Evans Blue Reduces Neuropathic Pain Behavior by Inhibiting Spinal ATP Release
Abstract
1. Introduction
2. Results
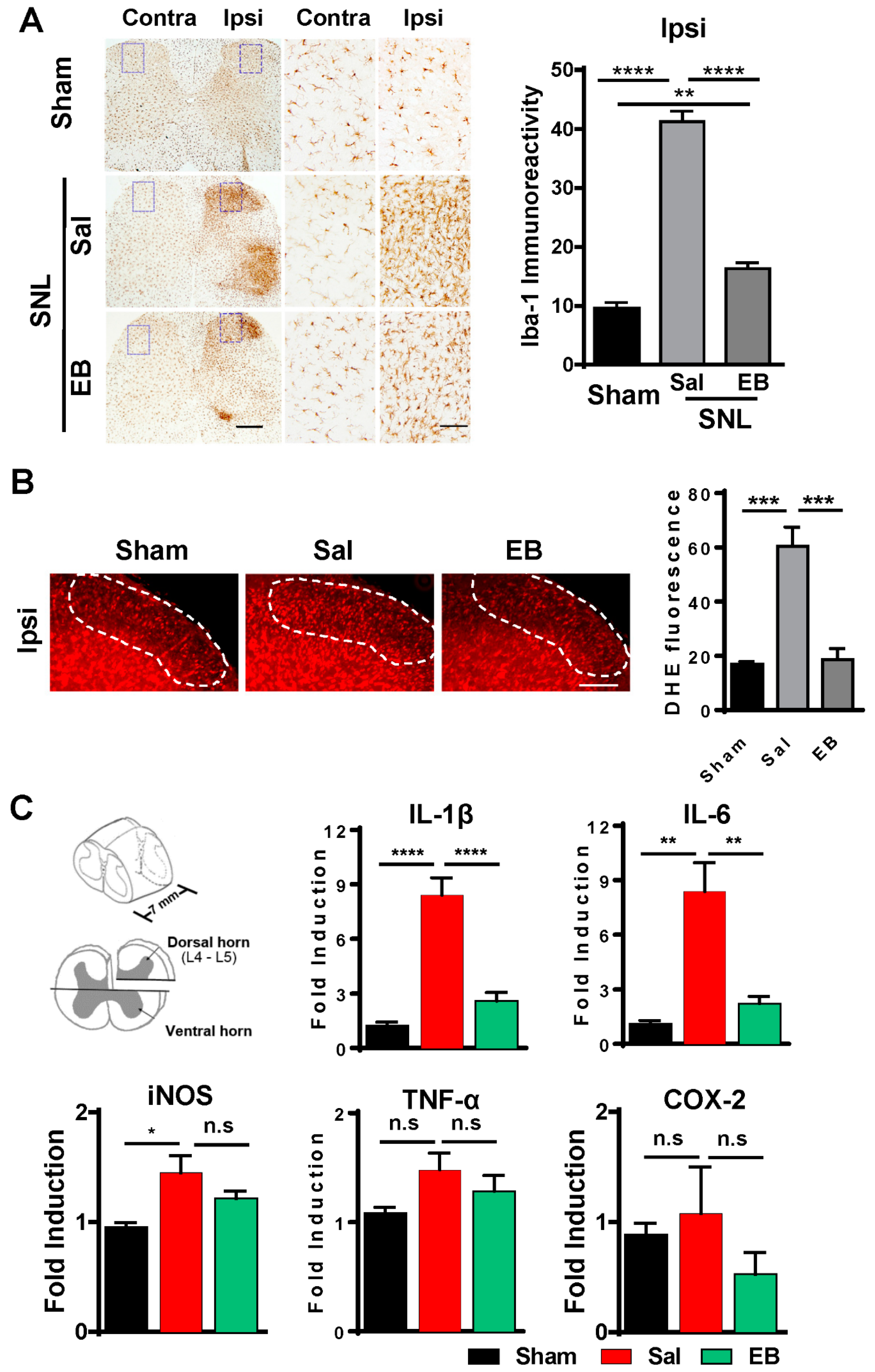
2.1. SNL Induces Neuropathic Pain Behavior and Activates Microglia in the Ipsilateral Dorsal Horn of the Spinal Cord
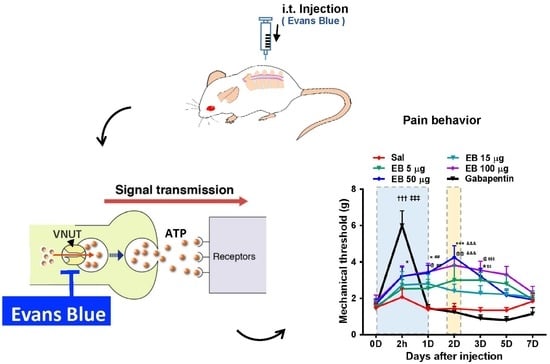
2.2. EB Attenuates Pain Behavior and Enhances Locomotive Activity in SNL-Induced Rats
2.3. EB Reduces the Level of ROS and Proinflammatory Mediators by Downregulating Microglial Activity in the Spinal Dorsal Horn
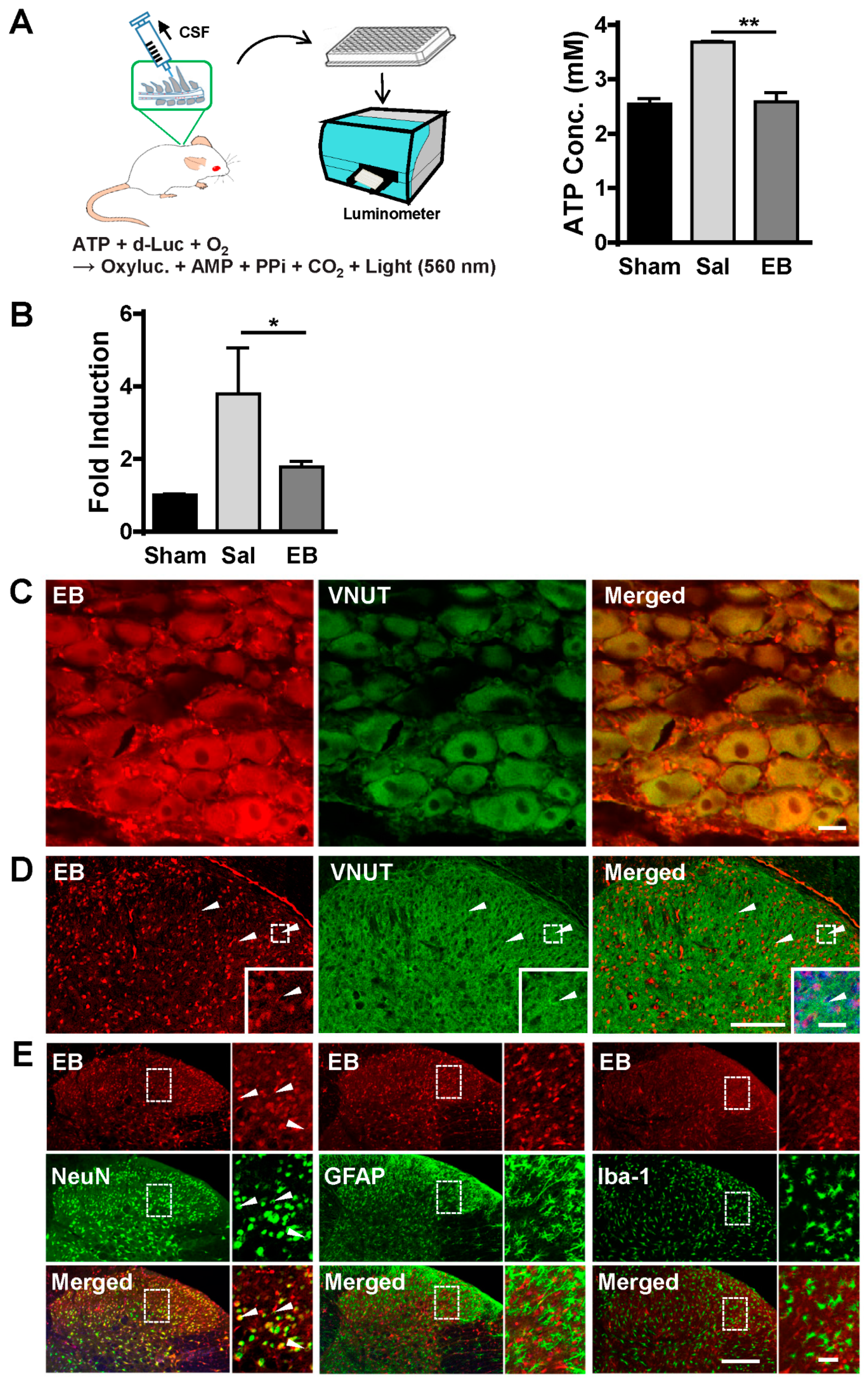
2.4. EB Blocks ATP Release from Neurons of the DRG and Spinal Dorsal Horn in a VNUT-Dependent Manner
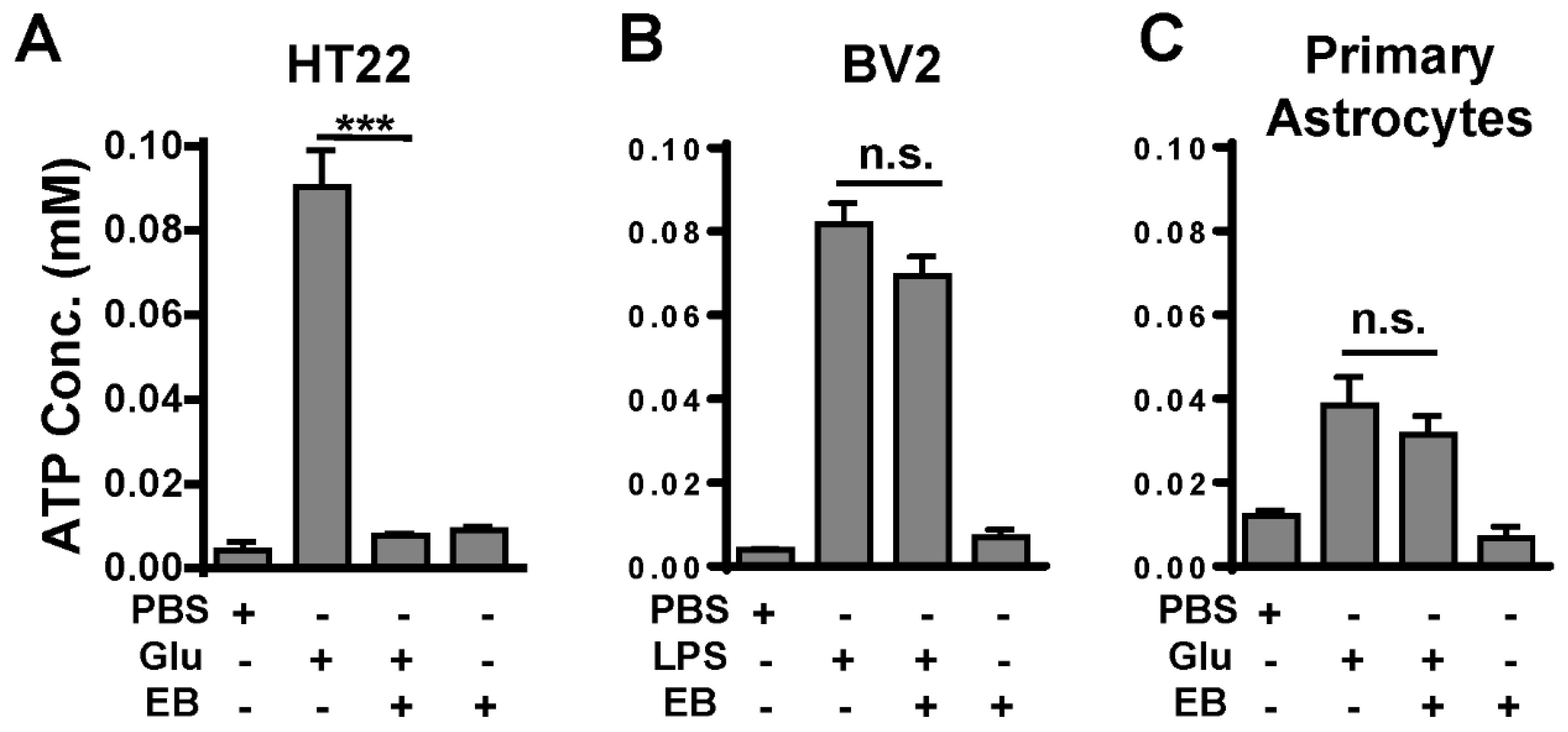
2.5. EB Inhibits ATP Release from Cultured Neurons, but Not from Cultured Microglia or Astrocytes
2.6. EB Significantly Alleviates Monoiodoacetate (MIA)-Induced OA Pain
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Pain Model, Behavioral Testing, and Intrathecal Injection
4.3. Tissue Processing and Immunohistochemistry
4.4. Reactive Oxygen Species (ROS) Detection Assay
4.5. Cerebrospinal Fluid (CSF) Collection
4.6. Cell Cultures
4.7. ATP Assay
4.8. Quantitative Polymerase Chain Reaction (qPCR)
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VNUT | Vesicular nucleotide transporter |
| EB | Evans blue |
| SNL | Spinal nerve ligation |
| IL | Interleukin |
| qPCR | Quantitative polymerase chain reaction (qPCR) |
| CSF | Cerebrospinal fluid |
| MIA | Monoiodoacetate |
| OA | Osteoarthritis |
References
- Marchand, F.; Perretti, M.; McMahon, S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 2005, 6, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Nomura, M.; Iwatsuki, K.; Moriyama, Y.; Gachet, C.; Koizumi, S. Microglia trigger astrocyte-mediated neuroprotection via purinergic gliotransmission. Sci. Rep. 2014, 4, 4329. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic. Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Nomura, M. Clodronate: A Vesicular ATP Release Blocker. Trends Pharm. Sci. 2018, 39, 13–23. [Google Scholar] [CrossRef]
