Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
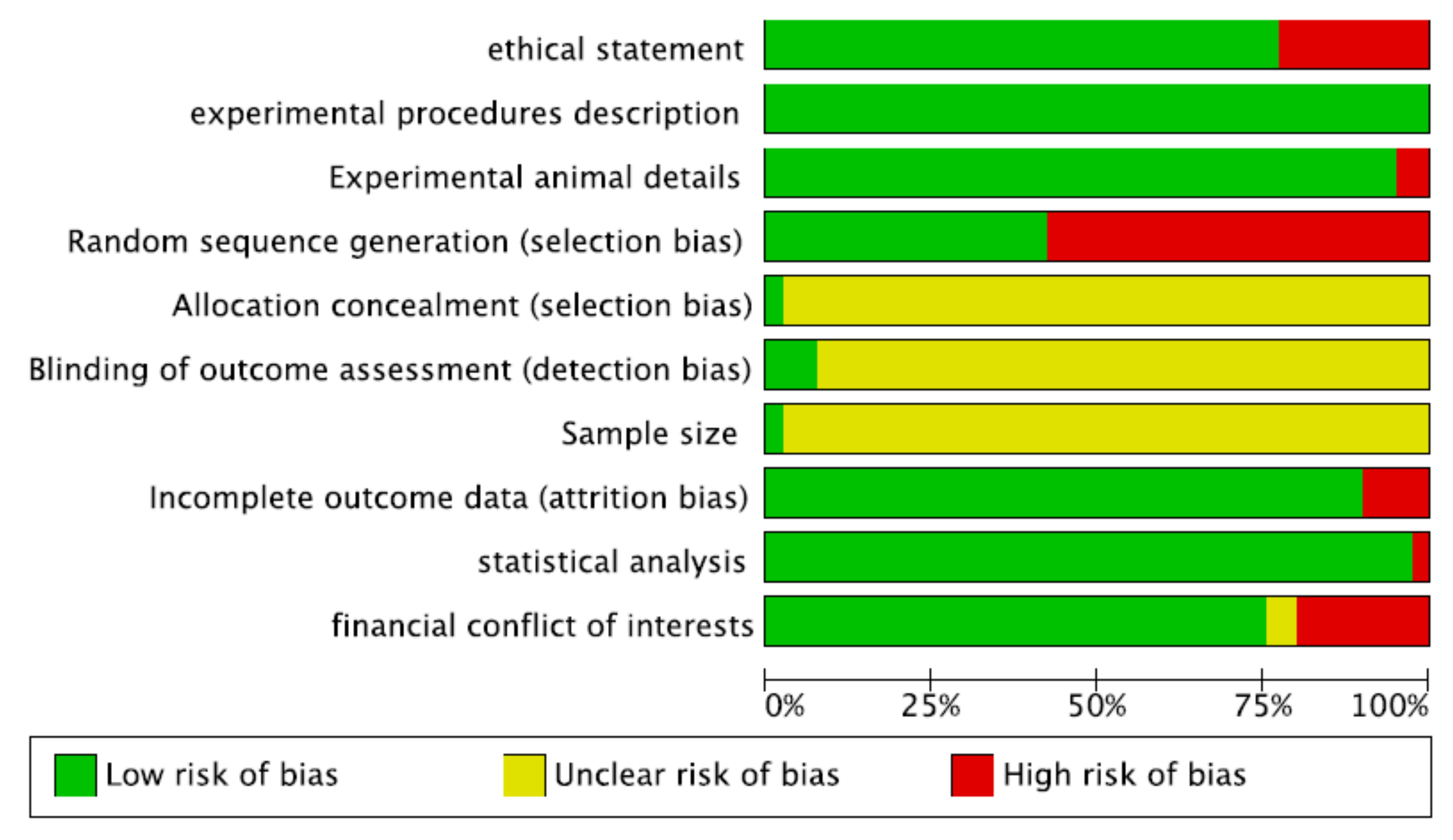
2.1. Risk-of-Bias Assessment
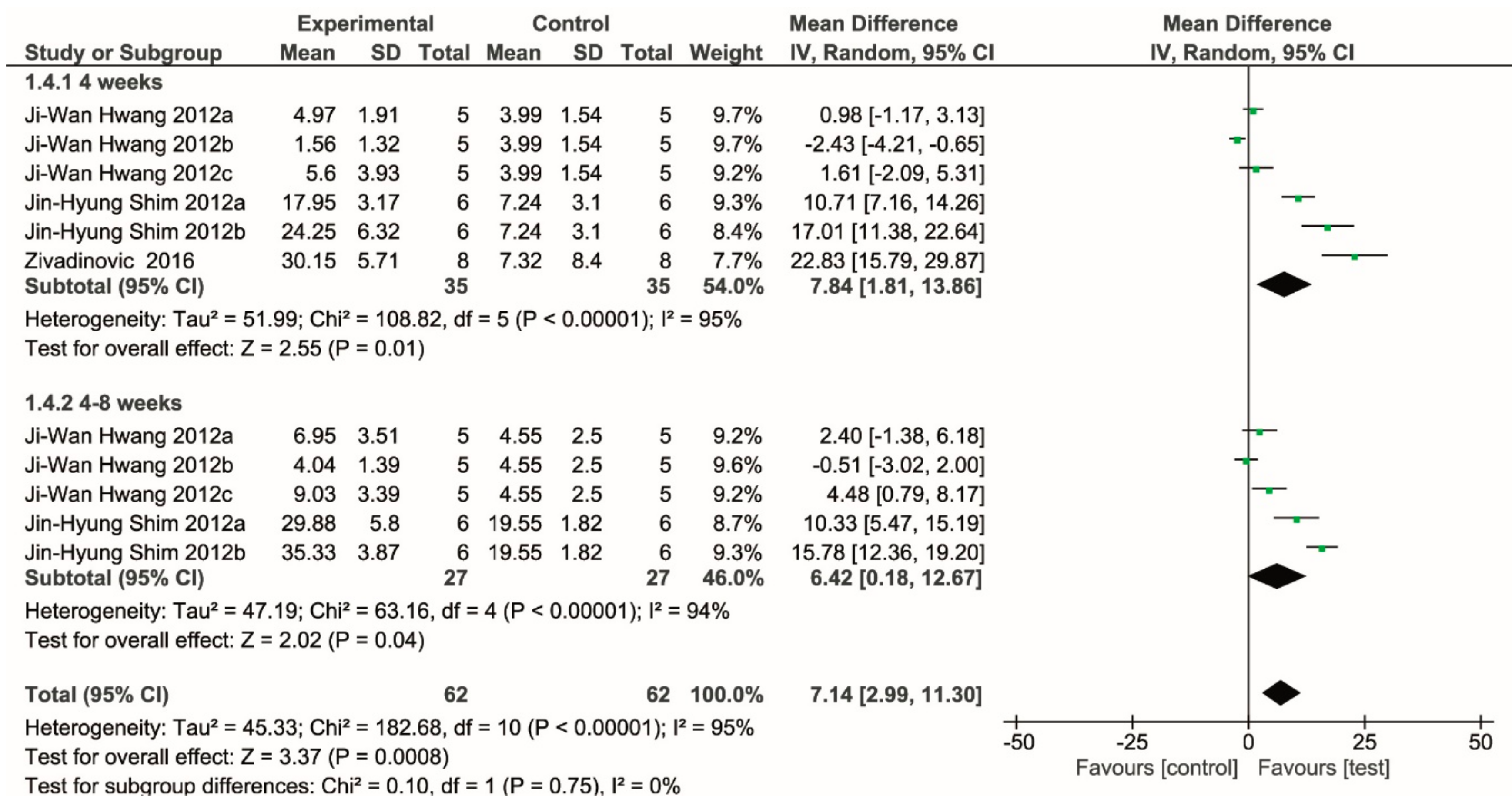
2.2. Meta-Analysis
2.3. Studies Not Included in the Meta-Analysis
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Inclusion Criteria
4.3. Selection of the Studies
4.4. Data Extraction
4.5. Risk-of-Bias Analysis
- Ethical statement (nature of ethical review permissions and national or institutional guidelines for the care and use of animals)
- Experimental procedures (precise details of all procedures performed)
- Experimental animals (details of animal used, including species, developmental stage or mean age, type of teeth, and diagnosis)
- Randomization
- Allocation concealment
- Blinding of the evaluator
- Sample size calculation
- Completeness of information on dropouts
- Statistical analysis appropriateness
- Financial conflict of interest
4.6. Meta-Analysis Inclusion Criteria
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009, 24, 237–259. [Google Scholar]
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implant. 2009, 24, 218–236. [Google Scholar]
- Urban, I.A.; Jovanovic, S.A.; Lozada, J.L. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: A retrospective study of 35 patients 12 to 72 months after loading. Int. J. Oral Maxillofac. Implant. 2009, 24, 502–510. [Google Scholar]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 136–159. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Schiegnitz, E. Augmentation procedures using bone substitute materials or autogenous bone - a systematic review and meta-analysis. Eur. J. Oral Implant. 2014, 7 (Suppl. 2), S219–S234. [Google Scholar]
- Kesireddy, V.; Kasper, F.K. Approaches for building bioactive elements into synthetic scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 6773–6786. [Google Scholar] [CrossRef]
- Sreejalekshmi, K.G.; Nair, P.D. Biomimeticity in tissue engineering scaffolds through synthetic peptide modifications-altering chemistry for enhanced biological response. J. Biomed. Mater. Res. A 2011, 96, 477–491. [Google Scholar] [CrossRef]
- Horowitz, R.A.; Leventis, M.D.; Rohrer, M.D.; Prasad, H.S. Bone grafting: History, rationale, and selection of materials and techniques. Compend Contin Educ Dent. 2014, 35, 1–6. [Google Scholar]
- Scarano, A.; Carinci, F.; Assenza, B.; Piattelli, M.; Murmura, G.; Piattelli, A. Vertical ridge augmentation of atrophic posterior mandible using an inlay technique with a xenograft without miniscrews and miniplates: Case series. Clin. Oral Implant. Res. 2011, 22, 1125–1130. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Chen, P.Y.; Yang, K.C.; Wu, C.C.; Yu, J.H.; Lin, F.H.; Sun, J.S. Fabrication of large perfusable macroporous cell-laden hydrogel scaffolds using microbial transglutaminase. Acta Biomater. 2014, 10, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Grigolo, B.; Fiorini, M.; Manferdini, C.; Cavallo, C.; Gabusi, E.; Zini, N.; Dolcini, L.; Nicoletti, A.; Pressato, D.; Facchini, A.; et al. Chemical-physical properties and in vitro cell culturing of a novel biphasic bio-mimetic scaffold for osteo-chondral tissue regeneration. J. Biol. Regul. Homeost. Agents 2011, 25, S3–S13. [Google Scholar] [PubMed]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implant. 2014, 7 (Suppl. 2), S203–S217. [Google Scholar]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J. Stem Cells 2015, 7, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Motamedian, S.R.; Tabatabaei, F.S.; Akhlaghi, F.; Torshabi, M.; Gholamin, P.; Khojasteh, A. Response of Dental Pulp Stem Cells to Synthetic, Allograft, and Xenograft Bone Scaffolds. Int. J. Periodontics Restor. Dent. 2017, 37, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Shand, J.M.; Heggie, A.A.C.; Holmes, A.D.; Holmes, W. Allogeneic bone grafting of calvarial defects: An experimental study in the rabbit. Int. J. Oral Maxillofac. Surg. 2002, 31, 525–531. [Google Scholar] [CrossRef]
