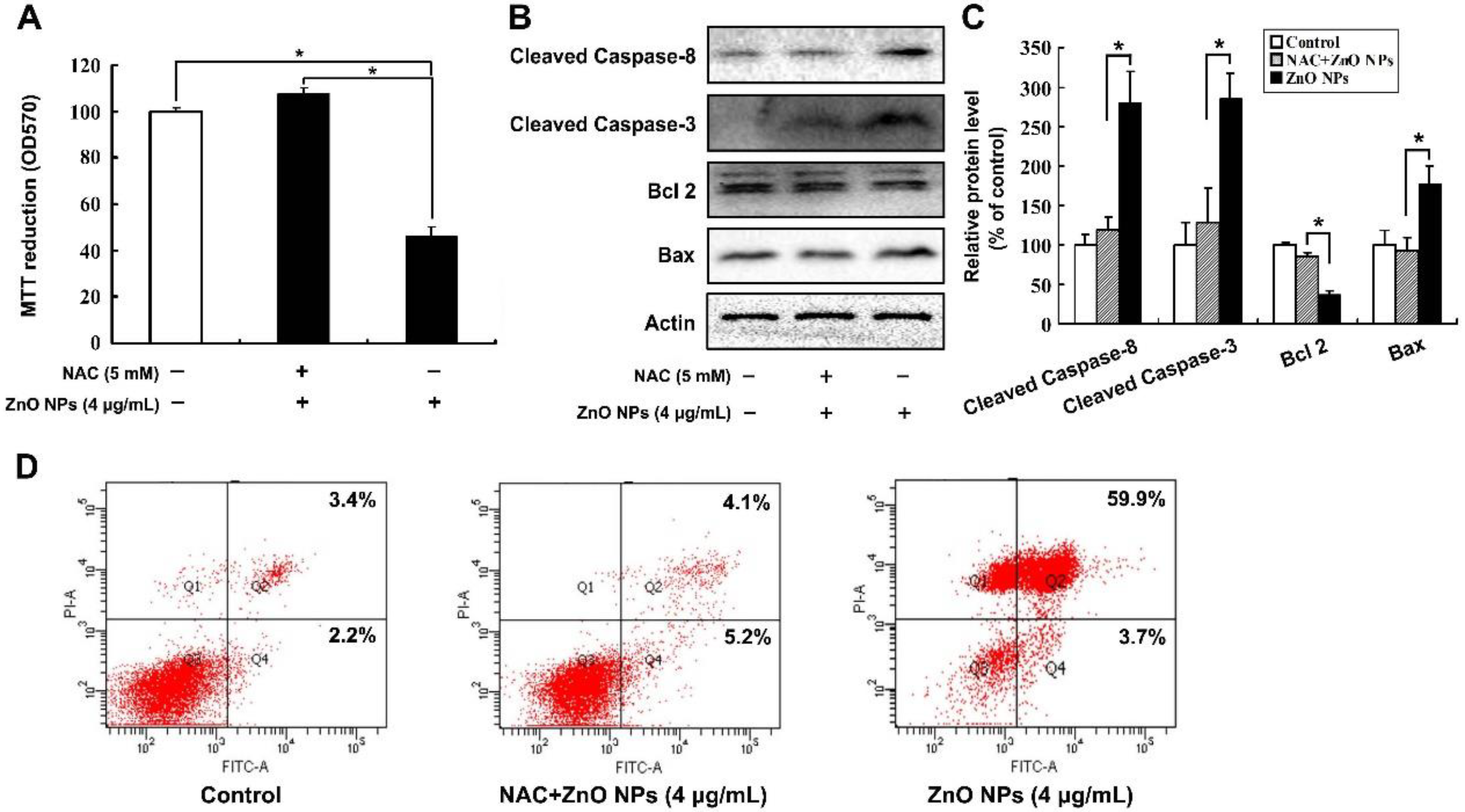
2.4. ZnO NPs Induce Apoptosis through Activation of Oxidative Stress
2.5. Oxidative Stress is Involved in ZnO NPs-Induced Autophagy
2.6. Inhibition of Autophagy Increases ZnO NP-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Nanoparticles and Characterization
4.3. Animal Administration
4.4. Histology
4.5. Western Blotting Analysis
4.6. Detection of Testosterone Content
4.7. Cell Culture and ZnO NP Treatment
4.8. Cell Viability Assay
4.9. AnnexinV-FITC/PI Apoptosis Assay
4.10. Oxidative Stress Measurement
4.11. Transmission Electron Microscopy (TEM) Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gambardella, C.; Ferrando, S.; Gatti, A.M.; Cataldi, E.; Ramoino, P.; Aluigi, M.G.; Faimali, M.; Diaspro, A.; Falugi, C. Review: Morphofunctional and biochemical markers of stress in sea urchin life stages exposed to engineered nanoparticles. Environ. Toxicol. 2016, 31, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Aluigi, M.G.; Ferrando, S.; Gallus, L.; Ramoino, P.; Gatti, A.M.; Rottigni, M.; Falugi, C. Developmental abnormalities and changes in cholinesterase activity in sea urchin embryos and larvae from sperm exposed to engineered nanoparticles. Aquat. Toxicol. 2013, 130, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1–11. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug. Discov. Today. 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health. Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Zinc oxide nanoparticles impacts: cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Yu, K.N.; Yoon, T.J.; Minai-Tehrani, A.; Kim, J.E.; Park, S.J.; Jeong, M.S.; Ha, S.W.; Lee, J.K.; Kim, J.S.; Cho, M.H. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol. In Vitro 2013, 27, 1187–1195. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef]
- Huo, L.; Chen, R.; Zhao, L.; Shi, X.; Bai, R.; Long, D.; Chen, F.; Zhao, Y.; Chang, Y.Z.; Chen, C. Silver nanoparticles activate endoplasmic reticulum stress signaling pathway in cell and mouse models: The role in toxicity evaluation. Biomaterials 2015, 61, 307–315. [Google Scholar] [CrossRef]
- Chen, R.; Ling, D.; Zhao, L.; Wang, S.; Liu, Y.; Bai, R.; Baik, S.; Zhao, Y.; Chen, C.; Hyeon, T. Parallel comparative studies on mouse toxicity of oxide nanoparticle- and gadolinium-based T1 MRI contrast agents. ACS. Nano. 2015, 9, 12425–12435. [Google Scholar] [CrossRef]
- Lin, L.; Xu, M.; Mu, H.; Wang, W.; Sun, J.; He, J.; Qiu, J.W.; Luan, T. Quantitative proteomic analysis to understand the mechanisms of zinc oxide nanoparticle toxicity to daphnia pulex (Crustacea: Daphniidae): comparing with bulk zinc oxide and zinc salt. Environ Sci. Technol. 2019, 53, 5436–5444. [Google Scholar] [CrossRef]
- Chen, R.; Huo, L.; Shi, X.; Bai, R.; Zhang, Z.; Zhao, Y.; Chang, Y.; Chen, C. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS. Nano. 2014, 8, 2562–2574. [Google Scholar] [CrossRef]
