Wnt Binding Affinity Prediction for Putative Frizzled-Type Cysteine-Rich Domains
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Homology Modelling, Complex Generation and Refinement
4.2. Prediction of Wnt Binding Affinities for Putative Fzd-Type CRDs
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANP | Atrial natriuretic peptide |
| APC | Adenomatous polyposis coli |
| COL18A1 | Collagen XVIII α-chain |
| CORIN | Atrial natriuretic peptide-converting enzyme |
| CPZ | Carboxypeptidase Z |
| CRD | Cysteine-rich domain |
| DP | Dermal papilla |
| Dvl | Dishevelled |
| Fzd | Frizzled |
| GSK | Glycogen synthase kinase |
| LRP | Low-density lipoprotein receptor |
| MFRP | Membrane Frizzled-related protein |
| MMGBSA | Molecular mechanics generalized Born surface area |
| MuSK | Muscle skeletal receptor tyrosine-protein kinase |
| PCP | Planar cell polarity |
| PDB | Protein Data Bank |
| ROR | Receptor tyrosine kinase-like orphan receptor |
| sFRP | Secreted Frizzled-related protein |
| SMO | Smoothened |
References
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; He, X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a007880. [Google Scholar] [CrossRef] [PubMed]
- Pohl, S.-G.; Brook, N.; Agostino, M.; Arfuso, F.; Kumar, A.P.; Dharmarajan, A. Wnt signaling in triple-negative breast cancer. Oncogenesis 2017, 6, e310. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hannoush, R.N. Single-cell imaging of Wnt palmitoylation by the acyltransferase Porcupine. Nat. Chem. Biol. 2014, 10, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.H.; Mukund, S.; Stanger, K.; Wang, W.; Hannoush, R.N. Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc. Natl. Acad. Sci. USA 2017, 114, 4147–4152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, S.; Scott, R.; Arfuso, F.; Perumal, V.; Dharmarajan, A. Secreted Frizzled-related protein 4 and its implications in cancer and apoptosis. Tumour Biol. 2015, 36, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Surana, R.; Sikka, S.; Cai, W.; Shin, E.M.; Warrier, S.R.; Tan, H.J.G.; Arfuso, F.; Fox, S.A.; Dharmarajan, A.M.; Kumar, A.P. Secreted Frizzled related proteins: Implications in cancers. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1845, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Grishin, N.V. Cysteine-rich domains related to Frizzled receptors and Hedgehog-interacting proteins. Protein Sci. 2012, 21, 1172–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, Y.; Oishi, I.; Endo, M.; Nishita, M. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: Their implications in developmental morphogenesis and human diseases. Dev. Dyn. 2010, 239, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Klemm, F.; Bleckmann, A.; Siam, L.; Chuang, H.N.; Rietkotter, E.; Behme, D.; Schulz, M.; Schaffrinski, M.; Schindler, S.; Trumper, L.; et al. β-catenin-independent Wnt signaling in basal-like breast cancer and brain metastasis. Carcinogenesis 2011, 32, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Mikels, A.; Minami, Y.; Nusse, R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 2009, 284, 30167–30176. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.H.; Paredes-Moscosso, S.R.; Harrasser, M.; Vezzalini, M.; Scarpa, A.; Morris, E.; Davidoff, A.M.; Sorio, C.; Nathwani, A.C.; Della Peruta, M. An ROR1 bi-specific T-cell engager provides effective targeting and cytotoxicity against a range of solid tumors. OncoImmunology 2017, 6, e1326437. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhu, R.; Hu, N.; Shi, J.; Zhang, J.; Jiang, L.; Jiang, H.; Guan, H. Association of frizzled-related protein (MFRP) and heat shock protein 70 (HSP70) single nucleotide polymorphisms with primary angle closure in a Han Chinese population: Jiangsu Eye Study. Mol. Vis. 2013, 19, 128–134. [Google Scholar] [PubMed]
- Matsushita, I.; Kondo, H.; Tawara, A. Novel compound heterozygous mutations in the MFRP gene in a Japanese patient with posterior microphthalmos. Jpn. J. Ophthalmol. 2012, 56, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Sergouniotis, P.I.; Mackay, D.S.; Day, A.C.; Wright, G.; Devery, S.; Leroy, B.P.; Robson, A.G.; Holder, G.E.; Li, Z.; et al. A detailed phenotypic assessment of individuals affected by MFRP-related oculopathy. Mol. Vis. 2010, 16, 540–548. [Google Scholar] [PubMed]
