Liver X Receptors and Their Implications in the Physiology and Pathology of the Peripheral Nervous System
Abstract
:1. Introduction
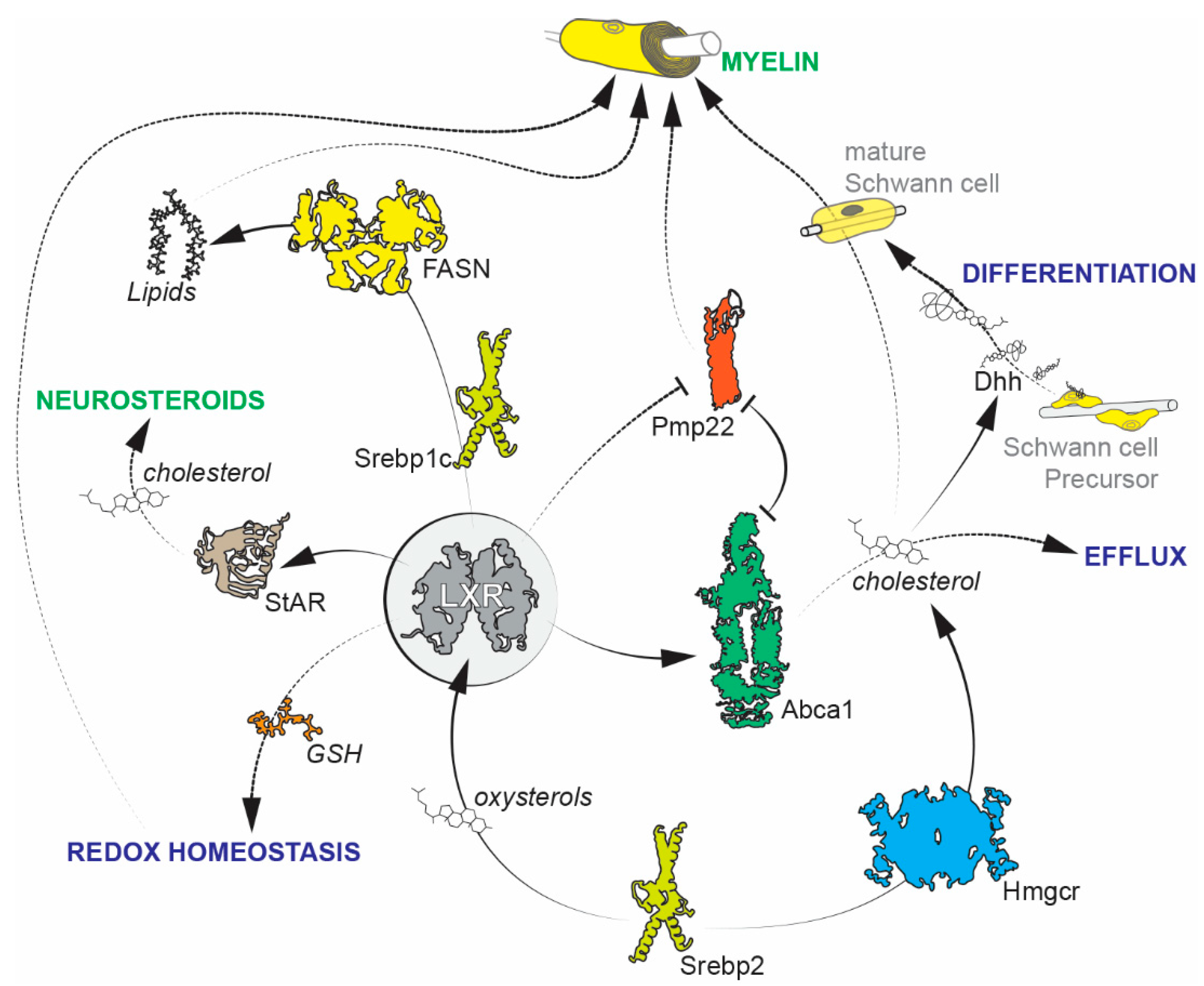
2. Schwann Cells
Future Avenues of Research on Schwann Cells
3. Sensory Neurons in the Ganglia
Future Avenues of Research on Sensory Neurons
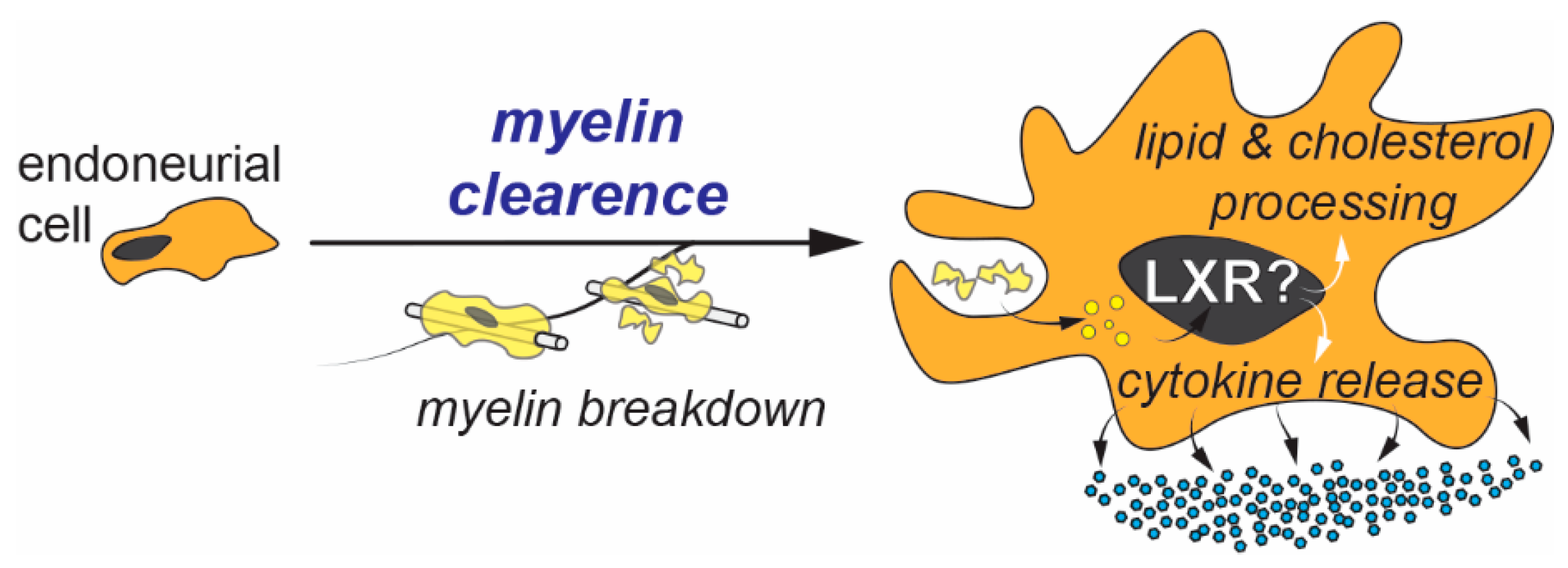
4. Endoneurial Cells
5. Perineurial Cells
6. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kainu, T.; Kononen, J.; Enmark, E.; Gustafsson, J.Å.; Pelto-Huikko, M. Localization and ontogeny of the orphan receptor OR-1 in the rat brain. J. Mol. Neurosci. 1996, 7, 29–39. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Kliewer, S.A.; Moore, L.B.; Smith-Oliver, T.A.; Oliver, B.B.; Su, J.L.; Sundseth, S.S.; Winegar, D.A.; Blanchard, D.E.; Spencer, T.A.; et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997, 272, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Grogan, M.J.; Jones, S.A.; Wisely, G.B.; Kliewer, S.A.; Corey, E.J.; Mangelsdorf, D.J. Structural requirements of ligands for the oxysterol liver X receptors LXR and LXR. Proc. Natl. Acad. Sci. USA 1999, 96, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Komati, R.; Spadoni, D.; Zheng, S.; Sridhar, J.; Riley, K.E.; Wang, G. Ligands of therapeutic utility for the liver X receptors. Molecules 2017, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, T.; Treuter, E.; Gustafsson, J.Å.; Steffensen, K.R. Liver X receptor biology and pharmacology: New pathways, challenges and opportunities. Trends Pharmacol. Sci. 2012, 33, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.G. Liver X receptors link lipid metabolism and inflammation. FEBS Lett. 2017, 591, 2978–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Vedin, L.L.; Steffensen, K.R. The emerging roles of liver X receptors and their ligands in cancer. Expert Opin. Ther. Targets 2016, 20, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Gabbi, C.; Warner, M.; Gustafsson, J.Å. Minireview: Liver X receptor β: Emerging roles in physiology and diseases. Mol. Endocrinol. 2009, 23, 129–136. [Google Scholar] [CrossRef]
- Luu, W.; Sharpe, L.J.; Capell-Hattam, I.; Gelissen, I.C.; Brown, A.J. Oxysterols: Old tale, new twists. Ann. Rev. Pharmacol. Toxicol. 2016, 56, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Courtney, R.; Landreth, G.E. LXR regulation of brain cholesterol: From development to disease. Trends Endocrinol. Metab. 2016, 27, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Schuster, G.U.; Hultenby, K.; Zhang, Q.; Andersson, S.; Gustafsson, J.A. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 13878–13883. [Google Scholar] [CrossRef] [PubMed]