- Masuda, T.; Ozono, Y.; Mikuriya, S.; Kohro, Y.; Tozaki-Saitoh, H.; Iwatsuki, K.; Uneyama, H.; Ichikawa, R.; Salter, M.W.; Tsuda, M.; et al. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat. Commun. 2016, 7, 12529. [Google Scholar] [CrossRef]
- Kato, Y.; Hiasa, M.; Ichikawa, R.; Hasuzawa, N.; Kadowaki, A.; Iwatsuki, K.; Shima, K.; Endo, Y.; Kitahara, Y.; Inoue, T.; et al. Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc. Natl. Acad. Sci. USA 2017, 114, E6297–E6305. [Google Scholar] [CrossRef]
- Norlund, L.K. A technique for the isolation of highly viable pancreatic B-cells from ob/ob mice. Acta Diabetol Lat 1986, 23, 43–49. [Google Scholar] [CrossRef]
- Malaowalla, A.M.; Fong, C. Toxicity of Evans blue dye in the monkey and tracing of it in the tooth pulp. Oral Surg Oral Med. Oral Pathol 1962, 15, 1259–1263. [Google Scholar] [CrossRef]
- Hatashita, S.; Hoff, J.T. Role of blood-brain barrier permeability in focal ischemic brain edema. Adv Neurol 1990, 52, 327–333. [Google Scholar] [PubMed]
- Sindrup, S.H.; Jensen, T.S. Efficacy of pharmacological treatments of neuropathic pain: An update and effect related to mechanism of drug action. Pain 1999, 83, 389–400. [Google Scholar] [CrossRef]
- Brumovsky, P.R. VGLUTs in Peripheral Neurons and the Spinal Cord: Time for a Review. Isrn Neurol 2013, 2013, 829753. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Davis, E.; Carrigan, C.N.; Cox, H.D.; Bridges, R.J.; Gerdes, J.M. Inhibitor of the glutamate vesicular transporter (VGLUT). Curr Med. Chem 2005, 12, 2041–2056. [Google Scholar] [CrossRef]
- Sawada, K.; Echigo, N.; Juge, N.; Miyaji, T.; Otsuka, M.; Omote, H.; Yamamoto, A.; Moriyama, Y. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. USA 2008, 105, 5683–5686. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Jancalek, R. Signaling mechanisms in mirror image pain pathogenesis. Ann. Neurosci. 2011, 18, 123–127. [Google Scholar] [CrossRef][Green Version]
