Oral Administration of Microencapsulated B. Longum BAA-999 and Lycopene Modulates IGF-1/IGF-1R/IGFBP3 Protein Expressions in a Colorectal Murine Model
Abstract
:1. Introduction
2. Results
2.1. General Observations in the AOM + DSS Carcinogenic Study
2.2. Microencapsulated Bifidobacterium Longum Viability and Gastrointestinal Tract (GIT) Distribution During the 16-Week AOM + DSS Carcinogenic Study
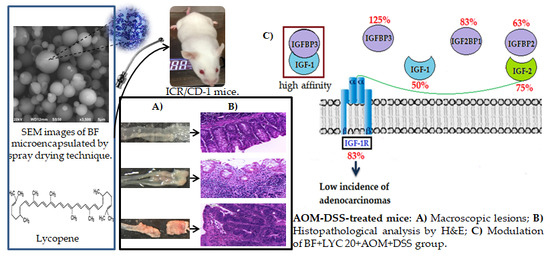
2.3. Macroscopic Evaluation and Histopathology Classification
2.4. Modulation of IGF System Components in Colorectal Tissue
3. Discussion
4. Materials and Methods
4.1. B. Longum (BF) Growth Conditions and its Microencapsulation by the SPRAY Drying Technique
4.2. Animal Studies
4.3. B. longum Viability in the Stomach, Small Intestine, Caecum, Colon, and Feces of CD-1 Mice
4.4. β-Glucuronidase Activity (β-GA) Assay and pH in the Cecal, Colonic and Fecal Content
4.5. Macroscopic and Histopathological Analyses
4.6. Protein Expressions of the IGF System by Immunohistochemistry Technique
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Aberrant crypt foci |
| AOM | Azoxymethane |
| BF | Bifidobacterium longum BAA-999 |
| β-GA | β-glucuronidase activity |
| CRC | Colorectal cancer |
| DMH | 1,2-Dimethylhydrazine |
| DSS | Sulfate sodium dextran |
| GIT | Gastrointestinal tract |
| H&E | Hematoxylin and eosin |
| IGF-1 | Insulin-like growth factor-I |
| IGF-2 | Insulin-like growth factor-II |
| IGF-1R | Insulin-like growth factor-I receptor |
| IGF2BP1 | Insulin-like growth factor-binding protein-1 |
| IGFBP2 | Insulin-like growth factor-binding protein-2 |
| IGFBP3 | Insulin-like growth factor-binding protein-3 |
| IGFBPs | Insulin-like growth factor-binding proteins |
| Log CFU | logarithm of Colony-forming unit |
| LYC | Lycopene |
References
- Gandomani, H.S.; Aghajani, M.; Mohammadian-Hafshejani, A.; Tarazoj, A.A.; Pouyesh, V.; Salehiniya, H. Colorectal cancer in the world: Incidence, mortality and risk factors. Biomed. Res. Ther. 2017, 4, 1656–1675. [Google Scholar] [CrossRef]
- Walfisch, S.; Walfisch, Y.; Kirilov, E.; Linde, N.; Mnitentag, H.; Agbaria, R.; Levy, J. Tomato lycopene extract supplementation decreases insulin-like growth factor-I levels in colon cancer patients. Eur. J. Cancer Prev. 2007, 16, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, P.G.; Tirrò, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The insulin/IGF system in colorectal cancer development and resistance to therapy. Front. Oncol. 2015, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, G.F.; Cheng, Y.H.; Zhao, Q.C. Correlations of insulin-like growth factor I and insulin-like growth factor I receptor with the clinicopathological features and prognosis of patients with colon cancer. Jpn. J. Clin. Oncol. 2016, 46, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, M.C.; Vieiralves, N.F.; Gomes, M.I.; Salvadori, D.M.; Rodrigues, M.A.; Barbisan, L.F. Effects of lycopene, synbiotic and their association on early biomarkers of rat colon carcinogenesis. Food Chem. Toxicol. 2010, 48, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M.; Jiménez-Farfán, D.; Cruz Salgado, A.; Serrano-García, N.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. -Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Kasdagly, M.; Radhakrishnan, S.; Reddivari, L.; Veeramachaneni, D.R.; Vanamala, J. Colon carcinogenesis: Influence of Western diet-induced obesity and targeting stem cells using dietary bioactive compounds. Nutrition 2014, 30, 1242–1256. [Google Scholar] [CrossRef]
- Ono, M.; Takeshima, M.; Nakano, S. Mechanism of the anticancer effect of lycopene (tetraterpenoids). In The Enzymes, 1st ed.; Bathaie, S., Tamanoi, F., Eds.; Academic Press: Waltham, MA, USA, 2015; Volume 37, pp. 139–166. [Google Scholar] [CrossRef]