- Yin, H.; Chen, R.; Casey, P.S.; Ke, P.C.; Davis, T.P.; Chen, C. Reducing the cytotoxicity of ZnO nanoparticles by a pre-formed protein corona in a supplemented cell culture medium. RSC Adv. 2015, 5, 73963–73973. [Google Scholar] [CrossRef]
- Le, T.C.; Yin, H.; Chen, R.; Chen, Y.; Zhao, L.; Casey, P.S.; Chen, C.; Winkler, D.A. An experimental and computational approach to the development of ZnO nanoparticles that are safe by design. Small 2016, 12, 3568–3577. [Google Scholar] [CrossRef]
- Huo, L.; Chen, R.; Shi, X.; Bai, R.; Wang, P.; Chang, Y.; Chen, C. High-content screening for assessing nanomaterial toxicity. J. Nanosci. Nanotechnol. 2015, 15, 1143–1149. [Google Scholar] [CrossRef]
- Wen, X.; Wu, J.; Wang, F.; Liu, B.; Huang, C.; Wei, Y. Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free. Radic. Biol. Med. 2013, 65, 402–410. [Google Scholar] [CrossRef]
- Jenkins, R.R.; Goldfarb, A. Introduction: oxidant stress, aging, and exercise. Med. Sci. Sports Exerc. 1993, 25, 210–212. [Google Scholar] [CrossRef]
- Chen, R.; Qiao, J.; Bai, R.; Zhao, Y.; Chen, C. Intelligent testing strategy and analytical techniques for the safety assessment of nanomaterials. Anal. Bioanal. Chem. 2018, 410, 6051–6066. [Google Scholar] [CrossRef]
- Chen, J.X.; Xu, L.L.; Mei, J.H.; Yu, X.B.; Kuang, H.B.; Liu, H.Y.; Wu, Y.J.; Wang, J.L. Involvement of neuropathy target esterase in tri-ortho-cresyl phosphate-induced testicular spermatogenesis failure and growth inhibition of spermatogonial stem cells in mice. Toxicol. Lett. 2012, 211, 54–61. [Google Scholar] [CrossRef]
- Chen, J.X.; Xu, L.L.; Wang, X.C.; Qin, H.Y.; Wang, J.L. Involvement of c-Src/STAT3 signal in EGF-induced proliferation of rat spermatogonial stem cells. Mol. Cell. Biochem. 2011, 358, 67–73. [Google Scholar] [CrossRef]
- Liu, M.L.; Wang, J.L.; Wei, J.; Xu, L.L.; Yu, M.; Liu, X.M.; Ruan, W.L.; Chen, J.X. Tri-ortho-cresyl phosphate induces autophagy of rat spermatogonial stem cells. Reproduction 2015, 149, 163–170. [Google Scholar] [CrossRef]
- Talebi, A.R.; Khorsandi, L.; Moridian, M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J. Assist. Reprod. Genet. 2013, 30, 1203–1209. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, M.; Rui, X.; Pu, S.; Li, Y.; Li, C. Supplemental dietary phytosterin protects against 4-nitrophenol-induced oxidative stress and apoptosis in rat testes. Toxicol. Rep. 2015, 2, 664–676. [Google Scholar] [CrossRef]
- Lone, Y.; Koiri, R.K.; Bhide, M. An overview of the toxic effect of potential human carcinogen Microcystin-LR on testis. Toxicol. Rep. 2015, 2, 289–296. [Google Scholar] [CrossRef]
- Han, Z.; Yan, Q.; Ge, W.; Liu, Z.G.; Gurunathan, S.; De Felici, M.; Shen, W.; Zhang, X.F. Cytotoxic effects of ZnO nanoparticles on mouse testicular cells. Int. J. Nanomed. 2016, 11, 5187–5203. [Google Scholar] [CrossRef]
- Bara, N.; Kaul, G. Enhanced steroidogenic and altered antioxidant response by ZnO nanoparticles in mouse testis Leydig cells. Toxicol. Ind. Health. 2018, 34, 571–588. [Google Scholar] [CrossRef]
