Ethanol Elevates Excitability of Superior Cervical Ganglion Neurons by Inhibiting Kv7 Channels in a Cell Type-Specific and PI(4,5)P2-Dependent Manner
Abstract
1. Introduction
2. Results
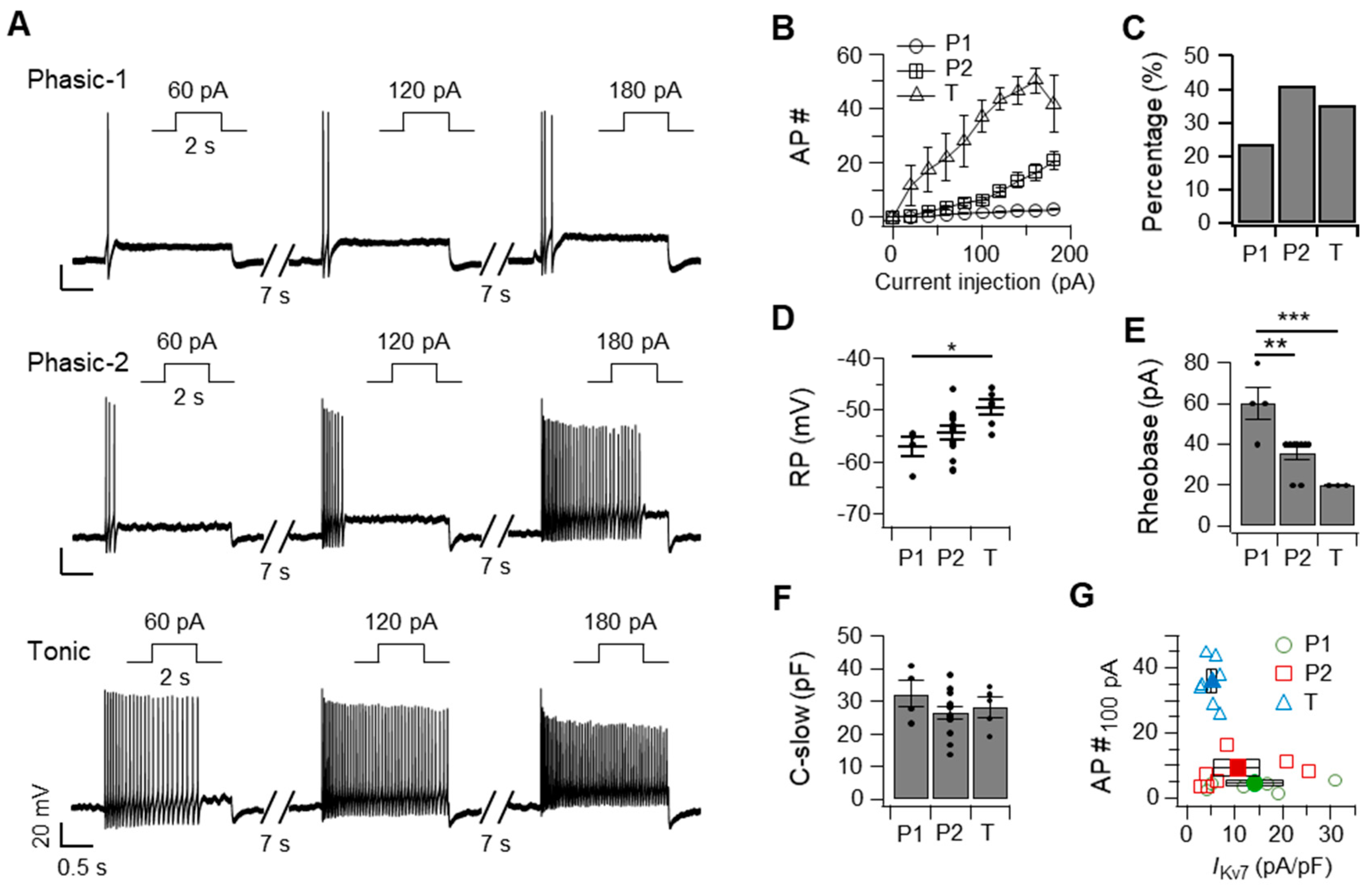
2.1. SCG Neurons Can Be Classified into Phasic-1, Phasic-2, and Tonic Neurons Based on Their Action Potential Firing Patterns and Kv7 Current (IKv7) Size
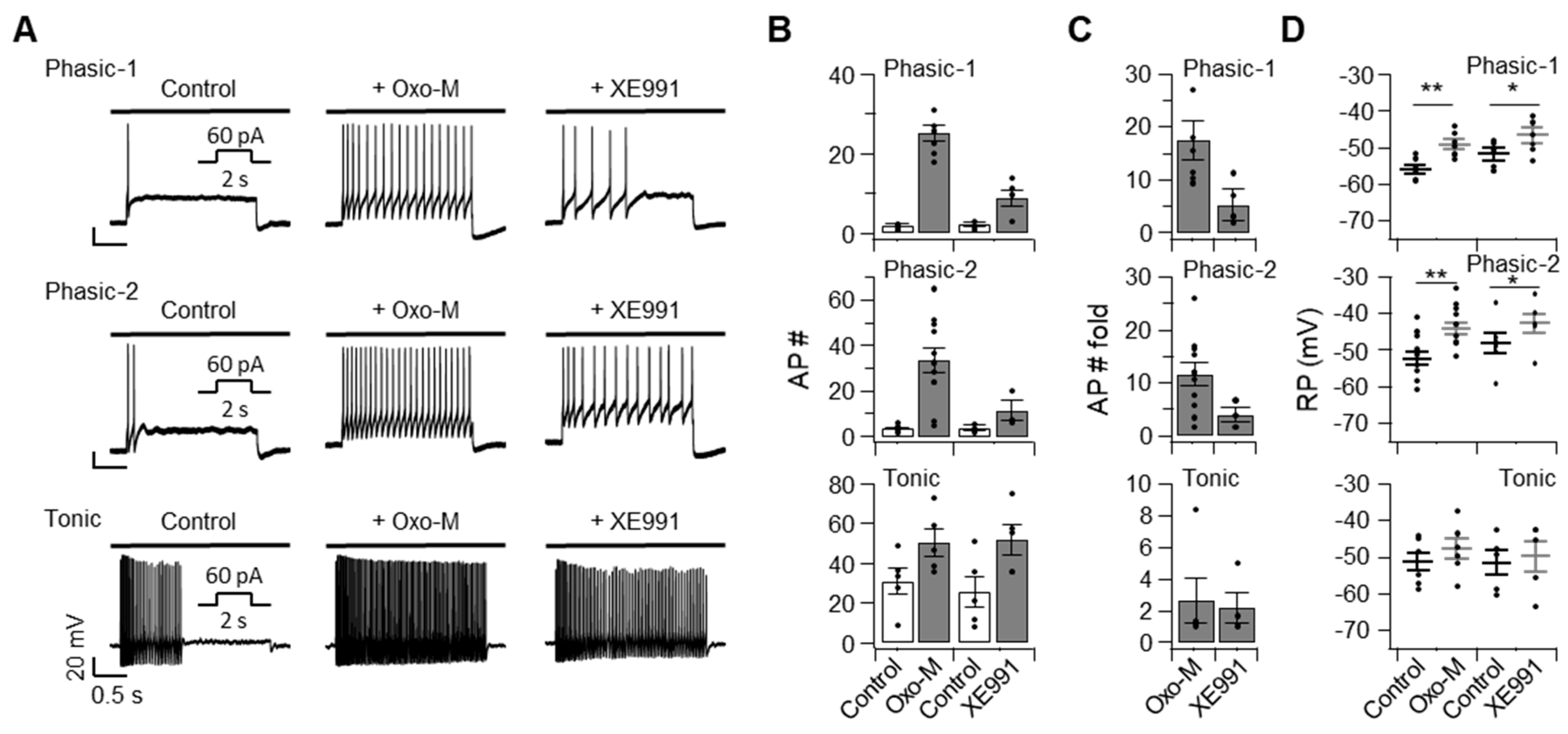
2.2. Excitability and the Resting Membrane Potential of SCG Neurons Are Affected by Inhibiting Kv7 Channels in a Cell Type-Specific Manner
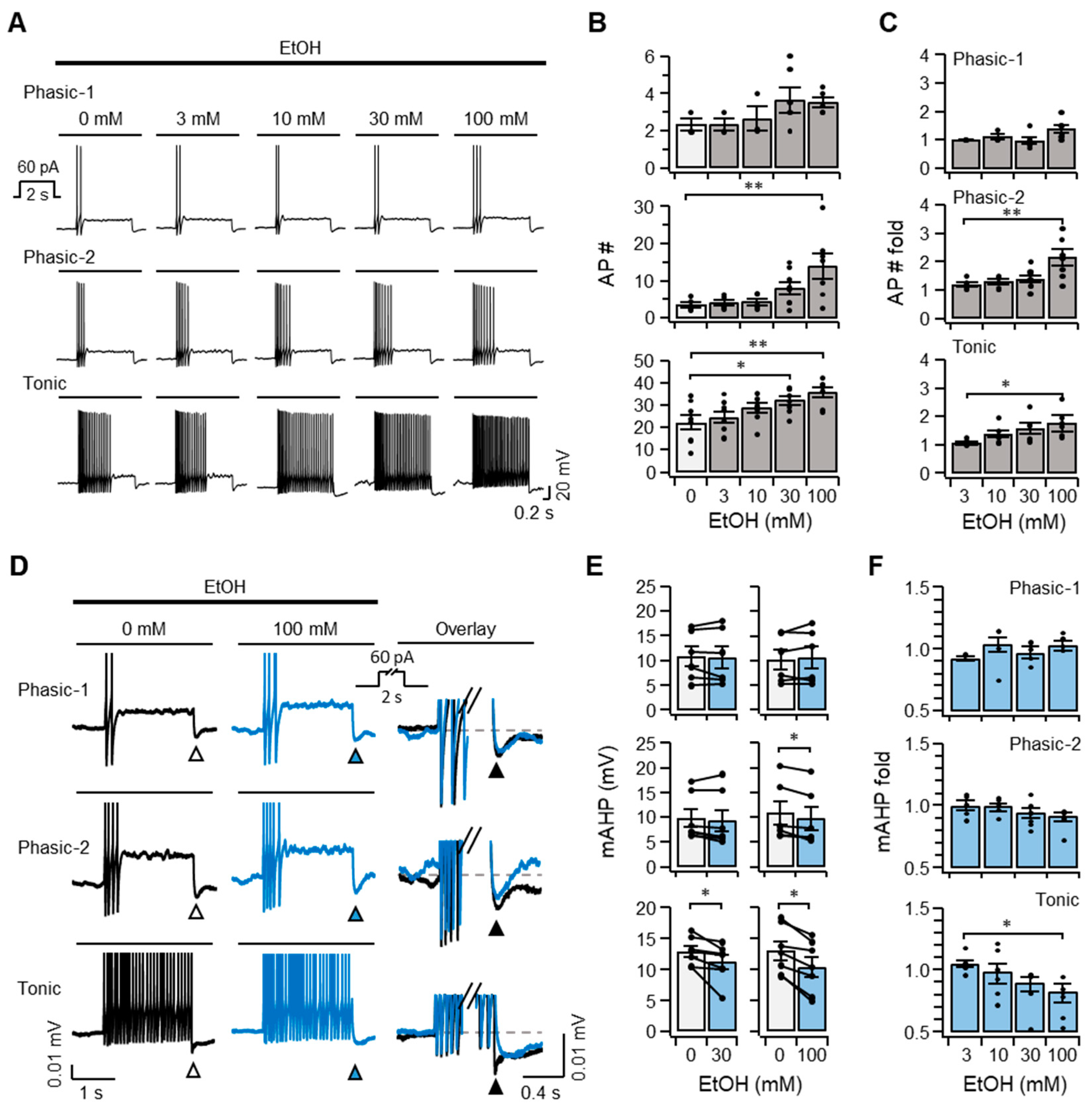
2.3. Ethanol Increases the Excitability of SCG Neurons
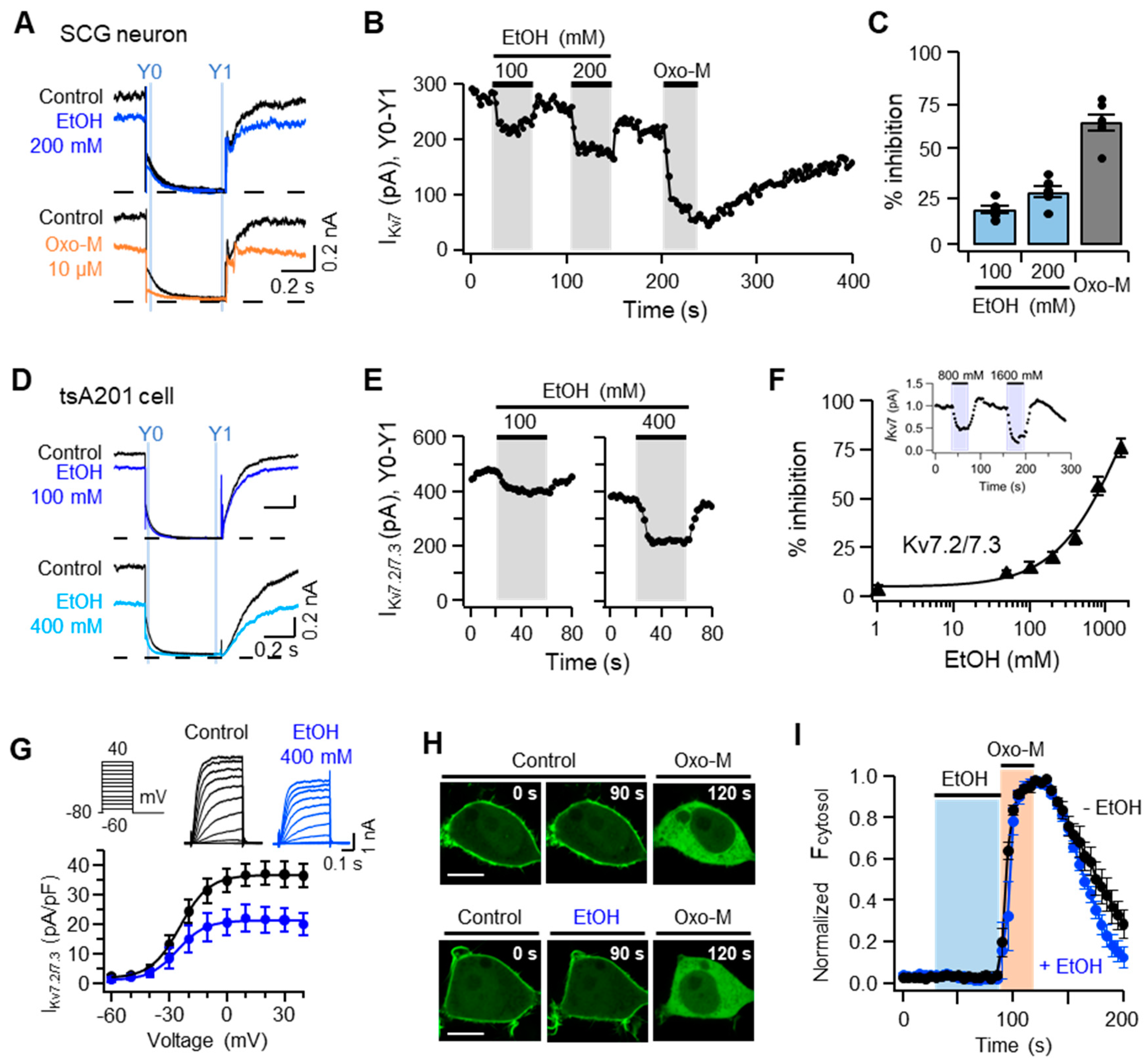
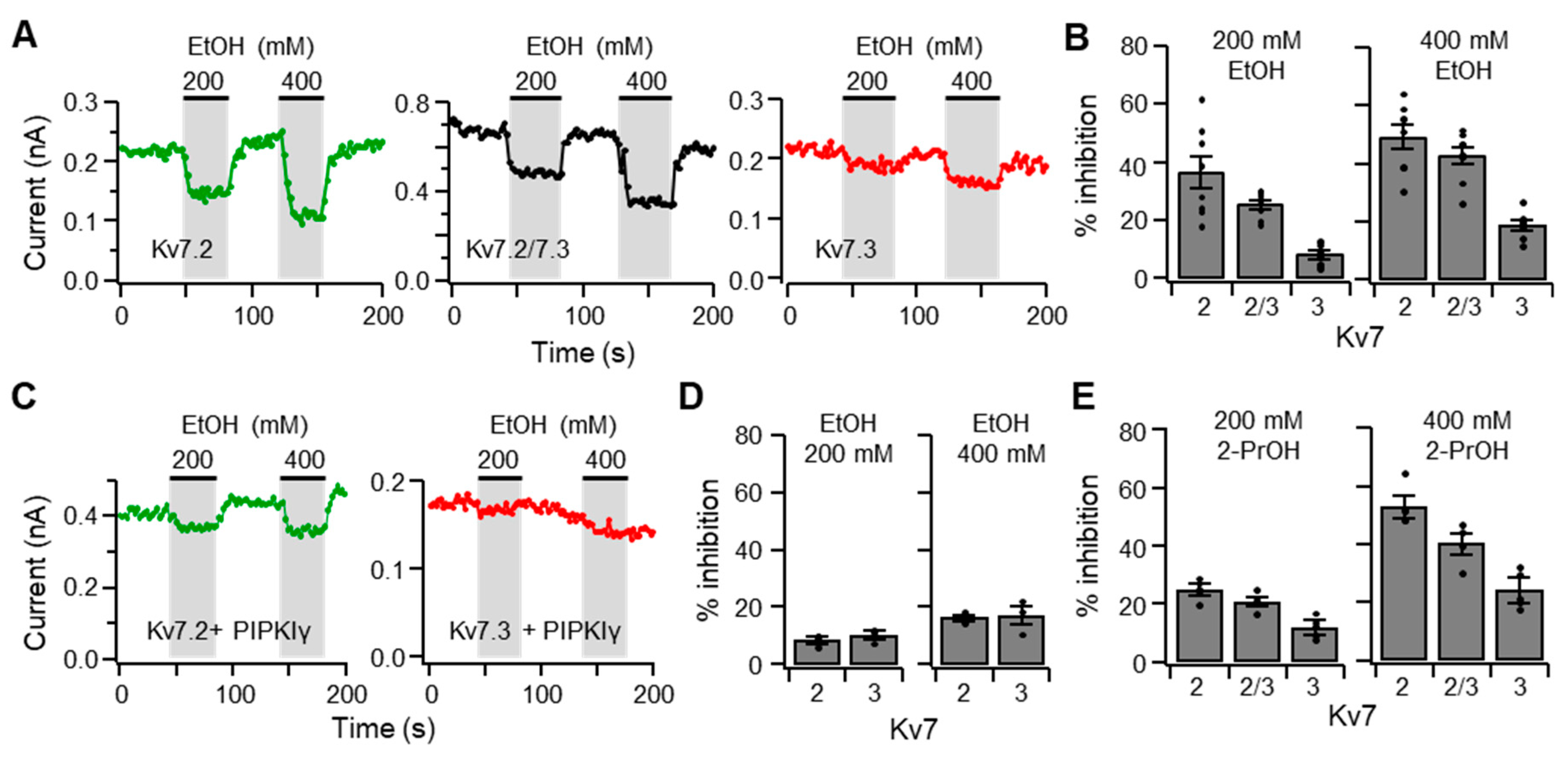
2.4. Ethanol Inhibits Kv7 Channels in Cultured SCG Neurons as Well as the Heterologous Kv7.2/7.3 Channels Expressed in TSA201 Cells
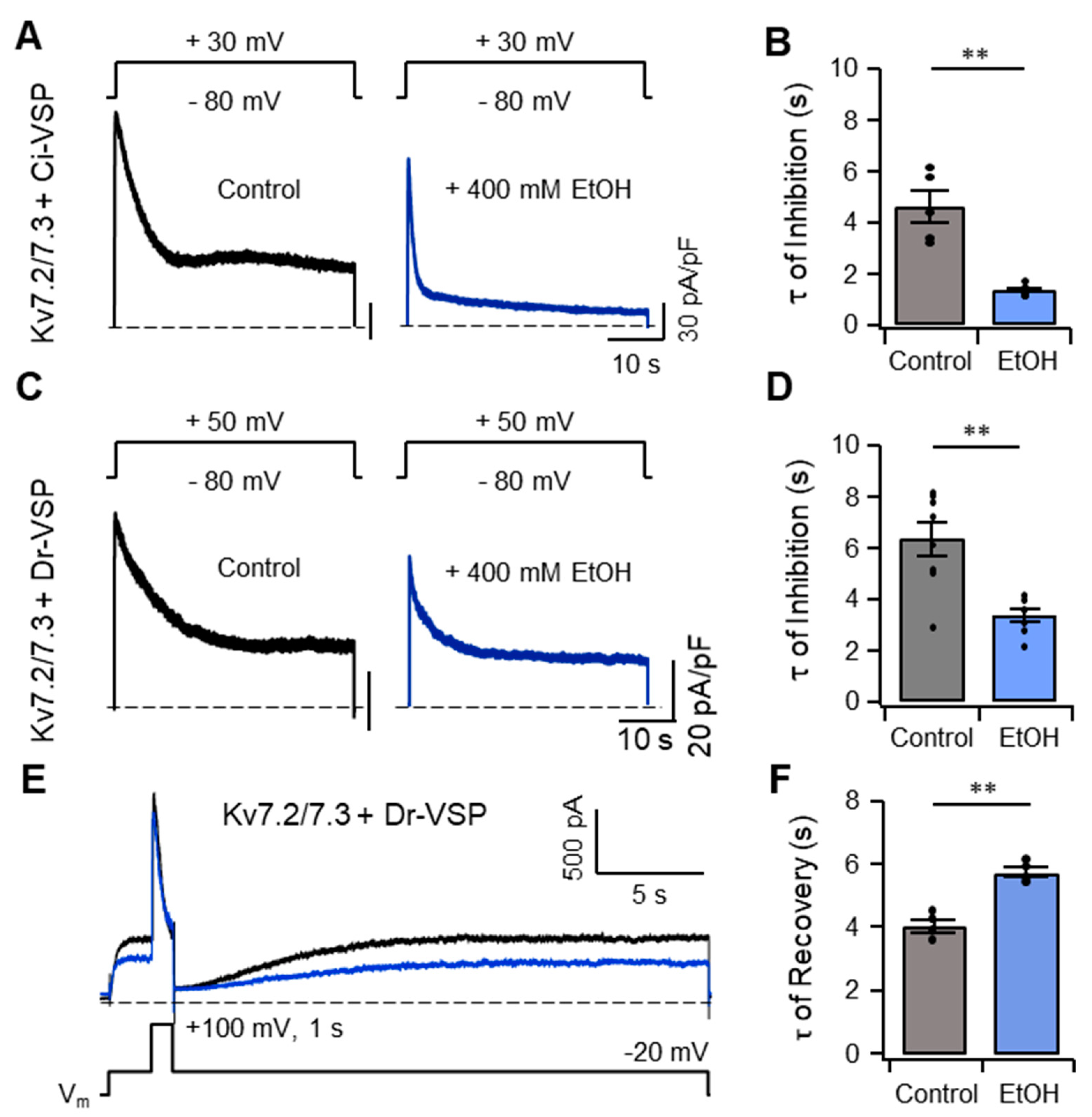
2.5. The Inhibition of IKv7 by Ethanol Is Not Due to Either Degradation or Translocation of Plasma Membrane PI(4,5)P2
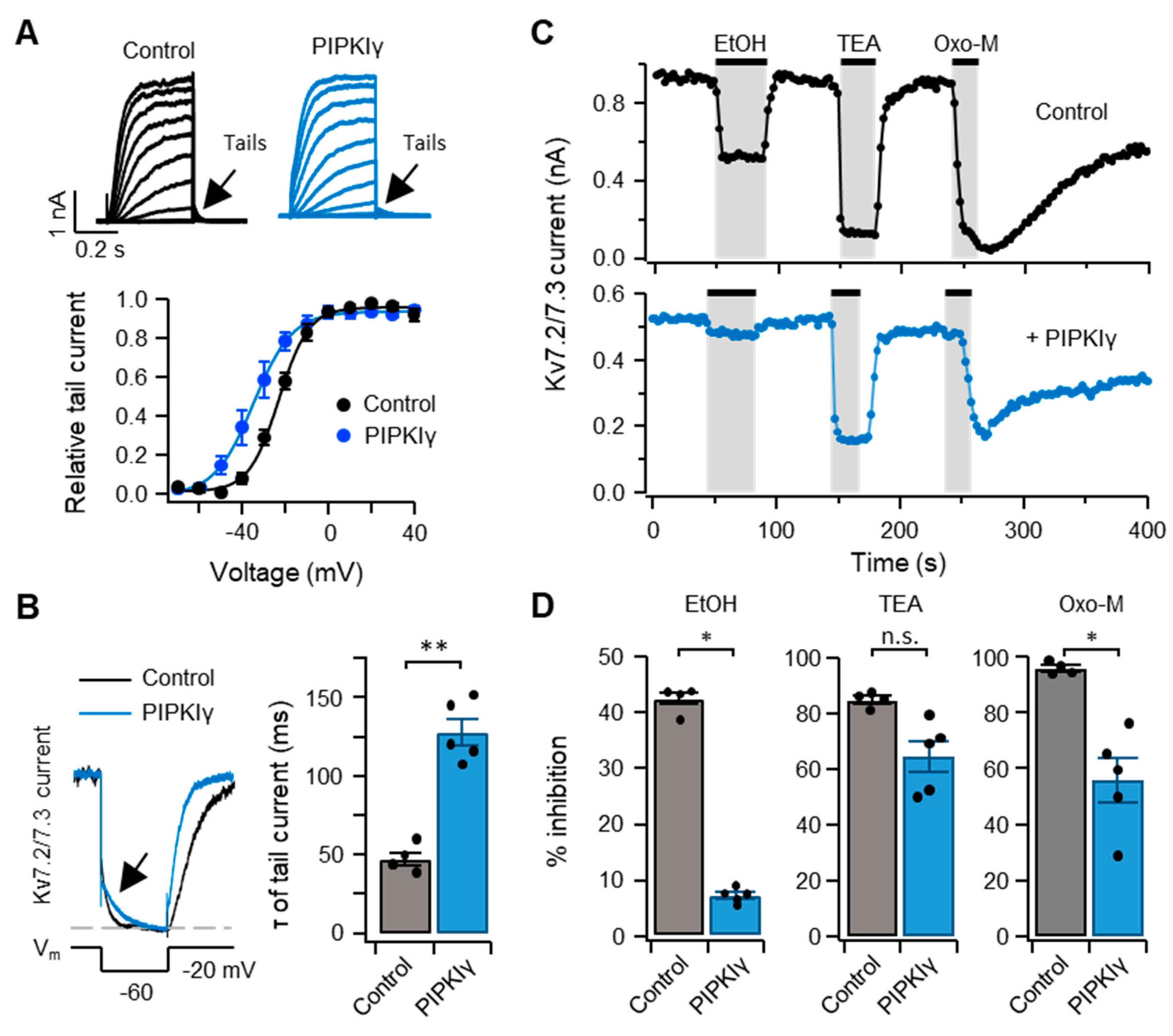
2.6. Inhibitory Effect of Ethanol on IKv7.2/7.3 is Modulated by the Level of the Plasma Membrane PI(4,5)P2
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfection
4.2. Electrophysiological Recording
4.3. Confocal Imaging and Analysis
4.4. Solutions and Materials
4.5. Data Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PI(4,5)P2 | Phosphatidylinositol 4,5-bisphosphate |
| SCG | Superior cervical ganglion |
| VSP | Voltage Sensitive phosphatase |
| FRET | Förster resonance energy transfer |
| VTA | Ventral tegmental area |
| IP3 | Inositol trisphosphate |
| DAG | Diacylglycerol |
| EtOH | Ethanol |
| PrOH | Propanol |
| TEA | Tetraethylammonium |
| M1R | M1 muscarinic acetylcholine receptor |
| Oxo-M | oxotremorine-M |
| PI(4)P | phosphatidylinositol 4-phosphate |
| PIPKIγ | phosphatidylinositol phosphate 5-kinase Iγ |
| mAHP | medium afterhyperpolarization |