- Wiffen, P.J.; Derry, S.; Bell, R.F.; Rice, A.S.; Tolle, T.R.; Phillips, T.; Moore, R.A. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 6, CD007938. [Google Scholar] [CrossRef]
- Bozkurt, A.; Deumens, R.; Scheffel, J.; O’Dey, D.M.; Weis, J.; Joosten, E.A.; Fuhrmann, T.; Brook, G.A.; Pallua, N. CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J. Neurosci. Methods 2008, 173, 91–98. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Uesugi, N.; Jeong, N.Y.; Park, B.S.; Konishi, H.; Kiyama, H. Increase of transcription factor EB (TFEB) and lysosomes in rat DRG neurons and their transportation to the central nerve terminal in dorsal horn after nerve injury. Neuroscience 2016, 313, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Hiasa, M.; Sakamoto, S.; Omote, H.; Nomura, M. Vesicular nucleotide transporter (VNUT): Appearance of an actress on the stage of purinergic signaling. Purinergic. Signal. 2017, 13, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Sawada, K.; Morland, C.; Hiasa, M.; Ormel, L.; Moriyama, Y.; Gundersen, V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb. Cortex. 2012, 22, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Sathe, M.N.; Woo, K.; Kresge, C.; Bugde, A.; Luby-Phelps, K.; Lewis, M.A.; Feranchak, A.P. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J. Biol. Chem. 2011, 286, 25363–25376. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, A.; Tsukimoto, M.; Harada, H.; Moriyama, Y.; Kojima, S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J. Biol. Chem. 2010, 285, 17406–17416. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Jobling, A.I.; Greferath, U.; Chuang, T.; Ramesh, A.; Fletcher, E.L.; Vessey, K.A. Vesicular expression and release of ATP from dopaminergic neurons of the mouse retina and midbrain. Front. Cell. Neurosci. 2015, 9, 389. [Google Scholar] [CrossRef]
- Imura, Y.; Morizawa, Y.; Komatsu, R.; Shibata, K.; Shinozaki, Y.; Kasai, H.; Moriishi, K.; Moriyama, Y.; Koizumi, S. Microglia release ATP by exocytosis. Glia 2013, 61, 1320–1330. [Google Scholar] [CrossRef]