- Deepa, S.; Murugananthkumar, R.; Raj Gupta, Y.; Gowda, K.S.M.; Senthilkumaran, B. Effects of zinc oxide nanoparticles and zinc sulfate on the testis of common carp, Cyprinus carpio. Nanotoxicology 2019, 13, 240–257. [Google Scholar] [CrossRef]
- Hu, Q.; Guo, F.; Zhao, F.; Fu, Z. Effects of titanium dioxide nanoparticles exposure on parkinsonism in zebrafish larvae and PC12. Chemosphere 2017, 173, 373–379. [Google Scholar] [CrossRef]
- Kononenko, V.; Repar, N.; Marušič, N.; Drašler, B.; Romih, T.; Hočevar, S.; Drobne, D. Comparative in vitro genotoxicity study of ZnO nanoparticles, ZnO macroparticles and ZnCl2 to MDCK kidney cells: Size matters. Toxicol. Vitro 2017, 40, 256–263. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.C.; Fang, C.; Russell, R.C.; Kim, J.H.; Fan, W.; Liu, R.; Zhong, Q.; Guan, K.L. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 2013, 152, 290–303. [Google Scholar] [CrossRef]
- Wong, P.M.; Puente, C.; Ganley, I.G.; Jiang, X. The ULK1 complex: Sensing nutrient signals for autophagy activation. Autophagy 2013, 9, 124–137. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Chen, J.X.; Sun, Y.J.; Wang, P.; Long, D.X.; Li, W.; Li, L.; Wu, Y.J. Induction of autophagy by TOCP in differentiated human neuroblastoma cells lead to degradation of cytoskeletal components and inhibition of neurite outgrowth. Toxicology 2013, 310, 92–97. [Google Scholar] [CrossRef]
- Long, D.X.; Hu, D.; Wang, P.; Wu, Y.J. Induction of autophagy in human neuroblastoma SH-SY5Y cells by tri-ortho-cresyl phosphate. Mol. Cell Biochem. 2014, 396, 33–40. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Agnello, M.; Bosco, L.; Roccheri, M.C. Autophagy as a defense strategy against stress: focus on Paracentrotus lividus sea urchinembryos exposed to cadmium. Cell Stress Chaperones 2016, 21, 19–27. [Google Scholar] [CrossRef]
- Xu, H.Y.; Wang, P.; Sun, Y.J.; Jiang, L.; Xu, M.Y.; Wu, Y.J. Autophagy in Tri-o-cresyl Phosphate-Induced Delayed Neurotoxicity. J. Neuropathol. Exp. Neurol. 2017, 76, 52–60. [Google Scholar] [CrossRef]
- Xu, L.L.; Liu, M.L.; Wang, J.L.; Yu, M.; Chen, J.X. Saligenin cyclic-o-tolyl phosphate (SCOTP) induces autophagy of rat spermatogonial stem cells. Reprod. Toxicol. 2016, 60, 62–68. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chiu, I.J.; Cheng, F.Y.; Lee, Y.H.; Wang, Y.J.; Hsu, Y.H.; Chiu, H.W. The role of hypoxia-inducible factor-1alpha in zinc oxide nanoparticle-induced nephrotoxicity in vitro and in vivo. Part. Fibre. Toxicol. 2016, 13, 52. [Google Scholar] [CrossRef]
- Johnson, B.M.; Fraietta, J.A.; Gracias, D.T.; Hope, J.L.; Stairiker, C.J.; Patel, P.R.; Mueller, Y.M.; McHugh, M.D.; Jablonowski, L.J.; Wheatley, M.A.; et al. Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology 2015, 9, 737–748. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Z.; Xie, Y.; Liu, L.; Cao, Y. Cyanidin chloride modestly protects Caco-2cells from ZnO nanoparticle exposure probably through the induction of autophagy. Food Chem. Toxicol. 2019, 127, 251–259. [Google Scholar] [CrossRef]