- Novikova, E.G. Carboxypeptidase Z is present in the regulated secretory pathway and extracellular matrix in cultured cells and in human tissues. J. Biol. Chem. 2000, 275, 4865–4870. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shao, Y.Y.; Ballock, R.T. Carboxypeptidase Z (CPZ) links thyroid hormone and Wnt signaling pathways in growth plate chondrocytes. J. Bone Miner. Res. 2009, 24, 265–273. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, L.D.; Conkrite, K.L.; Chang, X.; Capasso, M.; Vaksman, Z.; Oldridge, D.A.; Zachariou, A.; Horn, M.; Diamond, M.; Hou, C.; et al. Common variants upstream of MLF1 at 3q25 and within CPZ at 4p16 associated with neuroblastoma. PLoS Genet. 2017, 13, e1006787. [Google Scholar] [CrossRef] [PubMed]
- Serafino, A.; Moroni, N.; Psaila, R.; Zonfrillo, M.; Andreola, F.; Wannenes, F.; Mercuri, L.; Rasi, G.; Pierimarchi, P. Anti-proliferative effect of atrial natriuretic peptide on colorectal cancer cells: Evidence for an Akt-mediated cross-talk between NHE-1 activity and Wnt/β-catenin signaling. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 1004–1018. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Rehn, M.; Pihlajaniemi, T. Alpha 1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc. Natl. Acad. Sci. USA 1994, 91, 4234–4238. [Google Scholar] [CrossRef] [PubMed]
- Quelard, D.; Lavergne, E.; Hendaoui, I.; Elamaa, H.; Tiirola, U.; Heljasvaara, R.; Pihlajaniemi, T.; Clement, B.; Musso, O. A cryptic Frizzled module in cell surface collagen 18 inhibits Wnt/β-catenin signaling. PLoS ONE 2008, 3, e1878. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.F.; Sircar, R.; Miller, P.S.; Hedger, G.; Luchetti, G.; Nachtergaele, S.; Tully, M.D.; Mydock-McGrane, L.; Covey, D.F.; Rambo, R.P.; et al. Structural basis of Smoothened regulation by its extracellular domains. Nature 2016, 535, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Nedelcu, D.; Watanabe, M.; Jao, C.; Kim, Y.; Liu, J.; Salic, A. Cellular