| Ci-VSP | Ciona intestinalis VSP |
| GIRK | G protein-gated inwardly rectifying potassium |
| Dr-VSP | Danio rerio VSP |
| PH(PLCδ1) | pleckstrin homology domain of phospholipase C-δ1 |
| GABA | γ-Aminobutyric acid |
| NMDA | N-methyl-d-aspartate |
References
- Harrison, N.L.; Skelly, M.J.; Grosserode, E.K.; Lowes, D.C.; Zeric, T.; Phister, S.; Salling, M.C. Effects of acute alcohol on excitability in the CNS. Neuropharmacology 2017, 122, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, J.; Jaatinen, P.; Sarviharju, M.; Kiianmaa, K.; Hervonen, A. Effects of lifelong ethanol consumption on rat sympathetic neurons. Alcohol 1999, 17, 113–118. [Google Scholar] [CrossRef]
- Alderazi, Y.; Brett, F. Alcohol and the nervous system. Curr. Diagn. Pathol. 2007, 13, 203–209. [Google Scholar] [CrossRef]
- Hirota, Y. Effect of ethanol on contraction and relaxation of isolated rat ventricular muscle. J. Mol. Cell. Cardiol. 1976, 8, 727–732. [Google Scholar] [CrossRef]
- Alifimoff, J.K.; Firestone, L.L.; Miller, K.W. Anaesthetic potencies of primary alkanols: Implications for the molecular dimensions of the anaesthetic site. Br. J. Pharm. 1989, 96, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hendler, R.A.; Ramchandani, V.A.; Gilman, J.; Hommer, D.W. Stimulant and Sedative Effects of Alcohol. In Behavioral Neurobiology of Alcohol Addiction; Sommer, W.H., Spanagel, R., Eds.; Curr. Top. Behav. Neurosci.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 489–509. ISBN 978-3-642-28720-6. [Google Scholar]
- Lee, H.; Roh, S.; Kim, D.J. Alcohol-induced blackout. Int. J. Env. Res. Public Health 2009, 6, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; York, J.L. Age-related differences in response to ethanol in the rat. Psychobiology 1979, 7, 391–395. [Google Scholar] [CrossRef]
- Harris, R.A.; Trudell, J.R.; Mihic, S.J. Ethanol’s molecular targets. Sci. Signal. 2008, 1, re7. [Google Scholar] [CrossRef]