- Serban, D.E. Gastrointestinal cancers: Influence of gut microbiota, probiotics and prebiotics. Cancer Lett. 2014, 345, 258–270. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J.Z. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Eratte, D.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B. Survival, oxidative stability, and surface characteristics of spray dried co-microcapsules containing omega-3 fatty acids and probiotic bacteria. Dry Technol. 2016, 34, 1926–1935. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yu, Z.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Colonization and probiotic function of Bifidobacterium longum. J. Funct. Foods. 2019, 53, 157–165. [Google Scholar] [CrossRef]
- Shah, N.P.; Lankaputhra, W.E.; Britz, M.L.; Kyle, W.S. Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in commercial yoghurt during refrigerated storage. Int. Dairy J. 1995, 5, 515–521. [Google Scholar] [CrossRef]
- De Vos, P.; De Haan, B.; Pater, J.; Van Schilfgaarde, R. Association between capsule diameter, adequacy of encapsulation, and survival of microencapsulated rat islet allografts1. Transplantation 1996, 62, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Shoji, A. Viability of L. acidophilus microcapsules and their application to buffalo milk yoghurt. Food Bioprod. Process. 2013, 91, 83–88. [Google Scholar] [CrossRef]
- Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Campos-Vega, R.; Gallegos-Corona, M.A.; De Mejía, E.G.; Loarca-Piña, G. Physicochemical and nutraceutical properties of moringa (Moringa oleifera) leaves and their effects in an in vivo AOM/DSS-induced colorectal carcinogenesis model. Food Res. Int. 2018, 105, 159–168. [Google Scholar] [CrossRef]
- Valadez-Bustos, N.; Escamilla-Silva, E.M.; Gallegos-Corona, M.A.; Hernandez-Iturriaga, M.; García-Almendarez, B.E.; Ramos-Gómez, M. Impact of microencapsulated B. longum BAA-999 on gastrointestinal colonization in an acute model of inflammation. 2019; Unpublished; manuscript in preparation. [Google Scholar]
- Kohno, H.; Suzuki, R.; Sugie, S.; Tanaka, T. β-Catenin mutations in a mouse model of inflammation-related colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sodium sulfate. Cancer Sci. 2005, 96, 69–76. [Google Scholar] [CrossRef]
- Tanaka, T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int. J. Inflam. 2012, 2012, 658786. [Google Scholar] [CrossRef]
- Jin, M.; Long, Z.W.; Yang, J.; Lin, X. Correlations of IGF-1R and COX-2 expressions with Ras and BRAF genetic mutations, clinicopathological features and prognosis of colorectal cancer patients. Pathol. Oncol. Res. 2018, 24, 45–57. [Google Scholar] [CrossRef]
- Sipos, F.; Székely, H.; Kis, I.D.; Tulassay, Z.; Műzes, G. Relation of the IGF/IGF1R system to autophagy in colitis and colorectal cancer. World J. Gastroenterol. 2017, 23, 8109. [Google Scholar] [CrossRef]
- Wang, S.Q.; Cui, S.X.; Qu, X.J. Metformin inhibited colitis and colitis-associated cancer (CAC) through protecting mitochondrial structures of colorectal epithelial cells in mice. Cancer Biol. Ther. 2019, 20, 338–348. [Google Scholar] [CrossRef]
- Tanaka, T. Colorectal carcinogenesis: Review of human and experimental animal studies. J. Carcinog. 2009, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Grimm, V.; Radulovic, K.; Riedel, C.U. Colonization of C57BL/6 mice by a potential probiotic Bifidobacterium bifidum strain under germ-free and specific pathogen-free conditions and during experimental colitis. PLoS ONE 2015, 10, e0139935. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Arioli, S.; Wang, A.; Villa, C.; Jahani, R.; Song, Y.; Mora, D.; Guglielmetti, S.; Comelli, E. Impact of Bifidobacterium bifidum MIMBb75 on mouse intestinal microorganisms. Fems Microbiol. Ecol. 2013, 85, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Lievin, V.; Peiffer, I.; Hudault, S.; Rochat, F.; Brassart, D.; Neeser, J.R.; Servin, A.L. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 2000, 47, 646–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer sci. 2003, 94, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Angelopoulou, K.; Kaldrymidou, E.; Tsingotjidou, A.; Abas, Z.; Erdman, S.E.; Poutahidis, T. Cholera-toxin suppresses carcinogenesis in a mouse model of inflammation-driven sporadic colon cancer. Carcinogenesis 2015, 36, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Takasuka, N.; Naito, A.; Fukamachi, K.; Murakoshi, M.; Nishino, H.; Tsuda, H. Modifying effects of carotenoids in a rat multi-organ carcinogenesis model. Proc. Jpn. Acad. B-Phys. 2002, 78, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Yancu, D.; Blouin, M.J.; Birman, E.; Florianova, L.; Aleynikova, O.; Zakikhani, M.; Pollak, M. A phenotype of IGFBP-3 knockout mice revealed by dextran sulfate-induced colitis. J. Gastroenterol. Hepatol. 2017, 32, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Josse, C.; Bouznad, N.; Geurts, P.; Irrthum, A.; Huynh-Thu, V.A.; Servais, L.; Oury, C. Identification of a microRNA landscape targeting the PI3K/Akt signaling pathway in inflammation-induced colorectal carcinogenesis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 306, G229–G243. [Google Scholar] [CrossRef]
- Wu, Y.; Yakar, S.; Zhao, L.; Hennighausen, L.; LeRoith, D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002, 62, 1030–1035. [Google Scholar]
- Yücel, Ç.Y.; Erden, G.; Yılmaz, F.M.; Sezer, S.; Çalcı, E. IGF-1 and IGFBP-3 levels and their correlations with carcinoembryonic antigen in colorectal cancer patients. Ajm 2018, 54, 11–15. [Google Scholar] [CrossRef]
- Renehan, A.G.; Jones, J.; Potten, C.S.; Shalet, S.M.; O’Dwyer, S.T. Elevated serum insulin-like growth factor (IGF)-II and IGF binding protein-2 in patients with colorectal cancer. Br. J. Cancer. 2000, 83, 1344. [Google Scholar] [CrossRef] [PubMed]
- Šunderić, M.; Đukanović, B.; Malenković, V.; Nedić, O. Molecular forms of the insulin-like growth factor-binding protein-2 in patients with colorectal cancer. Exp. Mol. Pathol. 2014, 96, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Amir, H.; Karas, M.; Giat, J.; Danilenko, M.; Levy, R.; Yermiahu, T.; Sharoni, Y. Lycopene and 1, 25-dihydroxyvitamin D3 cooperate in the inhibition of cell cycle progression and induction of differentiation in HL-60 leukemic cells. Nutr. Cancer 1999, 33, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sharoni, Y.; Danilenko, M.; Levy, J. Molecular mechanisms for the anticancer activity of the carotenoid lycopene. Drug Dev. Res. 2000, 50, 448–456. [Google Scholar] [CrossRef]
- Bhuvaneswari, V.; Nagini, S. Lycopene: A review of its potential as an anticancer agent. Curr. Med. Chem. Anticancer Agents 2005, 5, 627–635. [Google Scholar] [CrossRef]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef]
- Dobrowolski, P. Short protocols in molecular biology. In A Compendium of Methods From “Current Protocols in Molecular Biology”, 1st ed.; Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, E., Eds.; John Wiley & Sons, Inc.: Weinheim, Germany, 1993; Volume 13.1, pp. 88–102. [Google Scholar] [CrossRef]
- Valadez-Bustos, N.; Ramos-Gómez, M.; Conde-Barajas, E.; Figueiras-Abdala, A.; Escamilla-Silva, E.M. Comparative study of microencapsulated Bifidobacterium longum BAA-999 by drip and spray drying techniques as probiotic enhancement in vitro. Bio-Med. Mater. Eng. 2019. Submitted. [Google Scholar]
- Lian, W.C.; Hsiao, H.C.; Chou, C.C. Survival of bifidobacteria after spray-drying. Int. J. Food Microbiol. 2002, 74, 79–86. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana. NOM-062-ZOO-1999. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. DOF 1999, 22. Available online: www.researchgate.net/profile/Aline_S_de_Aluja3/publication/11280294_Laboratory_animals_and_official_Mexican_norm_NOM-062-ZOO-1999 (accessed on 12 February 2018).