cholesterol directly activates Smoothened in Hedgehog signaling. Cell 2016, 166, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, Z.Y.; Liu, W.P.; Zhao, M.R. Crosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways in colon cancer and implications for therapy. Cancer Biol. Ther. 2015, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.G.; Wang, Q.; Zhou, J.Z.; Wang, J.; Luo, Z.; Liu, M.; He, X.; Wynshaw-Boris, A.; Xiong, W.C.; Lu, B.; et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 2002, 35, 489–505. [Google Scholar] [CrossRef]
- Yumoto, N.; Kim, N.; Burden, S.J. LRP4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature 2012, 489, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.R.; Gribble, K.D.; Syrett, C.M.; Granato, M. Initiation of synapse formation by Wnt-induced MuSK endocytosis. Development 2012, 139, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.; Lefebvre, J.L.; Gordon, L.R.; Granato, M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron 2009, 61, 721–733. [Google Scholar] [CrossRef]
- Agostino, M.; Pohl, S.Ö.-G.; Dharmarajan, A. Structure-based prediction of Wnt binding affinities for Frizzled-type cysteine-rich domains. J. Biol. Chem. 2017, 292, 11218–11229. [Google Scholar] [CrossRef] [Green Version]
- Dijksterhuis, J.P.; Baljinnyam, B.; Stanger, K.; Sercan, H.O.; Ji, Y.; Andres, O.; Rubin, J.S.; Hannoush, R.N.; Schulte, G. Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J. Biol. Chem. 2015, 290, 6789–6798. [Google Scholar] [CrossRef] [PubMed]
- Willert, K.; Brown, J.D.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R., 3rd; Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Speer, K.F.; Sommer, A.; Tajer, B.; Mullins, M.C.; Klein, P.S.; Lemmon, M.A. Non-acylated Wnts can promote signaling. Cell Rep. 2019, 26, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Tobi, D. Designing coarse grained-and atom based-potentials for protein-protein docking. BMC Struct. Biol. 2010, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Feliu, E.; Aloy, P.; Oliva, B. On