- Howard, R.J.; Slesinger, P.A.; Davies, D.L.; Das, J.; Trudell, J.R.; Harris, R.A. Alcohol-binding sites in distinct brain proteins: The quest for atomic level resolution. Alcohol. Clin. Exp. Res. 2011, 35, 1561–1573. [Google Scholar] [CrossRef]
- Koyama, S.; Brodie, M.S.; Appel, S.B. Ethanol inhibition of M-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J. Neurophysiol. 2007, 97, 1977–1985. [Google Scholar] [CrossRef]
- Bodhinathan, K.; Slesinger, P.A. Molecular mechanism underlying ethanol activation of G-protein–gated inwardly rectifying potassium channels. Proc. Natl. Acad. Sci. USA 2013, 110, 18309–18314. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Shahidullah, M.; Ellingson, J.S.; Covarrubias, M. General anesthetic action at an internal protein site involving the S4-S5 cytoplasmic loop of a neuronal K+ channel. J. Biol. Chem. 2000, 275, 4928–4936. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.L.; Hoshi, N. Modulation of Kv7 channels and excitability in the brain. Cell. Mol. Life Sci. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Klinger, F.; Gould, G.; Boehm, S.; Shapiro, M.S. Distribution of M-channel subunits KCNQ2 and KCNQ3 in rat hippocampus. NeuroImage 2011, 58, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S.; Pan, Z.; Shi, W.; Brown, B.S.; Wymore, R.S.; Cohen, I.S.; Dixon, J.E.; McKinnon, D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science 1998, 282, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Zaydman, M.A.; Cui, J. PIP2 regulation of KCNQ channels: Biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front. Physiol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Telezhkin, V.; Brown, D.A.; Gibb, A.J. Distinct subunit contributions to the activation of M-type potassium channels by PI(4,5)P2. J. Gen. Physiol. 2012, 140, 41–53. [Google Scholar] [CrossRef]
- Suh, B.-C.; Inoue, T.; Meyer, T.; Hille, B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science 2006, 314, 1454–1457. [Google Scholar] [CrossRef]