- Cermenati, G.; Brioschi, E.; Abbiati, F.; Melcangi, R.C.; Caruso, D.; Mitro, N. Liver X receptors, nervous system, and lipid metabolism. J. Endocrinol. Investig. 2013, 36, 435–443. [Google Scholar] [PubMed]
- Joseph, N.M.; Mukouyama, Y.; Mosher, J.T.; Jaegle, M.; Crone, S.A.; Dormand, E.-L.; Lee, K.F.; Meijer, D.; Anderson, D.J.; Morrison, S.J. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 2004, 131. [Google Scholar] [CrossRef] [PubMed]
- Verber, N.S.; Shepheard, S.R.; Sassani, M.; McDonough, H.E.; Moore, S.A.; Alix, J.J.P.; Wilkinson, I.D.; Jenkins, T.M.; Shaw, P.J. Biomarkers in motor neuron disease: A state of the art review. Front. Neurol. 2019, 10, 291. [Google Scholar] [CrossRef]
- Abdel-Khalik, J.; Yutuc, E.; Crick, P.J.; Gustafsson, J.Å.; Warner, M.; Roman, G.; Talbot, K.; Gray, E.; Griffiths, W.J.; Turner, M.R.; et al. Defective cholesterol metabolism in amyotrophic lateral sclerosis. J. Lipid Res. 2017, 58, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, F.; Hussain, G.; Dupuis, L.; Loeffler, J.P.; Henriques, A. A plural role for lipids in motor neuron diseases: energy, signaling and structure. Front. Cell. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef]
- Makoukji, J.; Shackleford, G.; Meffre, D.; Grenier, J.; Liere, P.; Lobaccaro, J.M.A.; Schumacher, M.; Massaad, C. Interplay between LXR and Wnt/β-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J. Neurosci. 2011, 31, 9620–9629. [Google Scholar] [CrossRef]
- Hichor, M.; Sampathkumar, N.K.; Montanaro, J.; Borderie, D.; Petit, P.X.; Gorgievski, V.; Tzavara, E.T.; Eid, A.A.; Charbonnier, F.; Grenier, J.; et al. Paraquat induces peripheral myelin disruption and locomotor defects: Crosstalk with LXR and wnt pathways. Antioxid. Redox Signal. 2017, 27, 168–183. [Google Scholar] [CrossRef]
- Hichor, M.; Sundaram, V.K.; Eid, S.A.; Abdel-Rassoul, R.; Petit, P.X.; Borderie, D.; Bastin, J.; Eid, A.A.; Manuel, M.; Grenier, J.; et al. Liver X receptor exerts a protective effect against the oxidative stress in the peripheral nerve. Sci. Rep. 2018, 8, 2524. [Google Scholar] [CrossRef]
- Tawk, M.; Makoukji, J.; Belle, M.; Fonte, C.; Trousson, A.; Hawkins, T.; Li, H.; Ghandour, S.; Schumacher, M.; Massaad, C. Wnt/β-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J. Neurosci. 2011, 31, 3729–3742. [Google Scholar] [CrossRef]
- Makoukji, J.; Belle, M.; Meffre, D.; Stassart, R.; Grenier, J.; Shackleford, G.; Fledrich, R.; Fonte, C.; Branchu, J.; Goulard, M.; et al. Lithium enhances remyelination of peripheral nerves. Proc. Natl. Acad. Sci. USA 2012, 109, 3973–3978. [Google Scholar] [CrossRef] [Green Version]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–26. [Google Scholar] [CrossRef]