the analysis of protein–protein interactions via knowledge-based potentials for the prediction of protein–protein docking. Protein Sci. 2011, 20, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Agostino, M.; Mancera, R.L.; Ramsland, P.A.; Fernández-Recio, J. Optimization of protein-protein docking for predicting Fc-protein interactions. J. Mol. Recognit. 2016, 29, 555–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, K.; Omura, N.; Hong, S.-M.; Griffith, M.; Vincent, A.; Borges, M.; Goggins, M. Overexpression of Smoothened activates the Sonic Hedgehog signaling pathway in pancreatic cancer associated fibroblasts. Clin. Cancer Res. 2010, 16, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.S.; Hui, M.N.; Elsworth, B.L.; Wu, S.Z.; Roden, D.; Chan, C.-L.; Skhinas, J.N.; Collot, R.; Yang, J.; Harvey, K.; et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 2018, 9, 2897. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L.; La, J.; Yum, K.W.; Desai, P.; Rodewald, L.-W.; Zhang, X.; Leblanc, M.; Nusse, R.; Lewis, M.T.; Wahl, G.M. Paracrine Wnt signaling both promotes and inhibits human breast tumor growth. Proc. Natl. Acad. Sci. USA 2013, 110, 6991–6996. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lin, P.; Wang, Q.; Zheng, M.; Pang, L. Wnt3a-regulated TCF4/β-catenin complex directly activates the key Hedgehog signalling genes Smo and Gli1. Exp. Ther. Med. 2018, 16, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- De, A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011, 43, 745–756. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Wu, E.; Taketo, M.M.; Morgan, B.A. β-catenin activity in the dermal papilla of the hair follicle regulates pigment-type switching. Proc. Natl. Acad. Sci. USA 2010, 107, 21564–21569. [Google Scholar] [CrossRef]
- Erijman, A.; Rosenthal, E.; Shifman, J.M. How structure defines affinity in protein-protein interactions. PLoS ONE 2014, 9, e110085. [Google Scholar] [CrossRef]