- Suh, B.-C.; Hille, B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 2002, 35, 507–520. [Google Scholar] [CrossRef]
- Brown, D.A.; Adams, P.R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 1980, 283, 673–676. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, P.; Chen, Z.; Li, M.; Jiang, H.; Gao, Z.; Yang, H. Dynamic PIP2 interactions with voltage sensor elements contribute to KCNQ2 channel gating. Proc. Natl. Acad. Sci. USA 2013, 110, 20093–20098. [Google Scholar] [CrossRef] [PubMed]
- Choveau, F.S.; De la Rosa, V.; Bierbower, S.M.; Hernandez, C.C.; Shapiro, M.S. Phosphatidylinositol 4,5-bisphosphate (PIP2) regulates KCNQ3 K+ channels by interacting with four cytoplasmic channel domains. J. Biol. Chem. 2018, 293, 19411–19428. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, I.L. Vasomotor, pilomotor and secretomotor neurons distinguished by size and neuropeptide content in superior cervical ganglia of mice. J. Auton. Nerv. Syst. 1991, 34, 171–183. [Google Scholar] [CrossRef]
- Li, C.; Horn, J.P. Physiological classification of sympathetic neurons in the rat superior cervical ganglion. J. Neurophysiol. 2006, 95, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Bei, J.; Rodat-Despoix, L.; Liu, B.; Jia, Q.; Delmas, P.; Zhang, H. NGF inhibits M/KCNQ currents and selectively alters neuronal excitability in subsets of sympathetic neurons depending on their M/KCNQ current background. J. Gen. Physiol. 2008, 131, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.H.; Eisenhofer, G.; Lambie, D.G. The effects of acute and chronic ingestion of ethanol on the autonomic nervous system. Drug Alcohol Depend. 1986, 18, 319–328. [Google Scholar] [CrossRef]
- Koob, G.F.; Arends, M.A.; Le Moal, M. Drugs, Addiction, and the Brain, 1st ed.; Chapter 6. Alcohol; Academic Press: Cambridge, MA, USA, 2014; pp. 173–219. ISBN 9780123869593. [Google Scholar]