- Oya, M.; Kitaguchi, T.; Yanagihara, Y.; Numano, R.; Kakeyama, M.; Ikematsu, K.; Tsuboi, T. Vesicular nucleotide transporter is involved in ATP storage of secretory lysosomes in astrocytes. Biochem. Biophys. Res. Commun. 2013, 438, 145–151. [Google Scholar] [CrossRef]
- Nishida, K.; Nomura, Y.; Kawamori, K.; Moriyama, Y.; Nagasawa, K. Expression profile of vesicular nucleotide transporter (VNUT, SLC17A9) in subpopulations of rat dorsal root ganglion neurons. Neurosci. Lett. 2014, 579, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.N.; Wall, P.D.; Ben-Dor, E.; Michaelis, M.; Amir, R.; Devor, M. Tactile allodynia in the absence of C-fiber activation: Altered firing properties of DRG neurons following spinal nerve injury. Pain 2000, 85, 503–521. [Google Scholar] [CrossRef]
- Tashima, R.; Koga, K.; Sekine, M.; Kanehisa, K.; Kohro, Y.; Tominaga, K.; Matsushita, K.; Tozaki-Saitoh, H.; Fukazawa, Y.; Inoue, K.; et al. Optogenetic Activation of Non-Nociceptive Abeta Fibers Induces Neuropathic Pain-Like Sensory and Emotional Behaviors after Nerve Injury in Rats. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Hochman, J.R.; Gagliese, L.; Davis, A.M.; Hawker, G.A. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthr. Cartil. 2011, 19, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Oteo-Alvaro, A.; Ruiz-Iban, M.A.; Miguens, X.; Stern, A.; Villoria, J.; Sanchez-Magro, I. High Prevalence of Neuropathic Pain Features in Patients with Knee Osteoarthritis: A Cross-Sectional Study. Pain Pr. 2015, 15, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Rahman, W.; Hobbs, C.; Dickenson, A.H.; Bennett, D.L. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS ONE 2012, 7, e33730. [Google Scholar] [CrossRef]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, X.M.; Zheng, B.J.; Wang, X.R. Electroacupuncture Relieves Nerve Injury-Induced Pain Hypersensitivity via the Inhibition of Spinal P2X7 Receptor-Positive Microglia. Anesth. Analg. 2016, 122, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.C.; Corbin, K.L.; Li, Q.; Feranchak, A.P.; Nunemaker, C.S.; Li, C. Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology 2013, 154, 675–684. [Google Scholar] [CrossRef]