- Agostino, M.; Velkov, T.; Dingjan, T.; Williams, S.J.; Yuriev, E.; Ramsland, P.A. The carbohydrate-binding promiscuity of Euonymus europaeus lectin is predicted to involve a single binding site. Glycobiology 2015, 25, 101–114. [Google Scholar] [CrossRef]
- Agostino, M.; Mancera, R.L.; Ramsland, P.A.; Yuriev, E. AutoMap: A tool for analyzing protein–ligand recognition using multiple ligand binding modes. J. Mol. Graph. Model. 2013, 40, 80–90. [Google Scholar] [CrossRef]
- Agostino, M.; Gandhi, N.S.; Mancera, R.L. Development and application of site mapping methods for the design of glycosaminoglycans. Glycobiology 2014, 24, 840–851. [Google Scholar] [CrossRef]
- Debruine, Z.J.; Ke, J.; Harikumar, K.G.; Gu, X.; Borowsky, P.; Williams, B.O.; Xu, W.; Miller, L.J.; Xu, H.E.; Melcher, K. Wnt5a promotes Frizzled-4 signalosome assembly by stabilizing cysteine-rich domain dimerization. Genes Dev. 2017, 31, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Hirai, H.; Matoba, K.; Mihara, E.; Arimori, T.; Takagi, J. Crystal structure of a mammalian Wnt–Frizzled complex. Nat. Struct. Mol. Biol. 2019, 26, 372–379. [Google Scholar] [CrossRef]
- Kaykas, A.; Yang-Snyder, J.; Heroux, M.; Shah, K.V.; Bouvier, M.; Moon, R.T. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat. Cell Biol. 2004, 6, 52–58. [Google Scholar] [CrossRef]
- Phesse, T.; Flanagan, D.; Vincan, E. Frizzled7: a promising Achilles’ heel for targeting the Wnt receptor complex to treat cancer. Cancers 2016, 8, 50. [Google Scholar] [CrossRef]
- Stiegler, A.L.; Burden, S.J.; Hubbard, S.R. Crystal Structure of the Frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK. J. Mol. Biol. 2009, 393, 1–9. [Google Scholar] [CrossRef]
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural basis of Wnt recognition by Frizzled. Science 2012, 337, 59–64. [Google Scholar] [CrossRef]
- Moal, I.H.; Jiménez-García, B.; Fernandez-Recio, J. CCharPPI web server: Computational characterisation of protein-protein interactions from structure. Bioinformatics 2015, 31, 123–125. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y. A novel side-chain orientation dependent potential derived from random-walk reference State for protein fold selection and structure prediction. PLoS ONE 2010, 5, e15386. [Google Scholar] [CrossRef]