- Mohamed, K.R.; Beherei, H.H.; El Bassyouni, G.T.; El Mahallawy, N. Fabrication and mechanical evaluation of hydroxyapatite/oxide nano-composite materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4126–4132. [Google Scholar] [CrossRef]
- Ormanci, O.; Akin, I.; Sahin, F.; Yucel, O.; Simon, V.; Cavalu, S.; Goller, G. Spark plasma sintered Al₂O₃-YSZ-TiO₂ composites: Processing, characterization and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 16–23. [Google Scholar] [CrossRef]
- Turkmen, A.; Cavalu, S.; Goller, G. Development of chitosan-hydroxyapatite-fibrinogen 3D scaffolds for bone tissue regeneration. J. Aust. Ceram. Soc. 2016, 2016, 52. [Google Scholar]
- Cavalu, S.; Simon, V. Microstructure and bioactivity of acrylic bone cements for prosthetic surgery. J. Optoelectron. Adv. Mater. 2006, 8, 1520. [Google Scholar]
- Hammouche, S.; Hammouche, D.; McNicholas, M. Biodegradable bone regeneration synthetic scaffolds: In tissue engineering. Curr. Stem Cell Res. Ther. 2012, 7, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Tamimi, F.; Alkhraisat, M.H.; Prados-Frutos, J.C.; Rastikerdar, E.; Gbureck, U.; Barralet, J.E.; López-Cabarcos, E. Vertical bone augmentation with 3D-synthetic monetite blocks in the rabbit calvaria. J. Clin. Periodontol. 2011, 38, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Živadinović, M.; Andrić, M.; Milošević, V.; Manojlović-Stojanoski, M.; Prokić, B.; Prokić, B.; Dimić, A.; Ćalasan, D.; Brković, B. Histomorphometric evaluation of bone regeneration using autogenous bone and beta-tricalcium phosphate in diabetic rabbits. Vojnosanit. Pregl. 2016, 73, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Torres, J.; Gbureck, U.; Lopez-Cabarcos, E.; Bassett, D.C.; Alkhraisat, M.H.; Barralet, J.E. Craniofacial vertical bone augmentation: A comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials 2009, 30, 6318–6326. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.W.; Park, J.S.; Lee, J.S.; Jung, U.W.; Kim, C.S.; Cho, K.S.; Lee, Y.K.; Choi, S.H. Comparative evaluation of three calcium phosphate synthetic block bone graft materials for bone regeneration in rabbit calvaria. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2012, 100, 2044–2052. [Google Scholar] [CrossRef]
- Bae, S.Y.; Park, J.C.; Shin, H.S.; Lee, Y.K.; Choi, S.H.; Jung, U.W. Tomographic and histometric analysis of autogenous bone block and synthetic hydroxyapatite block grafts without rigid fixation on rabbit calvaria. J. Periodontal Implant Sci. 2014, 44, 251–258. [Google Scholar] [CrossRef]
- Shim, J.H.; Moon, T.S.; Yun, M.J.; Jeon, Y.C.; Jeong, C.M.; Cho, D.W.; Huh, J.B. Stimulation of healing within a rabbit calvarial defect by a PCL/PLGA scaffold blended with TCP using solid freeform fabrication technology. J. Mater. Sci. Mater. Med. 2012, 23, 2993–3002. [Google Scholar] [CrossRef]
- Xu, S.; Lin, K.; Wang, Z.; Chang, J.; Wang, L.; Lu, J.; Ning, C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Bae, J.H.; Kim, S.E.; Bae, E.B.; Kim, S.Y.; Choi, K.H.; Moon, K.O.; Jeong, C.M.; Huh, J.B. The Effect of Bisphasic Calcium Phosphate Block Bone Graft Materials with Polysaccharides on Bone Regeneration. Materials (Basel) 2017, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Crincoli, V.; Di Benedetto, A.; Cozzolino, V.; Lorusso, F.; Podaliri Vulpiani, M.; Grano, M.; Kalemaj, Z.; Mori, G.; Grassi, F.R. Bone Regeneration Induced by Bone Porcine Block with Bone Marrow Stromal Stem Cells in a Minipig Model of Mandibular “Critical Size” Defect. Stem Cells Int. 2017, 2017, 9082869. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. A 2009, 90, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, A.; Mazon, P.; Del Fabbro, M.; Tumedei, M.; Aramburù, J.; Perez-Diaz, L.; De Aza, P. Histological and Histomorphometric Analyses of Two Bovine Bone Blocks Implanted in Rabbit Calvaria. Symmetry 2019, 11, 641. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lin, R.M.; Ju, C.P.; Chern Lin, J.H. Bioresorption behavior of tetracalcium phosphate-derived calcium phosphate cement implanted in femur of rabbits. Biomaterials 2008, 29, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Kawai, A.; Sugihara, S.; Yoshida, A.; Inoue, H. Resorption of apatite-wollastonite containing glass-ceramic and beta-tricalcium phosphate in vivo. Acta Med. Okayama 2005, 59, 201–207. [Google Scholar]
- Ohsawa, K.; Neo, M.; Okamoto, T.; Tamura, J.; Nakamura, T. In vivo absorption of porous apatite- and wollastonite-containing glass-ceramic. J. Mater. Sci. Mater. Med. 2004, 15, 859–864. [Google Scholar] [CrossRef]
- Guo, J.; Meng, Z.; Chen, G.; Xie, D.; Chen, Y.; Wang, H.; Tang, W.; Liu, L.; Jing, W.; Long, J.; et al. Restoration of critical-size defects in the rabbit mandible using porous nanohydroxyapatite-polyamide scaffolds. Tissue Eng. Part A 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Zhang, P.; Hong, Z.; Yu, T.; Chen, X.; Jing, X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(L-lactide). Biomaterials 2009, 30, 58–70. [Google Scholar] [CrossRef]
- Rismanchian, M.; Nosouhian, S.; Razavi, S.M.; Davoudi, A.; Sadeghiyan, H. Comparing three different three-dimensional scaffolds for bone tissue engineering: An in vivo study. J. Contemp. Dent. Pract. 2015, 16, 25–30. [Google Scholar] [PubMed]
- Du, B.; Liu, W.; Deng, Y.; Li, S.; Liu, X.; Gao, Y.; Zhou, L. Angiogenesis and bone regeneration of porous nano-hydroxyapatite/coralline blocks coated with rhVEGF165 in critical-size alveolar bone defects in vivo. Int J. Nanomedicine 2015, 10, 2555–2565. [Google Scholar] [PubMed] [Green Version]
- Nery, E.B.; LeGeros, R.Z.; Lynch, K.L.; Lee, K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J. Periodontol. 1992, 63, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Erbe, E.M.; Marx, J.G.; Clineff, T.D.; Bellincampi, L.D. Potential of an ultraporous beta-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft. Eur. Spine J. 2001, 10, S141–S146. [Google Scholar] [PubMed]
- Gabbai-Armelin, P.R.; Souza, M.T.; Kido, H.W.; Tim, C.R.; Bossini, P.S.; Magri, A.M.P.; Fernandes, K.R.; Pastor, F.A.C.; Zanotto, E.D.; Parizotto, N.A.; et al. Effect of a new bioactive fibrous glassy scaffold on bone repair. J. Mater. Sci Mater. Med. 2015, 26, 177. [Google Scholar] [CrossRef]
- Coraça, D.C.; Duek, E.A.R.; Padovani, C.A.; Camilli, J.A. Osteointegration of poly(l-lactic acid)PLLA and poly(l-lactic acid)PLLA/poly(ethylene oxide)PEO implants in rat tibiae. J. Mater. Sci. Mater. Med. 2008, 19, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Montjovent, M.O.; Mathieu, L.; Schmoekel, H.; Mark, S.; Bourban, P.E.; Zambelli, P.Y.; Laurent-Applegate, L.A.; Pioletti, D.P. Repair of critical size defects in the rat cranium using ceramic-reinforced PLA scaffolds obtained by supercritical gas foaming. J. Biomed. Mater. Res. A 2007, 83, 41–51. [Google Scholar] [CrossRef]
- Hulsart-Billström, G.; Hu, Q.; Bergman, K.; Jonsson, K.B.; Åberg, J.; Tang, R.; Larsson, S.; Hilborn, J. Calcium phosphates compounds in conjunction with hydrogel as carrier for BMP-2: A study on ectopic bone formation in rats. Acta Biomater. 2011, 7, 3042–3049. [Google Scholar] [CrossRef]