- Sakamoto, S.; Miyaji, T.; Hiasa, M.; Ichikawa, R.; Uematsu, A.; Iwatsuki, K.; Shibata, A.; Uneyama, H.; Takayanagi, R.; Yamamoto, A.; et al. Impairment of vesicular ATP release affects glucose metabolism and increases insulin sensitivity. Sci. Rep. 2014, 4, 6689. [Google Scholar] [CrossRef]
- Lessmann, V.; Gottmann, K.; Lux, H.D. Evans blue reduces macroscopic desensitization of non-NMDA receptor mediated currents and prolongs excitatory postsynaptic currents in cultured rat thalamic neurons. Neurosci. Lett. 1992, 146, 13–16. [Google Scholar] [CrossRef]
- Wallace, M.S.; Lam, V.; Schettler, J. NGX426, an oral AMPA-kainate antagonist, is effective in human capsaicin-induced pain and hyperalgesia. Pain Med. 2012, 13, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Attal, N.; Casale, R.; Dickenson, A.H.; Treede, R.D. Neuropathic low back pain in clinical practice. Eur. J. Pain 2016, 20, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Mu, A.; Weinberg, E.; Moulin, D.E.; Clarke, H. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can. Fam. Physician 2017, 63, 844–852. [Google Scholar] [PubMed]
- Kang, D.W.; Moon, J.Y.; Choi, J.G.; Kang, S.Y.; Ryu, Y.; Park, J.B.; Lee, J.H.; Kim, H.W. Antinociceptive Profile of Levo-tetrahydropalmatine in Acute and Chronic Pain Mice Models: Role of spinal sigma-1 receptor. Sci. Rep. 2016, 6, 37850. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Miller, A.; Roughan, J.; Malik, A.; Haylor, K.; Sandersen, C.; Flecknell, P.; Leach, M. Efficacy of Intrathecal Morphine in a Model of Surgical Pain in Rats. PLoS ONE 2016, 11, e0163909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yi, M.H.; Shin, N.; Baek, H.; Kim, S.; Kim, E.; Kwon, K.; Lee, S.; Kim, H.W.; Chul Bae, Y.; et al. Endoplasmic reticulum stress impairment in the spinal dorsal horn of a neuropathic pain model. Sci. Rep. 2015, 5, 