- Giovanni, M.; Yue, J.; Zhang, L.; Xie, J.; Ong, C.N.; Leong, D.T. Pro-inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. J. Hazard. Mater. 2015, 297, 146–152. [Google Scholar] [CrossRef]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; Johannessen, B.; Glover, C.J.; Kappen, P.; Kopittke, P.M. Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ. Sci. Technol. 2013, 47, 13822–13830. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Ge, C.; Tseng, M.T.; Bai, R.; Qu, Y.; Beer, C.; Autrup, H.; Chen, C. Subchronic toxicity and cardiovascular responses in spontaneously hypertensive rats after exposure to multiwalled carbon nanotubes by intratracheal instillation. Chem. Res. Toxicol. 2015, 28, 440–450. [Google Scholar] [CrossRef]
- Zhao, F.; Meng, H.; Yan, L.; Wang, B.; Zhao, Y. Nanosurface chemistry and dose govern the bioaccumulation and toxicity of carbon nanotubes, metal nanomaterials and quantum dots in vivo. Sci. Bull. 2015, 60, 3–20. [Google Scholar] [CrossRef]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Aboushoushah, S.F.; Alshammari, W.W. Long-term biodistribution and toxicity of curcumin capped iron oxide nanoparticles after single-dose administration in mice. Life Sci. 2019, 230, 76–83. [Google Scholar] [CrossRef]
- Qian, L.; Cheng, X.; Ji, G.; Hui, L.; Mo, Y.; Tollerud, D.J.; Gu, A.; Zhang, Q. Sublethal effects of zinc oxide nanoparticles on male reproductive cells. Toxicol. In Vitro 2016, 35, 131. [Google Scholar]
- Kim, Y.R.; Park, J.I.; Lee, E.J.; Park, S.H.; Seong, N.W.; Kim, J.H.; Kim, G.Y.; Meang, E.H.; Hong, J.S.; Kim, S.H.; et al. Toxicity of 100 nm zinc oxide nanoparticles: a report of 90-day repeated oral administration in Sprague Dawley rats. Int. J. Nanomed. 2014, 9, 109–126. [Google Scholar]
- Ko, J.W.; Hong, E.T.; Lee, I.C.; Park, S.H.; Park, J.I.; Seong, N.W.; Hong, J.S.; Yun, H.I.; Kim, J.C. Evaluation of 2-week repeated oral dose toxicity of 100 nm zinc oxide nanoparticles in rats. Lab. Anim. Res. 2015, 31, 139–147. [Google Scholar] [CrossRef]
- Hong, J.S.; Park, M.K.; Kim, M.S.; Lim, J.H.; Park, G.J.; Maeng, E.H.; Shin, J.H.; Kim, Y.R.; Kim, M.K.; Lee, J.K.; et al. Effect of zinc oxide nanoparticles on dams and embryo-fetal development in rats. Int. J. Nanomed. 2014, 9, 145–157. [Google Scholar]
- Fei, G.; Ma, N.; Hong, Z.; Wang, Q.; Hao, Z.; Pu, W.; Hou, H.; Wen, H.; Li, L. Zinc oxide nanoparticles-induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int. J. Nanomed. 2016, 11, 3859–3874. [Google Scholar]
- Liang, S.; Sun, K.; Wang, Y.; Dong, S.; Wang, C.; Liu, L.; Wu, Y. Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid. Chem. Biol. Interact. 2016, 258, 40–51. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, C.; Chen, X.; Yang, Z. Zinc oxide nanoparticles induce renal toxicity through reactive oxygen species. Food Chem. Toxicol. 2016, 90, 76–83. [Google Scholar] [CrossRef]
- Chen, P.; Wang, H.; He, M.; Chen, B.; Yang, B.; Hu, B. Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol. Environ. Saf. 2019, 171, 337–346. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Mizushima, N. Methods for monitoring autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2491–2502. [Google Scholar] [CrossRef]