- Do Carmo, A.B.X.; Sartoretto, S.C.; Alves, A.T.N.N.; Granjeiro, J.M.; Miguel, F.B.; Calasans-Maia, J.; Calasans-Maia, M.D. Alveolar bone repair with strontium-containing nanostructured carbonated hydroxyapatite. J. Appl. Oral Sci. 2018, 26, e20170084. [Google Scholar] [CrossRef]
- Yeo, A.; Cheok, C.; Teoh, S.H.; Zhang, Z.Y.; Buser, D.; Bosshardt, D.D. Lateral ridge augmentation using a PCL-TCP scaffold in a clinically relevant but challenging micropig model. Clin. Oral Implant. Res. 2012, 23, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, M.; Lenz, S.; Henkel, K.O.; Frerich, B.; Holzhüter, G.; Radefeldt, S.; Gerber, T. Lateral augmentation of the mandible in minipigs with a synthetic nanostructured hydroxyapatite block. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2011, 96, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Henkel, K.O.; Gerber, T.; Lenz, S.; Gundlach, K.K.H.; Bienengräber, V. Macroscopical, histological, and morphometric studies of porous bone-replacement materials in minipigs 8 months after implantation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Verket, A.; Müller, B.; Wohlfahrt, J.C.; Lyngstadaas, S.P.; Ellingsen, J.E.; Jostein Haugen, H.; Tiainen, H. TiO2 scaffolds in peri-implant dehiscence defects: An experimental pilot study. Clin. Oral Implant. Res. 2016, 27, 1200–1206. [Google Scholar] [CrossRef]
- Nandi, S.K.; Kundu, B.; Datta, S.; De, D.K.; Basu, D. The repair of segmental bone defects with porous bioglass: An experimental study in goat. Res. Vet. Sci. 2009, 86, 162–173. [Google Scholar] [CrossRef]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.C.; van Blitterswijk, C.A.; Barralet, J.E. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 2008, 29, 944–953. [Google Scholar] [CrossRef]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; van Blitterswijk, C.A. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar] [CrossRef]
- Koëter, S.; Tigchelaar, S.J.; Farla, P.; Driessen, L.; van Kampen, A.; Buma, P. Coralline hydroxyapatite is a suitable bone graft substitute in an intra-articular goat defect model. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2009, 90, 116–122. [Google Scholar] [CrossRef]
- von Doernberg, M.C.; von Rechenberg, B.; Bohner, M.; Grünenfelder, S.; van Lenthe, G.H.; Müller, R.; Gasser, B.; Mathys, R.; Baroud, G.; Auer, J. In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials 2006, 27, 5186–5198. [Google Scholar] [CrossRef]
- Gosain, A.K.; Riordan, P.A.; Song, L.; Amarante, M.T.; Kalantarian, B.; Nagy, P.G.; Wilson, C.R.; Toth, J.M.; McIntyre, B.L. A 1-year study of hydroxyapatite-derived biomaterials in an adult sheep model: III. Comparison with autogenous bone graft for facial augmentation. Plast. Reconstr. Surg. 2005, 116, 1044–1052. [Google Scholar] [CrossRef]
- Kanazawa, M.; Tsuru, K.; Fukuda, N.; Sakemi, Y.; Nakashima, Y.; Ishikawa, K. Evaluation of carbonate apatite blocks fabricated from dicalcium phosphate dihydrate blocks for reconstruction of rabbit femoral and tibial defects. J. Mater. Sci. Mater. Med. 2017, 28, 85. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.S.; Choi, J.W.; Kim, J.H.; Chung, H.Y.; Jin, S.; Shim, J.H.; Yun, W.S.; Jeong, C.M.; Huh, J.B. Comparative Efficacies of Collagen-Based 3D Printed PCL/PLGA/β-TCP Composite Block Bone Grafts and Biphasic Calcium Phosphate Bone Substitute for Bone Regeneration. Materials (Basel) 2017, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jung, I.H.; Lee, K.I.; Jung, U.W.; Kim, C.S.; Choi, S.H.; Cho, K.S.; Yun, J.H. Volumetric bone regenerative efficacy of biphasic calcium phosphate-collagen composite block loaded with rhBMP-2 in vertical bone augmentation model of a rabbit calvarium. J Biomed Mater Res A. 2012, 100, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Miyaji, H.; Otani, K.; Inoue, K.; Nakane, K.; Nishimura, H.; Ibara, A.; Shimada, A.; Ogawa, K.; Nishida, E.; et al. Bone augmentation using a highly porous PLGA/β-TCP scaffold containing fibroblast growth factor-2. J. Periodont. Res. 2015, 50, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Peric, M.; Dumic-Cule, I.; Grcevic, D.; Matijasic, M.; Verbanac, D.; Paul, R.; Grgurevic, L.; Trkulja, V.; Bagi, C.M.; Vukicevic, S. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 2015, 70, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcazzan, S.; Weinstein, R.L.; Del Fabbro, M. Efficacy of platelets in bone healing: A systematic review on animal studies. Platelets 2018, 29, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Prado, R.; Orive, G.; Tejero, R. Effects of calcium-modified titanium implant surfaces on platelet activation, clot formation, and osseointegration. J. Biomed. Mater. Res. A 2015, 103, 969–980. [Google Scholar] [CrossRef]
- Muschler, G.F.; Raut, V.P.; Patterson, T.E.; Wenke, J.C.; Hollinger, J.O. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng. Part. B Rev. 2010, 16, 123–145. [Google Scholar] [CrossRef]
- Moran, C.J.; Ramesh, A.; Brama, P.A.J.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 2016, 3, 1. [Google Scholar] [CrossRef]
- Hakimi, M.; Jungbluth, P.; Sager, M.; Betsch, M.; Herten, M.; Becker, J.; Windolf, J.; Wild, M. Combined use of platelet-rich plasma and autologous bone grafts in the treatment of long bone defects in mini-pigs. Injury 2010, 41, 717–723. [Google Scholar] [CrossRef]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 Suppl 21, 92–102. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Aludden, H.C.; Mordenfeld, A.; Hallman, M.; Dahlin, C.; Jensen, T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: A systematic review. Int. J. Oral Maxillofac. Surg. 2017, 46, 1030–1038. [Google Scholar] [CrossRef]
- Artzi, Z.; Nemcovsky, C.E.; Dayan, D. Bovine-HA spongiosa blocks and immediate implant placement in sinus augmentation procedures. Histopathological and histomorphometric observations on different histological stainings in 10 consecutive patients. Clin. Oral Implant. Res. 2002, 13, 420–427. [Google Scholar] [CrossRef]
- Mangano, C.; Scarano, A.; Perrotti, V.; Iezzi, G.; Piattelli, A. Maxillary sinus augmentation with a porous synthetic hydroxyapatite and bovine-derived hydroxyapatite: A comparative clinical and histologic study. Int. J. Oral Maxillofac. Implant. 2007, 22, 980–986. [Google Scholar]
- Santana, R.B.; Santana, C.M. A clinical comparison of guided bone regeneration with platelet-derived growth factor-enhanced bone ceramic versus autogenous bone block grafting. Int. J. Oral Maxillofac. Implant. 2015, 30, 700–706. [Google Scholar] [CrossRef]
- Cavalu, S.; Banica, F.; Simon, V.; Akin, I.; Goller, G. Surface Modification of Alumina/Zirconia Ceramics Upon Different Fluoride-Based Treatments. Int. J. Appl. Ceram. Technol. 2014, 11, 402–411. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Tolstunov, L. Classification of the alveolar ridge width: Implant-driven treatment considerations for the horizontally deficient alveolar ridges. J. Oral Implantol. 2014, 40, 365–370. [Google Scholar] [CrossRef] [PubMed]


| Authors | N. Animals | Defect | Test Block | Control Site | Analysis Time | Evaluation |
|---|---|---|---|---|---|---|
| Studies on Rabbit | ||||||
| Torres et al. 2011 [25] | 8 | Calvaria | Monetite in different thickness | - | 8 weeks | Histology and Histomorphometry |
| Zivadinovic et al. 2016 [26] | 8 | Calvaria | β-tricalcium phosphate | Unfilled, Autologous graft | 4 weeks | Histology and Histomorphometry |