- Hamilton, R.T.; Bhattacharya, A.; Walsh, M.E.; Shi, Y.; Wei, R.; Zhang, Y.; Rodriguez, K.A.; Buffenstein, R.; Chaudhuri, A.R.; Van Remmen, H. Elevated protein carbonylation, and misfolding in sciatic nerve from db/db and Sod1−/− mice: plausible link between oxidative stress and demyelination. PLoS ONE 2013, 8, e65725. [Google Scholar] [CrossRef]
- Zhou, Y.; Miles, J.R.; Tavori, H.; Lin, M.; Khoshbouei, H.; Borchelt, D.; Bazick, H.; Landreth, G.E.; Lee, S.; Fazio, S.; et al. PMP22 regulates cholesterol trafficking and ABCA1-mediated cholesterol efflux. J. Neurosci. 2019, 39, 5404–5418. [Google Scholar] [CrossRef]
- Cermenati, G.; Giatti, S.; Cavaletti, G.; Bianchi, R.; Maschi, O.; Pesaresi, M.; Abbiati, F.; Volonterio, A.; Saez, E.; Caruso, D.; et al. Activation of the liver X receptor increases neuroactive steroid levels and protects from diabetes-induced peripheral neuropathy. J. Neurosci. 2010, 30, 11896–11901. [Google Scholar] [CrossRef]
- Cermenati, G.; Abbiati, F.; Cermenati, S.; Brioschi, E.; Volonterio, A.; Cavaletti, G.; Saez, E.; De Fabiani, E.; Crestani, M.; Garcia-Segura, L.M.; et al. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: protective effects of LXR activation. J. Lipid Res. 2012, 53, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Melcangi, R.C.; Panzica, G.C. Neuroactive steroids: Old players in a new game. Neuroscience 2006, 138, 733–739. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Garcia-Segura, L.M.; Mensah-Nyagan, A.G. Neuroactive steroids: State of the art and new perspectives. Cell. Mol. Life Sci. 2008, 65, 777–797. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Garcia-Segura, L.M. Therapeutic approaches to peripheral neuropathy based on neuroactive steroids. Expert Rev. Neurother. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Montani, L.; Pereira, J.A.; Norrmén, C.; Pohl, H.B.F.; Tinelli, E.; Trötzmüller, M.; Figlia, G.; Dimas, P.; von Niederhäusern, B.; Schwager, R.; et al. De novo fatty acid synthesis by Schwann cells is essential for peripheral nervous system myelination. J. Cell Biol. 2018, 217, 1353–1368. [Google Scholar] [CrossRef] [Green Version]
- Saher, G.; Quintes, S.; Mobius, W.; Wehr, M.C.; Kramer-Albers, E.M.; Brugger, B.; Nave, K.A. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J. Neurosci. 2009, 29, 6094–6104. [Google Scholar] [CrossRef]