- Tremblay, J.J. Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids. 2015, 103, 3–10. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Akiri, O.F.; Durojaiye, M.A.; Alabi, A.F. Combined effects of repeated administration of Bretmont Wipeout (Glyphosate) and Ultrazin (Atrazine) on testosterone, oxidative stress and sperm quality of Wistar rats. Toxicol. Mech. Method 2014, 25, 1–31. [Google Scholar] [CrossRef]
- Asani, S.C.; Umrani, R.D.; Paknikar, K.M. Differential dose-dependent effects of zinc oxide nanoparticles on oxidative stress-mediated pancreatic beta-cell death. Nanomedicine 2017, 12, 745–759. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Zhang, P.; Chao, Z.; Xia, F.; Jiang, C.; Zhang, X.; Jiang, Z.; Liu, H. Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes. Cancer. 2014, 5, 100–112. [Google Scholar]
- Sharma, K.; Le, N.; Alotaibi, M.; Gewirtz, D.A. Cytotoxic autophagy in cancer therapy. Int. J. Mol. Sci. 2014, 15, 10034–10051. [Google Scholar] [CrossRef]
- Denton, D.; Nicolson, S.; Kumar, S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012, 19, 87–95. [Google Scholar] [CrossRef]
- Shintani, T.; Klionsky, D.J. Autophagy in health and disease: a double-edged sword. Science 2004, 306, 990–995. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, J.; Zeng, L.; Yang, D.; Shao, S.; Wang, J.; Wei, J.; Xiong, J.; Chen, J. Role of autophagy in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis in mouse Leydig cells. Environ Pollut. 2018, 243, 563–572. [Google Scholar] [CrossRef]
- Liu, X.; Xu, L.; Shen, J.; Wang, J.; Ruan, W.; Yu, M.; Chen, J. Involvement of oxidative stress in tri-ortho-cresyl phosphate-induced autophagy of mouse Leydig TM3 cells in vitro. Reprod. Biol. Endocrin. 2016, 14, 30. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Yang, D.; Zhou, X.; Wang, Y.; Tang, S.; Yin, H.; Wang, J.; Chen, R.; Chen, J. Role of Autophagy in Zinc Oxide Nanoparticles-Induced Apoptosis of Mouse LEYDIG Cells. Int. J. Mol. Sci. 2019, 20, 4042. https://doi.org/10.3390/ijms20164042
Shen J, Yang D, Zhou X, Wang Y, Tang S, Yin H, Wang J, Chen R, Chen J. Role of Autophagy in Zinc Oxide Nanoparticles-Induced Apoptosis of Mouse LEYDIG Cells. International Journal of Molecular Sciences. 2019; 20(16):4042. https://doi.org/10.3390/ijms20164042
Chicago/Turabian StyleShen, Jingcao, Dan Yang, Xingfan Zhou, Yuqian Wang, Shichuan Tang, Hong Yin, Jinglei Wang, Rui Chen, and Jiaxiang Chen. 2019. "Role of Autophagy in Zinc Oxide Nanoparticles-Induced Apoptosis of Mouse LEYDIG Cells" International Journal of Molecular Sciences 20, no. 16: 4042. https://doi.org/10.3390/ijms20164042
APA StyleShen, J., Yang, D., Zhou, X., Wang, Y., Tang, S., Yin, H., Wang, J., Chen, R., & Chen, J. (2019). Role of Autophagy in Zinc Oxide Nanoparticles-Induced Apoptosis of Mouse LEYDIG Cells. International Journal of Molecular Sciences, 20(16), 4042. https://doi.org/10.3390/ijms20164042