- Hu, H.; Vervaeke, K.; Storm, J.F. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J. Physiol. 2002, 545, 783–805. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Thapa, N.; Hedman, A.C.; Anderson, R.A. Phosphatidylinositol-4,5-bisphosphate: Targeted production and signaling. Bioessays 2013, 35, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.-C.; Hille, B. Electrostatic interaction of internal Mg2+ with membrane PIP2 seen with KCNQ K+ channels. J. Gen. Physiol. 2007, 130, 241–256. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Falkenburger, B.; Shapiro, M.S. Affinity for phosphatidylinositol 4,5-bisphosphate determines muscarinic agonist sensitivity of Kv7 K+ channels. J. Gen. Physiol. 2009, 134, 437–448. [Google Scholar] [CrossRef]
- Kosenko, A.; Hoshi, N. A change in configuration of the calmodulin-KCNQ channel complex underlies Ca2+-dependent modulation of KCNQ channel activity. PLoS ONE 2013, 8, e82290. [Google Scholar] [CrossRef] [PubMed]
- Ariwodola, O.J.; Crowder, T.L.; Grant, K.A.; Daunais, J.B.; Friedman, D.P.; Weiner, J.L. Ethanol modulation of excitatory and inhibitory synaptic transmission in rat and monkey dentate granule neurons. Alcohol. Clin. Exp. Res. 2003, 27, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Mameli, M.; Valenzuela, C.F. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J. Neurosci. 2004, 24, 3746–3751. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Tao, L.; Ren, J.; Schaefer, R.; Krnjevic, K.; Liu, P.L.; Schiller, D.A.; Mcardle, J.J. Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J. Pharm. Exp. 2001, 296, 77–83. [Google Scholar]
- Badanich, K.A.; Mulholland, P.J.; Beckley, J.T.; Trantham-Davidson, H.; Woodward, J.J. Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology 2013, 38, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- McDaid, J.; McElvain, M.A.; Brodie, M.S. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: Involvement of barium-sensitive potassium currents. J. Neurophysiol. 