- Verheijen, M.H.G.; Camargo, N.; Verdier, V.; Nadra, K.; de Preux Charles, A.S.; Médard, J.J.; Luoma, A.; Crowther, M.; Inouye, H.; Shimano, H.; et al. SCAP is required for timely and proper myelin membrane synthesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21383–21388. [Google Scholar] [CrossRef] [Green Version]
- Valentijn, L.J.; Bolhuis, P.A.; Zorn, I.; Hoogendijk, J.E.; van den Bosch, N.; Hensels, G.W.; Stanton, V.P.; Housman, D.E.; Fischbeck, K.H.; Ross, D.A.; et al. The peripheral myelin gene PMP–22/GAS–3 is duplicated in charcot–marie–tooth disease type 1A. Nat. Genet. 1992, 1, 166–170. [Google Scholar] [CrossRef]
- Li, J. Caveats in the established understanding of CMT1A. Ann. Clin. Transl. Neurol. 2017, 4, 601–607. [Google Scholar] [CrossRef]
- Fledrich, R.; Abdelaal, T.; Rasch, L.; Bansal, V.; Schütza, V.; Brügger, B.; Lüchtenborg, C.; Prukop, T.; Stenzel, J.; Rahman, R.U.; et al. Targeting myelin lipid metabolism as a potential therapeutic strategy in a model of CMT1A neuropathy. Nat. Commun. 2018, 9, 3025. [Google Scholar] [CrossRef]
- Farid, M.; Demicco, E.G.; Garcia, R.; Ahn, L.; Merola, P.R.; Cioffi, A.; Maki, R.G. Malignant peripheral nerve sheath tumors. Oncologist 2014, 19, 193–201. [Google Scholar] [CrossRef]
- Brohl, A.S.; Kahen, E.; Yoder, S.J.; Teer, J.K.; Reed, D.R. The genomic landscape of malignant peripheral nerve sheath tumors: diverse drivers of ras pathway activation. Sci. Rep. 2017, 7, 14992. [Google Scholar] [CrossRef]
- Patel, A.V.; Johansson, G.; Colbert, M.C.; Dasgupta, B.; Ratner, N. Fatty acid synthase is a metabolic oncogene targetable in malignant peripheral nerve sheath tumors. Neuro. Oncol. 2015, 17, 1599–1608. [Google Scholar] [CrossRef] [Green Version]
- Varin, J.; Poulain, L.; Hivelin, M.; Nusbaum, P.; Hubas, A.; Laurendeau, I.; Lantieri, L.; Wolkenstein, P.; Vidaud, M.; Pasmant, E.; et al. Dual mTORC1/2 inhibition induces anti-proliferative effect in NF1-associated plexiform neurofibroma and malignant peripheral nerve sheath tumor cells. Oncotarget 2016, 7, 35753–35767. [Google Scholar] [CrossRef]
- Johansson, G.; Mahller, Y.Y.; Collins, M.H.; Kim, M.O.; Nobukuni, T.; Perentesis, J.; Cripe, T.P.; Lane, H.A.; Kozma, S.C.; Thomas, G.; et al. Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol. Cancer Ther. 2008, 7, 1237–1245. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3281–3282. [Google Scholar] [CrossRef] [Green Version]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Repa, J.J.; Liang, G.; Ou, J.; Bashmakov, Y.; Lobaccaro, J.M.; Shimomura, I.; Shan, B.; Brown, M.S.; Goldstein, J.L.; Mangelsdorf, D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000, 14, 2819–2830. [Google Scholar] [CrossRef]