- Hosono, K.; Endo, H.; Takahashi, H. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol. Carcinog. 2010, 49, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Quartieri, A.; Simone, M.; Gozzoli, C.; Popovic, M.; D’Auria, G.; Amaretti, A.; Rossi, M. Comparison of culture-dependent and independent approaches to characterize fecal bifidobacteria and lactobacilli. Anaerobe 2016, 38, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Washington, DC, USA, 2010; pp. 216–217. [Google Scholar]
- Jenab, M.; Thompson, L.U. The influence of flaxseed and lignans on colon carcinogenesis and β-glucuronidase activity. Carcinogenesis 1996, 17, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Cerar, A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J. Biomed. Biotechnol. 2011, 2011, 473964. [Google Scholar] [CrossRef] [PubMed]




| Groups | Final Body Weight (g) | Final Body Weight Gain (g) | Caecum | Colon | Feces | |||
|---|---|---|---|---|---|---|---|---|
| pH | β-GA 1 | pH | β-GA 1 | pH | β-GA 1 | |||
| Normal | 39.56 ± 1.01 a | 8.41 ± 0.95 b | 7.12 ± 0.04 b | 3.22 ± 0.33 c | 7.21 ± 0.07 ab | 2.61 ± 0.16 bc | 6.99 ± 0.10 b | 5.72 ± 0.03 de |
| AOM + DSS control | 40.46 ± 1.01 a | 9.85 ± 0.68 b | 7.47 ± 0.04 a | 6.56 ± 0.35 a | 7.42 ± 0.03 a | 5.34 ± 0.53 a | 8.08 ± 0.13 a | 9.52 ± 0.08 a |
| BF + AOM + DSS | 41.76 ± 1.29 a | 12.05 ± 1.03 a | 7.20 ± 0.14 ab | 3.32 ± 0.34 bc | 7.20 ± 0.06 ab | 1.85 ± 0.15 bc | 6.96 ± 0.10 b | 4.88 ± 0.14 ef |
| BF + LYC 20 + AOM + DSS | 41.71 ± 0.97 a | 11.71 ± 0.65 ab | 7.24 ± 0.04 ab | 2.68 ± 0.25 c | 7.20 ± 0.06 ab | 1.66 ± 0.09 c | 7.07 ± 0.20 b | 6.71 ± 0.26 c |
| BF + LYC 50 + AOM + DSS | 41.42 ± 0.97 a | 11.61 ± 0.71 ab | 7.17 ± 0.06 ab | 2.37 ± 0.24 c | 7.19 ± 0.07 b | 2.44 ± 0.27 bc | 7.04 ± 0.11 b | 4.71 ± 0.12 f |
| LYC 20 + AOM + DSS | 39.78 ± 0.70 a | 10.01 ± 0.44 ab | 7.39 ± 0.09 ab | 3.65 ± 0.24 bc | 7.36 ± 0.09 ab | 2.73 ± 0.21 bc | 7.19 ± 0.13 b | 6.26 ± 0.27 cd |
| LYC 50 + AOM + DSS | 41.19 ± 1.29 a | 12.10 ± 1.08 a | 7.29 ± 0.09 ab | 4.74 ± 0.22 b | 7.18 ± 0.07 b | 3.01 ± 0.31 b | 7.18 ± 0.16 b | 6.20 ± 0.10 cd |
| Metformin + AOM + DSS | 39.03 ± 1.01 a | 9.74 ± 1.14 b | 7.27 ± 0.11 ab | 2.99 ± 0.31 c | 7.28 ± 0.04 ab | 2.46 ± 0.27 bc | 7.02 ± 0.25 b | 7.62 ± 0.33 b |
| Group | n | Incidence of Early Lesions a (%) | Mean Number b | Colon Distribution of Early Lesions a | Incidence of Tumors c (%) | Mean Number b | Colon Distribution of Tumors c | ||
|---|---|---|---|---|---|---|---|---|---|
| Proximal | Distal | Proximal | Distal | ||||||
| Normal | 10 | 10% * | 0.1 ± 0.1 b | 1 (100%) | 0 (0%) | 0% * | 0.0 ± 0.0 b | 0 (0%) | 0 (0%) |
| AOM + DSS control | 10 | 60% | 1.2 ± 0.4 ab | 2 (17%) | 10 (83%) | 80% | 4.2 ± 1.0 a | 0 (0%) | 42 (100%) |
| BF + AOM + DSS | 7 | 86% | 2.4 ± 0.7 ab | 1 (6%) | 16 (94%) | 14% * | 0.1 ± 0.1 b | 0 (0%) | 1 (100%) |
| BF + LYC 20 AOM + DSS | 8 | 88% | 3.0 ± 0.6 a | 4 (17%) | 20 (83%) | 13% * | 0.1 ± 0.1 b | 0 (0%) | 1 (100%) |
| BF + LYC 50 AOM + DSS | 8 | 75% | 2.3 ± 0.7 ab | 4 (25) | 12 (75%) | 38% * | 0.4 ± 0.2 b | 1 (33%) | 2 (67%) |
| LYC 20 + AOM + DSS | 7 | 71% | 1.1 ± 0.5 ab | 0 (0%) | 8 (100%) | 43% | 1.7 ± 1.0 ab | 0 (0%) | 12 (100%) |