| Tamimi et al. 2009 [27] | 8 | Calvaria | Monetite | Autologous graft | 8 weeks | Histology and Histomorphometry |
| Kim et al. 2012 [63] | 16 | Calvaria | Block-type biphasic calcium phosphate (BCP), rhBMP-2, collagene | - | 8 weeks | Histology and Histomorphometry |
| Hwang et al. 2012 [28] | 10 | Calvaria | Hydroxyapatite, β-tricalcium phosphate, Biphasic calcium phosphate synthetic block-type bone graft | Unfilled | 4–8 weeks | micro-CT, Histology and Histomorphometry |
| Bae et al. 2014 [29] | 16 | Calvaria | Hydroxyapatite bone block | Autologous graft | 4–8 weeks | micro-CT, Histology and Histomorphometry |
| Shim et al. 2012 [30] | 18 | Calvaria | polycaprolactone(PCL)/poly(lactic-co-glycolic acid) (PLGA) scaffold blended with tri-calcium phosphate (TCP) | Unfilled | 4–8 weeks | micro-CT, Histology and Histomorphometry |
| Xu et al. 2008 [31] | 12 | Calvaria | β-calcium silicate (b-CaSiO3, b-CS), porous β-tricalcium phosphate (b-Ca3(PO4)2; β -TCP | - | 4–8 and 16 weeks | micro-CT, Histology and Histomorphometry |
| Yoo et al. 2017 [32] | 7 | Calvaria | Biphasic calcium phosphate(BCP); Biphasic calcium phosphate phosphate/carboxymethyl cellulose (BCP/CMC); Biphasic calcium phosphate/cross-linked carboxymethyl cellulose (BCP/c-CMC); Biphasic calcium phosphate/hyaluronic acid (BCP/HyA) | - | 4 weeks | micro-CT, Histology and Histomorphometry |
| Lam et al. 2009 [34] | 6 | Calvaria | PCL scaffolds (NaOH treated for 12h); Predegraded PCL scaffolds (PD-PCL, NaOH treated for 7 days); Untreated PCL/TCP scaffolds | - | 18–24 weeks | Histology |
| Gehrke et al. 2019 [35] | 20 | Calvaria | Sintered bovine bone blocks Nonsintered bovine bone blocks | - | 6 and 8 weeks | Histology and Histomorphometry |
| Kanazawa et al. 2017 [61] | 19 | Femur and tibia defects | Carbonate apatite (CO3Ap); Hydroxyapatite (HA) block | - | 4, 12, and 24 weeks | micro -CT |
| Tsai et al. 2008 [36] | 16 | Femur defects | CPC (amorphous calcium phosphate, DCPD powders mixed with physiological saline) in different concentration | - | 1, 4, 12, and 24 weeks | Histology |
| Teramoto et al. 2005 [37] | 38 | Femur defects | β-tricalciumphosphate (75% porosity); Apatite-wollastonite glass-ceramic (70, 80, and 90% porosity) | - | 8, 12, 24, and 36 weeks | Histology |
| Ohsawa et al. 2004 [38] | 3 Femur defects | Porous apatite Wollastonite-containing glass-ceramic (AW) | - | 3, 6, 12 months | Radiographs, Histology | |
| Guo et al. 2012 [39] | 6 | Mandible defects (angle and body) | Composite nano-HA/polyamide (n-HA/PA); Composite n-HA/PA+ BMSC bone marrow stromal cells | - | 4–12 weeks | Histology, Histomorphometry, SEM, micro-CT |
| Zhang et al. 2009 [40] | 12 | Radius defects | Nanocomposite of hydroxyapatite surface-grafted with poly(L-lactide and Poly- Glycolidee (g-HAP) | - | 4, 8, 12, and 20 weeks | Radiographs, Histology |
| Authors | N. Animals | Defect | Test Block | Control Site | Analysis Time | Evaluation |
|---|---|---|---|---|---|---|
| Studies on Dogs | ||||||
| Rismanchian et al. 2015 [41] | 4 | Maxillary defects | Bioglass (BG), Demineralized bone matrix (DBM), Forstrite (FR) | Unfilled | 15, 30, 45, and 60 days | Histology and Histomorphometrry |
| Du et al. 2015 [42] | 4 | Mandibular critical-size defect | Nano Hydroxyapatite (nHA) coral blocks; recombinant human vascular endothelial growth factor165 (rhVEGF), Nano Hydroxyapatite (nHA)/coral blocks | - | 3 and 8 weeks | Histology, Histomorphometry |
| Nery et al. 1992 [43] | 21 | Mandibular and maxillary periodontal defects (Canines and 1st molar) | Hydroxyapatite/beta tricalcium phosphate (HA/ßTCP) in different macroporosity, Biphasic calcium phosphate ceramic | Unfilled | 6 months | Histology, Histomorphometry |