- Higuchi, N.; Kato, M.; Shundo, Y.; Tajiri, H.; Tanaka, M.; Yamashita, N.; Kohjima, M.; Kotoh, K.; Nakamuta, M.; Takayanagi, R.; et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 2008, 38, 1122–1129. [Google Scholar] [CrossRef]
- Rong, S.; Cortés, V.A.; Rashid, S.; Anderson, N.N.; McDonald, J.G.; Liang, G.; Moon, Y.-A.; Hammer, R.E.; Horton, J.D. Expression of SREBP-1c Requires SREBP-2-mediated generation of a sterol ligand for LXR in livers of mice. Elife 2017, 6. [Google Scholar] [CrossRef]
- Gavini, C.K.; Bookout, A.L.; Bonomo, R.; Gautron, L.; Lee, S.; Mansuy-Aubert, V. Liver X receptors protect dorsal root ganglia from obesity-induced endoplasmic reticulum stress and mechanical allodynia. Cell Rep. 2018, 25, 271–277. [Google Scholar] [CrossRef]
- Mansuy-Aubert, V.; Gautron, L.; Lee, S.; Bookout, A.L.; Kusminski, C.M.; Sun, K.; Zhang, Y.; Scherer, P.E.; Mangelsdorf, D.J.; Elmquist, J.K. Loss of the liver X receptor LXRα/β in peripheral sensory neurons modifies energy expenditure. Elife 2015, 4. [Google Scholar] [CrossRef]
- Richard, L.; Topilko, P.; Magy, L.; Decouvelaere, A.V.; Charnay, P.; Funalot, B.; Vallat, J.-M. Endoneurial fibroblast-like cells. J. Neuropathol. Exp. Neurol. 2012, 71, 938–947. [Google Scholar] [CrossRef]
- Richard, L.; Védrenne, N.; Vallat, J.M.; Funalot, B. Characterization of endoneurial fibroblast-like cells from human and rat peripheral nerves. J. Histochem. Cytochem. 2014, 62, 424–435. [Google Scholar] [CrossRef]
- Woodhoo, A.; Sommer, L. Development of the schwann cell lineage: From the neural crest to the myelinated nerve. Glia 2008, 56, 1481–1490. [Google Scholar] [CrossRef]
- Schubert, T.; Friede, R.L. The role of endoneurial fibroblasts in myelin degradation. J. Neuropathol. Exp. Neurol. 1981, 40, 134–154. [Google Scholar] [CrossRef]
- Stevens, A.; Schabet, M.; Schott, K.; Wiethölter, H. Role of endoneural cells in experimental allergic neuritis and characterisation of a resident phagocytic cell. Acta Neuropathol. 1989, 77, 412–419. [Google Scholar] [CrossRef]
- Goodrum, J.F.; Earnhardt, T.; Goines, N.; Bouldin, T.W. Fate of myelin lipids during degeneration and regeneration of peripheral nerve: an autoradiographic study. J. Neurosci. 1994, 14, 357–367. [Google Scholar] [CrossRef]
- Müller, M.; Stenner, M.; Wacker, K.; Ringelstein, E.B.; Hickey, W.F.; Kiefer, R. Contribution of resident endoneurial macrophages to the local cellular response in experimental autoimmune neuritis. J. Neuropathol. Exp. Neurol. 2006, 65, 499–507. [Google Scholar] [CrossRef]
- Pascual-García, M.; Valledor, A.F. Biological roles of liver X receptors in immune cells. Arch. Immunol. Ther. Exp. (Warsz). 2012, 60, 235–249. [Google Scholar] [CrossRef]