2008, 100, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Q.; Madison, R.; Wilson, W.A.; Swartzwelder, H.S. Differential sensitivity of hippocampal interneurons to ethanol in adolescent and adult rats. J. Pharm. Exp. 2010, 335, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Q.; Fleming, R.; Madison, R.D.; Wilson, W.A.; Swartzwelder, H.S. Developmental sensitivity of hippocampal interneurons to ethanol: Involvement of the hyperpolarization-activated current, Ih. J. Neurophysiol. 2009, 101, 67–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, G.E.; Hendrickson, L.M.; Penta, K.L.; Friesen, R.M.; Pietrzykowski, A.Z.; Tapper, A.R.; Treistman, S.N. Identification of a BK channel auxiliary protein controlling molecular and behavioral tolerance to alcohol. Proc. Natl. Acad. Sci. USA 2008, 105, 17543–17548. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, K.P.; Chancey, J.H.; Chan, C.S.; Lovinger, D.M. Ethanol-Sensitive Pacemaker Neurons in the Mouse External Globus Pallidus. Neuropsychopharmacology 2017, 42, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.D.; Madamba, S.G.; Siggins, G.R. Ethanol diminishes a voltage-dependent K+ current, the M-current, in CA1 hippocampal pyramidal neurons in vitro. Brain Res. 1990, 516, 222–228. [Google Scholar] [CrossRef]
- Gu, N.; Vervaeke, K.; Hu, H.; Storm, J.F. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J. Physiol. 2005, 566, 689–715. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Benton, D.C.H.; Dunn, P.M.; Jenkinson, D.H.; Moss, G.W.J. SK3 is an important component of K+ channels mediating the afterhyperpolarization in cultured rat SCG neurones. J. Physiol. 2001, 535, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Suzuki, T. The identification of the sympathetic neurons innervating the hamster submandibular gland and their electrophysiological membrane properties. Bull. Tokyo Dent. Coll. 2001, 42, 15–33. [Google Scholar] [CrossRef][Green Version]