- Chaudhury, S.; Lyskov, S.; Gray, J.J. PyRosetta: A script-based interface for implementing molecular modeling algorithms using Rosetta. Bioinformatics 2010, 26, 689–691. [Google Scholar] [CrossRef]
- Andrusier, N.; Nussinov, R.; Wolfson, H.J. FireDock: Fast interaction refinement in molecular docking. Proteins Struct. Funct. Bioinform. 2007, 69, 139–159. [Google Scholar] [CrossRef]
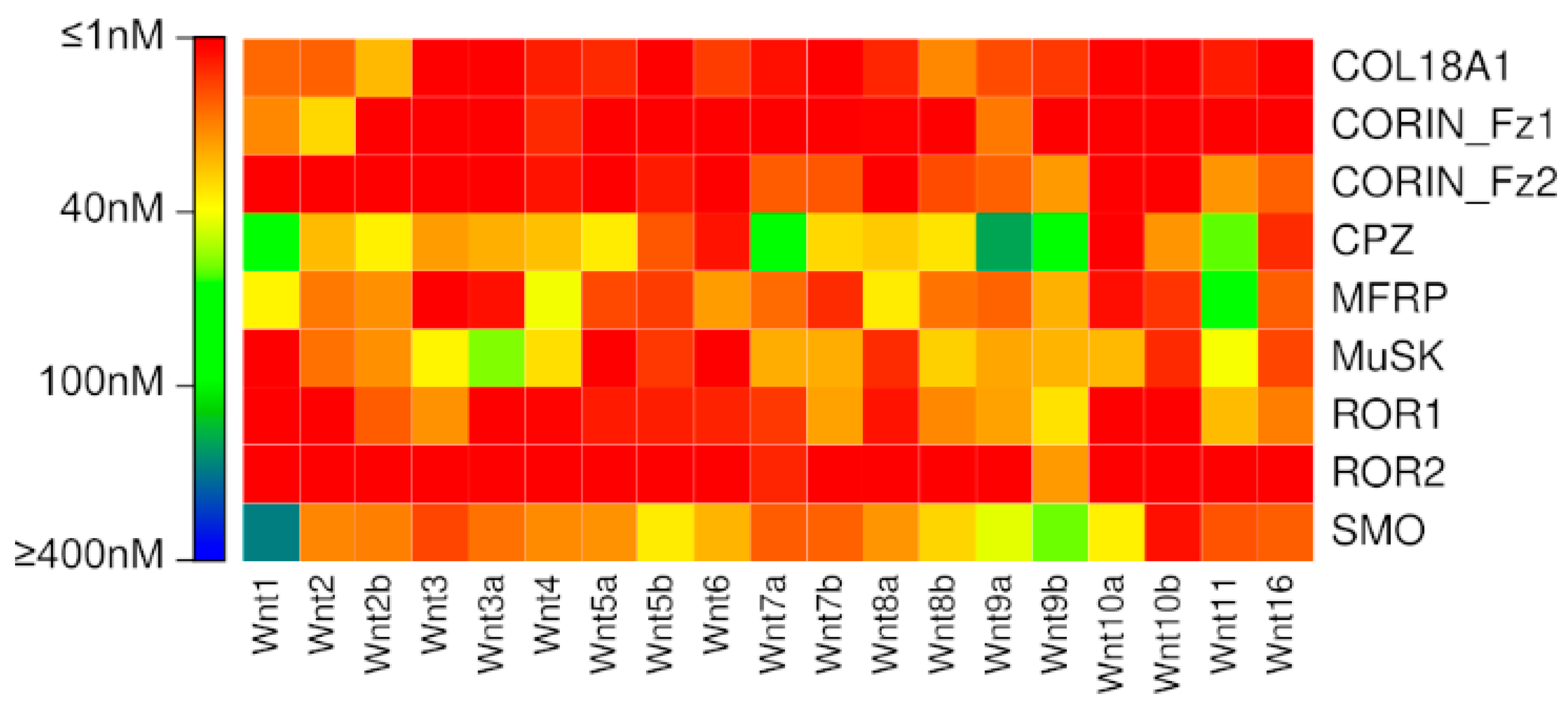
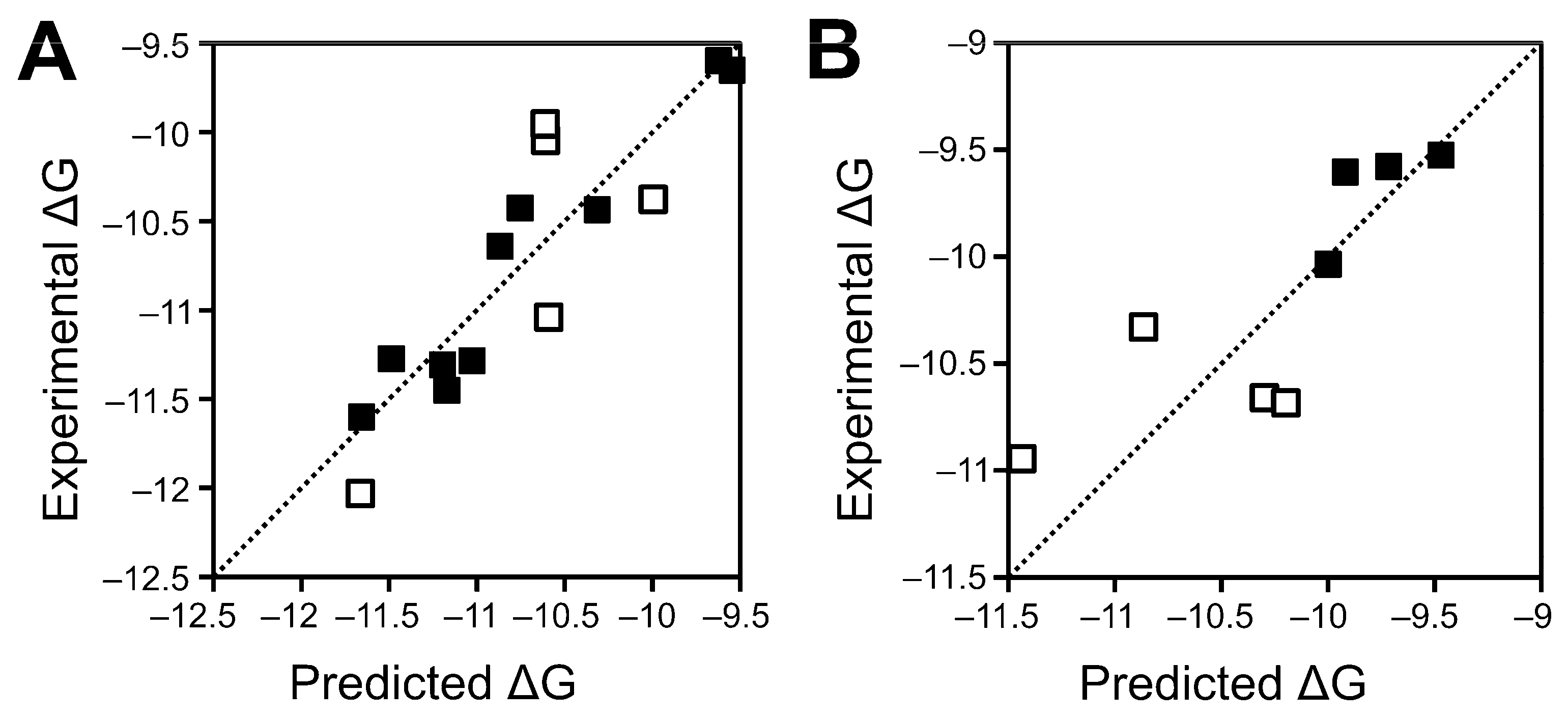

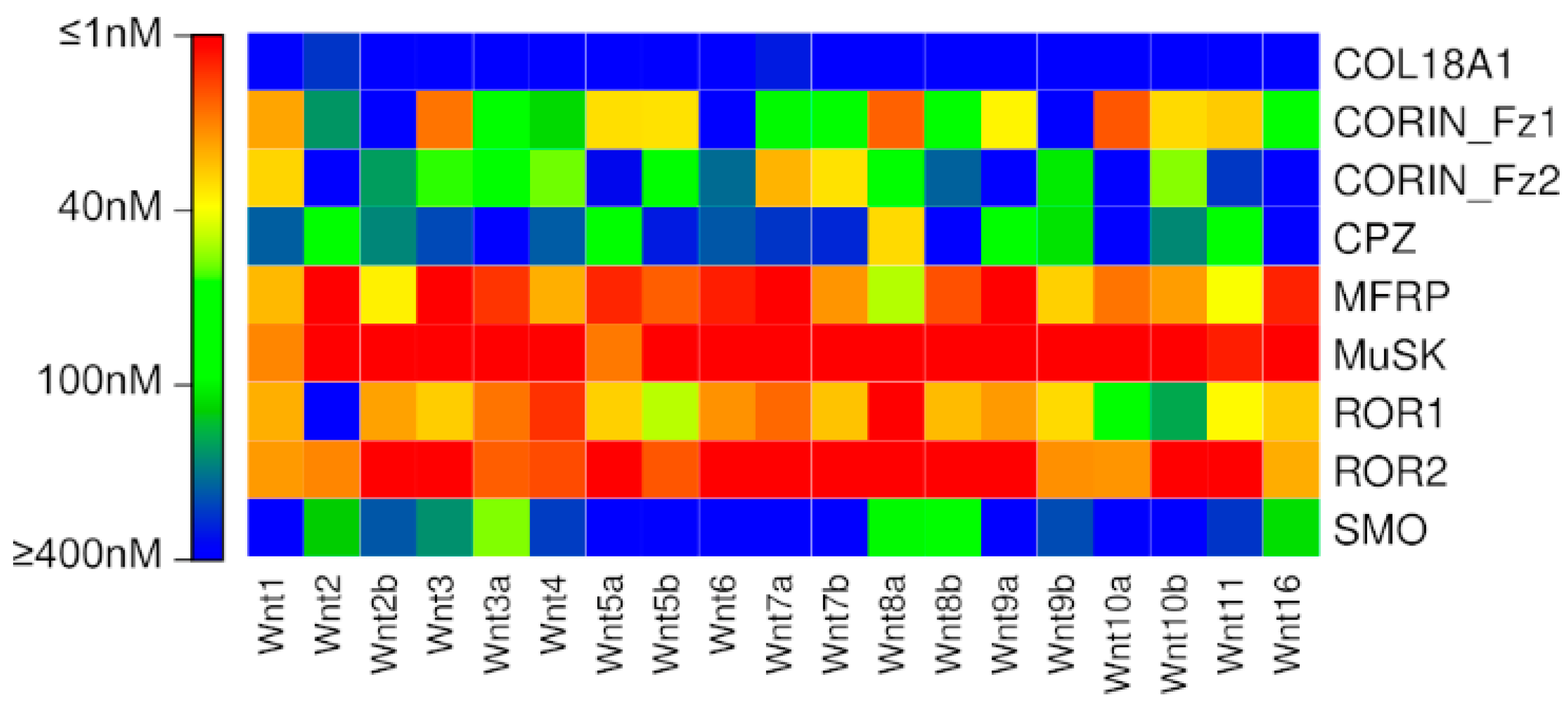
| Number | Model | RMSEtrain | RMSEtest | InExptrain | InExptest |
|---|---|---|---|---|---|
| 1 | ΔG = 0.06715 × CP_TSC + 0.001913 × CP_ELOCAL_CB − 0.01128 × CP_ELOCAL_MIN − 0.3072 × MMGBSA dG Bind Solv GB − 6.2941 | 0.33 | 0.36 | 9/15 | 4/8 |
| 2 | ΔG = 0.002936 × CP_ELOCAL_CB − 0.01811 × CP_ELOCAL_MIN − 0.6022 × CP_ZLOCAL_CB − 0.2115 × MMGBSA dG Bind Solv GB − 7.2704 | 0.40 | 0.32 | 6/15 | 4/8 |
| 3 | ΔG = 0.0840 × CP_TSC + 0.2258 × INSIDE − 0.06487 × FA_PP − 0.4274 × MMGBSA dG Bind Solv GB − 4.6833 | 0.36 | 0.40 | 7/15 | 6/8 |
| Interaction | ΔGexp a | ΔGpred a | |ΔGexp − ΔGpred| a,b | Experimental Kd c | Predicted Kd c | Experimental Range (Predicted Range) d | Set |
|---|---|---|---|---|---|---|---|
| mWnt3a–mFzd2 | −10.64 | −10.87 | 0.23 | 15.7 | 10.7 | +++ (+++) | Training |
| mWnt3a–mFzd4 | −11.27 | −11.49 | 0.22 | 5.4 | 3.7 | ++++ (++++) | Training |
| mWnt3a–mFzd5 | −11.60 | −11.66 | 0.06 | 3.1 | 2.8 | ++++ (++++) | Training |
| mWnt3a–mFzd7 | −11.28 | −11.03 | 0.25 | 5.3 | 8.1 | ++++ (++++) | Training |
| mWnt3a–mFzd8 | −12.03 | −11.66 | 0.37 | 1.5 | 2.8 | ++++ (++++) | Training |
| mWnt5–mFzd2 | −10.38 | −10.00 | 0.38 | 24.4 | 46.3 | +++ (++) | Training |
| mWnt5–mFzd4 | −10.38 | −10.75 | 0.37 | 24.4 | 13.0 | +++ (+++) | Training |
| mWnt5–mFzd5 | −11.31 | −11.20 | 0.11 | 5.1 | 6.1 | ++++ (++++) | Training |
| mWnt5–mFzd7 | −10.05 | −10.61 | 0.56 | 42.6 | 16.5 | ++ (+++) | Training |
| mWnt5–mFzd8 | −11.45 | −11.17 | 0.28 | 4.0 | 6.4 | ++++ (++++) | Training |