- Bunge, M.; Wood, P.; Tynan, L.; Bates, M.; Sanes, J.R. Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science 1989, 243, 229–231. [Google Scholar] [CrossRef]
- Kucenas, S. Perineurial glia. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef]
- Fontenas, L.; Kucenas, S. Livin′ on the edge: Glia shape nervous system transition zones. Curr. Opin. Neurobiol. 2017, 47, 44–51. [Google Scholar] [CrossRef]
- Fontenas, L.; Kucenas, S. Motor exit point (MEP) glia: Novel myelinating glia that bridge CNS and PNS myelin. Front. Cell. Neurosci. 2018, 12, 333. [Google Scholar] [CrossRef]
- Parmantier, E.; Lynn, B.; Lawson, D.; Turmaine, M.; Namini, S.S.; Chakrabarti, L.; McMahon, A.P.; Jessen, K.R.; Mirsky, R. Schwann cell-derived desert hedgehog controls the development of peripheral nerve sheaths. Neuron 1999, 23, 713–724. [Google Scholar] [CrossRef]
- Sharghi-Namini, S.; Turmaine, M.; Meier, C.; Sahni, V.; Umehara, F.; Jessen, K.R.; Mirsky, R. The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J. Neurosci. 2006, 26, 6364–6376. [Google Scholar] [CrossRef]
- Petrov, K.; Wierbowski, B.M.; Salic, A. Sending and receiving hedgehog signals. Ann. Rev. Cell Dev. Biol. 2017, 33, 145–168. [Google Scholar] [CrossRef]
- Huang, P.; Nedelcu, D.; Watanabe, M.; Jao, C.; Kim, Y.; Liu, J.; Salic, A. Cellular cholesterol directly activates smoothened in hedgehog signaling. Cell 2016, 166, 1176–1187. [Google Scholar] [CrossRef]
- Nedelcu, D.; Liu, J.; Xu, Y.; Jao, C.; Salic, A. Oxysterol binding to the extracellular domain of smoothened in hedgehog signaling. Nat. Chem. Biol. 2013, 9, 557–564. [Google Scholar] [CrossRef]
- Xu, P.; Xu, H.; Tang, X.; Xu, L.; Wang, Y.; Guo, L.; Yang, Z.; Xing, Y.; Wu, Y.; Warner, M.; et al. Liver X receptor β is essential for the differentiation of radial glial cells to oligodendrocytes in the dorsal cortex. Mol. Psychiatry 2014, 19, 947–957. [Google Scholar] [CrossRef]
- Andersson, S.; Gustafsson, N.; Warner, M.; Gustafsson, J.A. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc. Natl. Acad. Sci. USA 2005, 102, 3857–3862. [Google Scholar] [CrossRef]
- Sacchetti, P.; Sousa, K.M.; Hall, A.C.; Liste, I.; Steffensen, K.R.; Theofilopoulos, S.; Parish, C.L.; Hazenberg, C.; Richter, L.Ä.; Hovatta, O.; et al. Liver X receptors and oxysterols promote ventral midbrain neurogenesis in vivo and in human embryonic stem cells. Cell Stem Cell 2009, 5, 409–419. [Google Scholar] [CrossRef]
- Wouters, E.; de Wit, N.M.; Vanmol, J.; van der Pol, S.M.A.; van het Hof, B.J.; Sommer, D.; Loix, M.; Geerts, D.; Gustafsson, J.A.; Steffensen, K.R.; et al. Liver X receptor alpha is important in maintaining blood-brain barrier function. Front. Immunol. 2019, 10, 1811. [Google Scholar] [CrossRef]