- Dopico, A.M.; Bukiya, A.N.; Kuntamallappanavar, G.; Liu, J. Modulation of BK channels by ethanol. Int. Rev. Neurobiol. 2016, 128, 239–279. [Google Scholar]
- Hopf, F.W.; Bowers, M.S.; Chang, S.-J.; Chen, B.T.; Martin, M.; Seif, T.; Cho, S.L.; Tye, K.; Bonci, A. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron 2010, 65, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lu, Z.; Liu, Z.; Liu, W.; Li, L.; Yin, S.; Yu, S.; Dong, H.; Zhu, F. Ethanol inhibits voltage-gated sodium channels in cultured superior cervical ganglion neurons. Neuroreport 2008, 19, 1773–1776. [Google Scholar] [CrossRef]
- Pegan, S.; Arrabit, C.; Slesinger, P.A.; Choe, S. Andersen’s syndrome mutation effects on the structure and assembly of the cytoplasmic domains of Kir2.1. Biochemistry 2006, 45, 8599–8606. [Google Scholar] [CrossRef]
- Aryal, P.; Dvir, H.; Choe, S.; Slesinger, P.A. A discrete alcohol pocket involved in GIRK channel activation. Nat. Neurosci. 2009, 12, 988–995. [Google Scholar] [CrossRef]
- Mahajan, R.; Ha, J.; Zhang, M.; Kawano, T.; Kozasa, T.; Logothetis, D.E. A computational model predicts that Gβγ acts at a cleft between channel subunits to activate GIRK1 channels. Sci. Signal. 2013, 6, ra69. [Google Scholar] [CrossRef]
- Covarrubias, M.; Vyas, T.B.; Escobar, L.; Wei, A. Alcohols inhibit a cloned potassium channel at a discrete saturable site. J. Biol. Chem. 1995, 270, 19408–19416. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Graber, A.R.; Covarrubias, M. Allosteric modulation of a neuronal K+ channel by 1-alkanols is linked to a key residue in the activation gate. Am. J. Physiol.-Cell Ph. 2003, 285, C788–C796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Craciun, L.C.; Mirshahi, T.; Rohács, T.; Lopes, C.M.B.; Jin, T.; Logothetis, D.E. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 2003, 37, 963–975. [Google Scholar] [CrossRef]