| mWnt5b–mFzd2 | −9.60 | −9.62 | 0.02 | 91.0 | 87.9 | ++ (++) | Training |
| mWnt5b–mFzd4 | −9.95 | −10.61 | 0.66 | 50.4 | 16.5 | ++ (+++) | Training |
| mWnt5b–mFzd5 | −10.44 | −10.32 | 0.12 | 22.0 | 27.0 | +++ (+++) | Training |
| mWnt5b–mFzd7 | −9.65 | −9.55 | 0.10 | 83.7 | 99.0 | ++ (++) | Training |
| mWnt5b–mFzd8 | −11.04 | −10.59 | 0.45 | 8.0 | 17.1 | ++++ (+++) | Training |
| mWnt3a–mFzd1 | −10.66 | −10.30 | 0.36 | 15.2 | 27.9 | +++ (+++) | Test |
| mWnt4–mFzd2 | −9.53 | −9.47 | 0.06 | 102.5 | 113.3 | + (+) | Test |
| mWnt4–mFzd4 | −10.04 | −10.00 | 0.04 | 43.3 | 46.3 | ++ (++) | Test |
| mWnt4–mFzd5 | −10.68 | −10.20 | 0.38 | 14.7 | 33.0 | +++ (+++) | Test |
| mWnt4–mFzd7 | −9.58 | −9.72 | 0.14 | 94.2 | 74.3 | ++ (++) | Test |
| mWnt4–mFzd8 | −10.95 | −11.44 | 0.49 | 9.3 | 4.1 | ++++ (++++) | Test |
| mWnt5–mFzd1 | −10.33 | −10.87 | 0.44 | 26.5 | 10.7 | +++ (+++) | Test |
| mWnt5b–mFzd1 | −9.60 | −9.92 | 0.32 | 91.0 | 53.0 | ++ (++) | Test |
| Protein | UniProt Accession | Sequence Used | Modelled Against a |
|---|---|---|---|
| MFRP | Q9BY79 | 461–579 | 5URV |
| CPZ | Q66K79 | 27–160 | 5URV |
| CORIN (Fzd 1) | Q9Y5Q5 | 134–259 | 5URV |
| CORIN (Fzd 2) | Q9Y5Q5 | 450–573 | 4F0A |
| COL18A1 | P39060 | 329–446 | 4F0A |
| SMO | Q99835 | 65–181 | 5L7D b |
| MuSK | O15146 | 312–450 | 3HKL |
| ROR1 | Q01973 | 165–299 | 3HKL |
| ROR2 | Q01974 | 169–303 | 3HKL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostino, M.; Pohl, S.Ö.-G. Wnt Binding Affinity Prediction for Putative Frizzled-Type Cysteine-Rich Domains. Int. J. Mol. Sci. 2019, 20, 4168. https://doi.org/10.3390/ijms20174168
Agostino M, Pohl SÖ-G. Wnt Binding Affinity Prediction for Putative Frizzled-Type Cysteine-Rich Domains. International Journal of Molecular Sciences. 2019; 20(17):4168. https://doi.org/10.3390/ijms20174168
Chicago/Turabian StyleAgostino, Mark, and Sebastian Öther-Gee Pohl. 2019. "Wnt Binding Affinity Prediction for Putative Frizzled-Type Cysteine-Rich Domains" International Journal of Molecular Sciences 20, no. 17: 4168. https://doi.org/10.3390/ijms20174168