- Lund, E.G.; Peterson, L.B.; Adams, A.D.; Lam, M.H.N.; Burton, C.A.; Chin, J.; Guo, Q.; Huang, S.; Latham, M.; Lopez, J.C.; et al. Different roles of liver X receptor α and β in lipid metabolism: Effects of an α-selective and a dual agonist in mice deficient in each subtype. Biochem. Pharmacol. 2006, 71, 453–463. [Google Scholar] [CrossRef]
- Alberti, S.; Schuster, G.; Parini, P.; Feltkamp, D.; Diczfalusy, U.; Rudling, M.; Angelin, B.; Björkhem, I.; Pettersson, S.; Gustafsson, J. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRβ-deficient mice. J. Clin. Investig. 2001, 107, 565–573. [Google Scholar] [CrossRef]
- Zhang, Y.; Repa, J.J.; Gauthier, K.; Mangelsdorf, D.J. Regulation of lipoprotein lipase by the oxysterol receptors, LXRα and LXRβ. J. Biol. Chem. 2001, 276, 43018–43024. [Google Scholar] [CrossRef]
- Magida, J.A.; Evans, R.M. Rational application of macrophage-specific LXR agonists avoids the pitfalls of SREBP-induced lipogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, 5051–5053. [Google Scholar] [CrossRef] [Green Version]
- Muse, E.D.; Yu, S.; Edillor, C.R.; Tao, J.; Spann, N.J.; Troutman, T.D.; Seidman, J.S.; Henke, A.; Roland, J.T.; Ozeki, K.A.; et al. Cell-specific discrimination of desmosterol and desmosterol mimetics confers selective regulation of LXR and SREBP in macrophages. Proc. Natl. Acad. Sci. USA 2018, 115, E4680–E4689. [Google Scholar] [CrossRef] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaram, V.K.; Massaad, C.; Grenier, J. Liver X Receptors and Their Implications in the Physiology and Pathology of the Peripheral Nervous System. Int. J. Mol. Sci. 2019, 20, 4192. https://doi.org/10.3390/ijms20174192
Sundaram VK, Massaad C, Grenier J. Liver X Receptors and Their Implications in the Physiology and Pathology of the Peripheral Nervous System. International Journal of Molecular Sciences. 2019; 20(17):4192. https://doi.org/10.3390/ijms20174192
Chicago/Turabian StyleSundaram, Venkat Krishnan, Charbel Massaad, and Julien Grenier. 2019. "Liver X Receptors and Their Implications in the Physiology and Pathology of the Peripheral Nervous System" International Journal of Molecular Sciences 20, no. 17: 4192. https://doi.org/10.3390/ijms20174192
APA StyleSundaram, V. K., Massaad, C., & Grenier, J. (2019). Liver X Receptors and Their Implications in the Physiology and Pathology of the Peripheral Nervous System. International Journal of Molecular Sciences, 20(17), 4192. https://doi.org/10.3390/ijms20174192