11555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yi, M.H.; Ko, Y.; Kim, H.W.; Seo, J.H.; Lee, Y.H.; Lee, W.; Kim, D.W. Expression of LC3 and Beclin 1 in the spinal dorsal horn following spinal nerve ligation-induced neuropathic pain. Brain Res. 2013, 1519, 31–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.J.; Chen, W.S.; Gao, H.L.; Liu, K.L.; Shi, X.L.; Fan, X.Y.; Jia, L.L.; Cui, W.; Zhu, G.Q.; et al. Exercise training attenuates renovascular hypertension partly via RAS- ROS- glutamate pathway in the hypothalamic paraventricular nucleus. Sci. Rep. 2016, 6, 37467. [Google Scholar] [CrossRef]
- Lin, L.; Desai, R.; Wang, X.; Lo, E.H.; Xing, C. Characteristics of primary rat microglia isolated from mixed cultures using two different methods. J. Neuroinflammation 2017, 14, 101. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Hong, J.; Phạm, T.L.; Shin, J.; Gwon, D.H.; Kwon, H.H.; Shin, N.; Shin, H.J.; Lee, S.Y.; Lee, W.-h.; et al. Evans Blue Reduces Neuropathic Pain Behavior by Inhibiting Spinal ATP Release. Int. J. Mol. Sci. 2019, 20, 4443. https://doi.org/10.3390/ijms20184443
Yin Y, Hong J, Phạm TL, Shin J, Gwon DH, Kwon HH, Shin N, Shin HJ, Lee SY, Lee W-h, et al. Evans Blue Reduces Neuropathic Pain Behavior by Inhibiting Spinal ATP Release. International Journal of Molecular Sciences. 2019; 20(18):4443. https://doi.org/10.3390/ijms20184443
Chicago/Turabian StyleYin, Yuhua, Jinpyo Hong, Thuỳ Linh Phạm, Juhee Shin, Do Hyeong Gwon, Hyeok Hee Kwon, Nara Shin, Hyo Jung Shin, Sun Yeul Lee, Won-hyung Lee, and et al. 2019. "Evans Blue Reduces Neuropathic Pain Behavior by Inhibiting Spinal ATP Release" International Journal of Molecular Sciences 20, no. 18: 4443. https://doi.org/10.3390/ijms20184443
APA StyleYin, Y., Hong, J., Phạm, T. L., Shin, J., Gwon, D. H., Kwon, H. H., Shin, N., Shin, H. J., Lee, S. Y., Lee, W.-h., & Kim, D. W. (2019). Evans Blue Reduces Neuropathic Pain Behavior by Inhibiting Spinal ATP Release. International Journal of Molecular Sciences, 20(18), 4443. https://doi.org/10.3390/ijms20184443