| Erbe et al. 2001 [44] | 4 | Cylindrical metaphyseal defects | B-TCP synthetic cancellous bone | - | 12, 24, and 52 week | Radiograph,X-ray diffraction (XRD), Histology and Histomorphometry t 12, 24, and 52 week |
| Authors | N. Animals | Defect | Test Block | Control Site | Analysis Time | Evaluation |
|---|---|---|---|---|---|---|
| Studies on Rats | ||||||
| Gabbai-Armelin et al. 2015 [45] | 60 | Tibia defect | Bioactive fibrous glassy scaffold | Unfilled | 15, 30, and 60 days | Histology, Histomorphometry |
| Coraca ̧ et al. 2008 [46] | 44 | Tibia defect | Poly(l-lactic acid)PLLA/poly(ethylene oxide) PEO blend, poly(l-lactic acid) PLLA | - | 2, 4, 6, 8 weeks | Histology, Histomorphometry |
| Inzana et al. 2014 [47] | 18 | Femural defect | Calcium phosphate scaffold; CPh scaffold, collagen binder; CPh scaffold collagen coating; Devitalized allograft | Unfilled | 9 weeks | Histology, Histomorphometry, SEM evaluation |
| Hwang et al. 2017 [62] | 32 | Calvaria | Polycaprolactone(PCL) polylactic-co-glycolic acid PLGA) β-tricalcium phosphate in a 4:4:2 ratio, Biphasic calcium phosphate | - | 2, 4, 6, 8 weeks | Histology, Histomorphometry |
| Montjovent et al. 2007 [48] | 24 | Calvaria | Bioresorbable scaffolds made of polylactic acid/beta tricalcium phosphate; PLa/Hydroxyapatite; Beta tricalcium phosphate | - | 18 weeks | Radiographs, Histology |
| Hulsart-Billström et al. 2011 [49] | 18 | Ectopic bone formation | Beta tricalcium phosphate (ß-TCP)/nano hydroxyapatite, hydroxyapatite, Clods of hydroxyapatite, HAP biomimetic | Unfilled | 4 weeks | Radiographs, Histology |
| do Carmo et al. 2017 [50] | 20 | Maxillary dental socket | Nanostructured carbonated hydroxyapatite/sodium alginate 5% strontium microspheres, Nanostructured carbonated hydroxyapatite/sodium alginate | - | 1 and 6 weeks | Histology |
| Authors | N. Animals | Defect | Test Block | Control Site | Analysis Time | Evaluation |
|---|---|---|---|---|---|---|
| Studies on Minipigs | ||||||
| Yeo et al. 2011 [51] | 10 | Mandible lateral defect | PCL-TCP scaffold, Collagen membrane | Autologous graft+ collagen membrane | 24 weeks | micro-CT, Histology and Histomorphometry |
| Kirchhoff et al. 2011 [52] | 6 | Mandible defect | Nanostructured hydroxyapatite (HA) porous matrix of silica (SiO2) gel | Autologous graft | 5 and 10 weeks | Histology, Histomorphometry |
| Henkel et al. 2006 [53] | 15 | Critical-size mandible defects | CaP matrix (HA:TCP = 60%:40%); Monophasic CaP matrix (HA: 100%), Pure hydroxyapatite; Beta tricalcium phosphate Gelatin sponge | - | 32 weeks | Histology, Histomorphometry |
| Verket et al. 2016 [54] | 5 | Implants with dehiscence defects | TiO2 scaffold | Autologous graft | 12 weeks | Histology, Histomorphometry |
| Authors | N. Animals | Defect | Test Block | Control Site | Analysis Time | Evaluation |
|---|---|---|---|---|---|---|
| Studies on Goats | ||||||
| Nandi et al. 2009 [55] | 12 | Radius defects | Porous bioactive glass blocks | Unfilled | 90 days | Histology, Radiographs and Angiogram |
| Habibovic et al. 2008 [56] | 12 | Lumbar transverse processes | BCPA, BCPB, BCPC, one composite (BCPCþ) of BCPC reinforced with PLA, HA, and CA ceramic. | - | 3, 6, 9, and 12 weeks | Histology, Histomorphometry |
| Habibovic et al. 2008 [57] | 12 | Transverse processes (L1–L4) | Ceramic tricalcium phosphate (TCP) brushite, monetite | - | 3, 6, and 9 weeks | Histology, and Histomorphometry, SEM evaluation |
| Koeter et al. 2008 [58] | 20 | Knee defects | Coralline hydroxyapatite (CHA) | Autologous bone | 12 weeks | Histology, Histomorphometry |
| Authors | N. Animals | Defect | Test Block | Control Site | Analysis Time | Evaluation |