| LYC 50 + AOM + DSS | 7 | 71% | 1.3 ± 0.4 ab | 3 (33%) | 6 (67%) | 71% | 1.6 ± 0.6 ab | 1 (9%) | 10 (91%) |
| Metformin + AOM +DSS | 6 | 83% | 2.2 ± 0.9 ab | 11 (85%) | 2 (15%) | 50% | 0.5 ± 0.2 b | 2 (67%) | 1 (33%) |
| Group | n | Normal Tissue | Lymphocyte Infiltration | Eosinophils | Calceiform Cells | Epithelial Ridges | Peyer’s Patches | Defined Tissue | Necrosis | Mitosis | Inflammation Grade | Incidence of Inflammation (%) | Adenocarcinomas | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Yes | No | Few | Moderate | Intense | Yes | No | Yes | No | + | ++ | +++ | ||||||||
| Normal | 10 | 9 (90%) * | None | × | × | × | × | Yes | No | No | 0 | 1 | 0 | 10% * | 0 (0%) * | |||||||
| AOM + DSS control | 10 | 0 (0%) | × | × | None | × | × | No | Yes | 1% | 0 | 0 | 2 | 20% * | 8 (80%) | |||||||
| BF + AOM + DSS | 7 | 0 (0%) | × | × | × | × | × | Yes | No | −1% | 3 | 3 | 0 | 86% | 1 (14%) * | |||||||
| BF + LYC 20 AOM + DSS | 8 | 1 (13%) | Focal | × | × | × | × | Yes | Focal | −1% | 2 | 4 | 0 | 75% | 1 (13%) * | |||||||
| BF + LYC 50 AOM + DSS | 8 | 0 (0%) | × | × | × | × | × | Yes | No | −1% | 4 | 3 | 0 | 88% | 1 (13%) * | |||||||
| LYC 20 + AOM + DSS | 7 | 0 (0%) | × | × | None | × | × | Focal | Focal | 1% | 0 | 2 | 1 | 43% * | 4 (57%) | |||||||
| LYC 50 + AOM + DSS | 7 | 0 (0%) | × | × | × | × | × | No | Focal | −1% | 0 | 2 | 1 | 43% * | 4 (57%) | |||||||
| Metformin + AOM + DSS | 6 | 2 (33%) | × | × | × | × | × | Focal | Focal | −1% | 1 | 0 | 0 | 17% * | 3 (50%) * | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valadez-Bustos, N.; Escamilla-Silva, E.M.; García-Vázquez, F.J.; Gallegos-Corona, M.A.; Amaya-Llano, S.L.; Ramos-Gómez, M. Oral Administration of Microencapsulated B. Longum BAA-999 and Lycopene Modulates IGF-1/IGF-1R/IGFBP3 Protein Expressions in a Colorectal Murine Model. Int. J. Mol. Sci. 2019, 20, 4275. https://doi.org/10.3390/ijms20174275
Valadez-Bustos N, Escamilla-Silva EM, García-Vázquez FJ, Gallegos-Corona MA, Amaya-Llano SL, Ramos-Gómez M. Oral Administration of Microencapsulated B. Longum BAA-999 and Lycopene Modulates IGF-1/IGF-1R/IGFBP3 Protein Expressions in a Colorectal Murine Model. International Journal of Molecular Sciences. 2019; 20(17):4275. https://doi.org/10.3390/ijms20174275
Chicago/Turabian StyleValadez-Bustos, Nancy, Eleazar M. Escamilla-Silva, Francisco J. García-Vázquez, Marco A. Gallegos-Corona, Silvia L. Amaya-Llano, and Minerva Ramos-Gómez. 2019. "Oral Administration of Microencapsulated B. Longum BAA-999 and Lycopene Modulates IGF-1/IGF-1R/IGFBP3 Protein Expressions in a Colorectal Murine Model" International Journal of Molecular Sciences 20, no. 17: 4275. https://doi.org/10.3390/ijms20174275
APA StyleValadez-Bustos, N., Escamilla-Silva, E. M., García-Vázquez, F. J., Gallegos-Corona, M. A., Amaya-Llano, S. L., & Ramos-Gómez, M. (2019). Oral Administration of Microencapsulated B. Longum BAA-999 and Lycopene Modulates IGF-1/IGF-1R/IGFBP3 Protein Expressions in a Colorectal Murine Model. International Journal of Molecular Sciences, 20(17), 4275. https://doi.org/10.3390/ijms20174275