- Lacin, E.; Aryal, P.; Glaaser, I.W.; Bodhinathan, K.; Tsai, E.; Marsh, N.; Tucker, S.J.; Sansom, M.S.P.; Slesinger, P.A. Dynamic role of the tether helix in PIP2-dependent gating of a G protein–gated potassium channel. J. Gen. Physiol. 2017, 149, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Bodhinathan, K.; Slesinger, P.A. Alcohol modulation of G-protein-gated inwardly rectifying potassium channels: From binding to therapeutics. Front. Physiol. 2014, 5, 76. [Google Scholar] [CrossRef]
- Rossi, M.A.; Zucoloto, S. Effect of alcohol on ganglion cells in the superior cervical ganglia of young rats. Beitr. Pathol. 1976, 157, 183–188. [Google Scholar] [CrossRef]
- Zareen, N.; Greene, L.A. Protocol for culturing sympathetic neurons from rat superior cervical ganglia (SCG). J. Vis. Exp. 2009, 23, 988. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-W.; Kim, K.; Lee, H.; Suh, B.-C. Ethanol Elevates Excitability of Superior Cervical Ganglion Neurons by Inhibiting Kv7 Channels in a Cell Type-Specific and PI(4,5)P2-Dependent Manner. Int. J. Mol. Sci. 2019, 20, 4419. https://doi.org/10.3390/ijms20184419
Kim K-W, Kim K, Lee H, Suh B-C. Ethanol Elevates Excitability of Superior Cervical Ganglion Neurons by Inhibiting Kv7 Channels in a Cell Type-Specific and PI(4,5)P2-Dependent Manner. International Journal of Molecular Sciences. 2019; 20(18):4419. https://doi.org/10.3390/ijms20184419
Chicago/Turabian StyleKim, Kwon-Woo, Keetae Kim, Hyosang Lee, and Byung-Chang Suh. 2019. "Ethanol Elevates Excitability of Superior Cervical Ganglion Neurons by Inhibiting Kv7 Channels in a Cell Type-Specific and PI(4,5)P2-Dependent Manner" International Journal of Molecular Sciences 20, no. 18: 4419. https://doi.org/10.3390/ijms20184419
APA StyleKim, K.-W., Kim, K., Lee, H., & Suh, B.-C. (2019). Ethanol Elevates Excitability of Superior Cervical Ganglion Neurons by Inhibiting Kv7 Channels in a Cell Type-Specific and PI(4,5)P2-Dependent Manner. International Journal of Molecular Sciences, 20(18), 4419. https://doi.org/10.3390/ijms20184419