|---|---|---|---|---|---|---|
| Studies on Sheep | ||||||
| von Doernberg et al. 2006 [59] | 9 | Methaphysial or epiphysial long bones | Beta-TCP cylinders at different pore size | Unfilled | 90 days | Histology, Radiographs and Angiogram |
| Gosain et al. 2004 [60] | 10 | Calvaria | 60% hydroxyapatite and 40%-TCP; 60% hydroxyapatite–cement and 40%-TCP; 20% hydroxyapatite–cement and 80%-TCP; Pure hydroxyapatite; | - | 3, 6, 9, 12 weeks | Histology, Histomorphometry |
| Author | N. TEST | Model TEST | Mean% NBF TEST | SD TEST | N CTR | Mean% NBF CTR | SD CTR | CTR | Mean Difference% (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Zivadinovic et al. 2016 [26] | 8 | Rabbit (β-TCP) | 30.15% (4 weeks) | 5.71 | 8 | 7.32 % (4 weeks) | 8.40 | UNFILLED | 22.83 (15.79, 29.87) |
| Tamimi et al. 2009 [27] | 8 | Rabbit (monetite) | 43.4% (8 weeks) | 8.1 | 8 | 60.1% (8 weeks) | 6.0 | AUTOLOGOUS | −16.30 (−23.29, −9.31) |
| Hwang et al. 2012 [28] | 5 | Rabbit (HA) | 4.97% (4 weeks) | 1.91 | 5 | 3.99% (4 weeks) | 1.54 | UNFILLED | 0.98 (−1.17, 3.13) |
| 5 | Rabbit (β-TCP) | 1.56% (4 weeks) | 1.32 | −2.43 (−4.21, −0.65) | |||||
| 5 | Rabbit (BCP) | 5.60% (4 weeks) | 3.93 | 1.61 (−2.09, 5.31) | |||||
| 5 | Rabbit (HA) | 6.95% (8 weeks) | 3.51 | 5 | 4.55% (8 weeks) | 2.50 | UNFILLED | 2.40 (−1.38, 6.18) | |
| 5 | Rabbit (β-TCP) | 4.04% (8 weeks) | 1.39 | −0.51 (−3.02, 2.00) | |||||
| 5 | Rabbit (BCP) | 9.03% (8 weeks) | 3.39 | 4.48 (0.79, 8.17) | |||||
| Shim et al. 2012 [30] | 6 | Rabbit (PCL/PLGA) | 10.74% (4 weeks) | 1.86 | 6 | 4.06% (4 weeks) | 2.03 | UNFILLED | 6.68 (4.48, 8.88) |
| 6 | Rabbit (PCL/PLGA/TCP) | 14.29% (4 weeks) | 4.59 | 10.23 (6.21, 14.25) | |||||
| 6 | Rabbit (PCL/PLGA) | 15.68% (8 weeks) | 2.89 | 6 | 10.08% (8weeks) | 1.86 | UNFILLED | 5.60 (2.85, 8.35) | |
| 6 | Rabbit (PCL/PLGA/TCP) | 20.75 % (8 weeks) | 4.20 | 10.67 (6.99, 14.35) | |||||
| Rismanchian et al. 2015 [41] | 4 | Dog (BG) | 22.65% (8 weeks) | 2.76 | 4 | 23.43% (8 weeks) | 5.26 | UNFILLED | −0.78 (−6.60, 5.04) |
| 4 | Dog (FR) | 26.65% (8 weeks) | 4.51 | 3.22 (-3.57, 10.01) | |||||
| 4 | Dog (BG) | 21.21 (4 weeks) | 0.94 | 4 | 22.37 (4 weeks) | 3.44 | UNFILLED | −1.16 (−4.65, 2.33) | |
| 4 | Dog (FR) | 26.56 (4 weeks) | 6.97 | 4.19 −3.43, 11.81) | |||||
| Gabbai-Armelin et al. 2015 [45] | 60 | Rat (BG) | 21.3 % (8 weeks) | 2.4 | 60 | 46.8% (8 weeks) | 7.1 | UNFILLED | −25.50 (−27.40, −23.60) |
| Gosain et al. 2004 [60] | 10 | Sheep (60%HA-CC) | 13.6% (1 year) | 2.0 | 10 | 0 (1 year) | 0 | UNFILLED | N.E. |
| 10 | Sheep (60%HA-CP) | 11.2% (1 year) | 2.3 | N.E. | |||||
| 10 | Sheep (20%HA-CP) | 28.5% (1 year) | 4.5 | N.E. | |||||
| 10 | Sheep (100%HA-CP) | 4.8% (1 year) | 1.4 | N.E. | |||||
| Kirchhoff 2010 [52] | 6 | Minipig (nHA-A) | 7.6% (5 weeks) | 6.0 | - | - | - | NO CONTROL | N.E. |
| 6 | Minipig (nHA-B) | 15.3% (5 weeks) | 8.3 | N.E. | |||||
| 6 | Minipig (nHA-A) | 34.1% (10 weeks) | 10.8 | N.E. | |||||
| 6 | Minipig (nHA-B) | 39.9% (10 weeks) | 13.5 | N.E. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumedei, M.; Savadori, P.; Del Fabbro, M. Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 4221. https://doi.org/10.3390/ijms20174221
Tumedei M, Savadori P, Del Fabbro M. Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2019; 20(17):4221. https://doi.org/10.3390/ijms20174221
Chicago/Turabian StyleTumedei, Margherita, Paolo Savadori, and Massimo Del Fabbro. 2019. "Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 20, no. 17: 4221. https://doi.org/10.3390/ijms20174221
APA StyleTumedei, M., Savadori, P., & Del Fabbro, M. (2019). Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 20(17), 4221. https://doi.org/10.3390/ijms20174221







