Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond
Abstract
:1. Introduction
1.1. Probiotics
1.2. Prebiotics
1.3. Synbiotics
1.4. Postbiotics
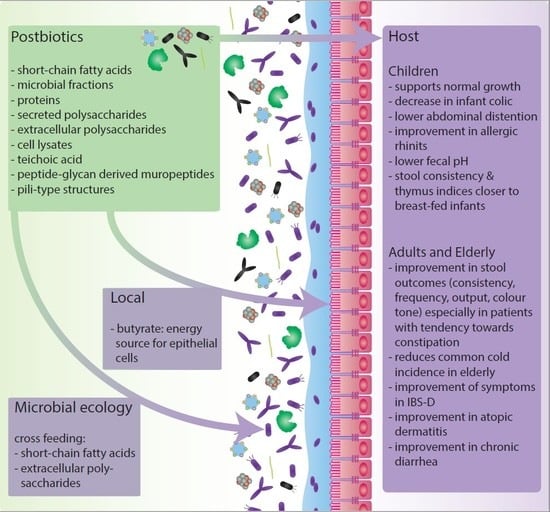
2. The Impact of Postbiotics on Host-Microbiota Interactions
2.1. Human Intervention Studies with Postbiotics in Early Life, Including New-Borns, Infants and Toddlers, until Adulthood (<18 Years)
2.1.1. Studies Using B. breve C50 and S. thermophilus 065 Combined with Prebiotics (scGOS/lcFOS)
2.1.2. Studies Using B. breve C50 and S. thermophilus
2.1.3. Studies Using Other Postbiotic Products
2.2. Human Intervention Studies with Postbiotics in Later Life, Including Adults and Elderly (>18 years).
3. Effects of Postbiotics on Microbial Community Interactions
4. Discussion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEs | Adverse events |
| BHT | Breath hydrogen test |
| CID | common infectious disease |
| EPS | Extracellular polysaccharides |
| EVs | Extracellular vesicles |
| FAO-WHO | Food and Agriculture Organization of the United Nations—World Health Organization |
| FIFs | Fermented infant formulas |
| F6PK | fructose-6-phosphate phosphoketolase |
| GPRs | G-protein coupled receptors |
| GRAS | Generally recognized as safe |
| HDAC | Histone deacetylase |
| HePS | Heteropolysaccharides |
| HoPS | Homopolysaccharides |
| HMOs | Human milk oligosaccharides |
| HRV | Human rhinovirus |
| IBS | Irritable bowel syndrome |
| IFN | interferon |
| LAB | Lactic acid bacteria |
| lcFOS | Long-chain fructooligosaccharides |
| MVs | Membrane vesicles |
| OMVs | Outer membrane vesicles |
| ORS | oral rehydration solution |
| PEP | Phosphoenolpyruvate |
| PRQLQ | pediatric rhino conjunctivitis quality of life questionnaire |
| SCFAs | Short-chain fatty acids |
| scGOS | Shot-chain galactooligosaccharides |
| SIgA | secretory immunoglobulin A |
| TLR | Toll-like receptors |
| TNF-α | Tumor Necrosis Factor α |
| UC | Ulcerative colitis |
References
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Segre, J.A. Signaling in host-associated microbial communities. Cell 2016, 164, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Dictionary.com. Biotic. Available online: https://www.dictionary.com/browse/biotical (accessed on 5 February 2019).
- FAO/WHO. Food and Agriculture Organization of the United Nations/World Health Organization Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. Available online: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (accessed on 5 February 2019).
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food. In Joint Fao/Who Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; WHO: London, ON, Canada, 2002. [Google Scholar]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Salminen, S.; Sanz, Y. The impact of probiotic on gut health. Curr. Drug Metab. 2009, 10, 68–78. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Vyas, U.; Ranganathan, N. Probiotics, prebiotics, and synbiotics: Gut and beyond. Gastroenterol. Res. Pract. 2012, 2012, 872716. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroentero. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hotel, A.C.P.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Thomas, D.W.; Greer, F.R. Probiotics and prebiotics in pediatrics. Pediatrics 2010, 126, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Amp Hepatol. 2017, 14, 491. [Google Scholar] [CrossRef]
- Giovannini, M.; Verduci, E.; Gregori, D.; Ballali, S.; Soldi, S.; Ghisleni, D.; Riva, E.; Group, P.T.S. Prebiotic effect of an infant formula supplemented with galacto-oligosaccharides: Randomized multicenter trial. J. Am. Coll. Nutr. 2014, 33, 385–393. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.; Bernal, M.-J.; Blasco, J.; Martínez, R.; Dalmau, J.; Ortuno, I.; Espín, B.; Vasallo, M.-I.; Gil, D.; Vidal, M.-L. Prebiotic effect during the first year of life in healthy infants fed formula containing gos as the only prebiotic: A multicentre, randomised, double-blind and placebo-controlled trial. Eur. J. Nutr. 2015, 54, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.; Schoterman, M.H.; Vaughan, E.E.; Belzer, C.; Benninga, M.A. The effect of fiber and prebiotics on children’s gastrointestinal disorders and microbiome. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, R.J.; Jensen, E.T.; Ringel-Kulka, T. Use of probiotics and prebiotics in infant feeding. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.; Cummings, J.T. Prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2006, 24, 701–714. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef]
- Gurry, T. Synbiotic approaches to human health and well-being. Microb. Biotechnol. 2017, 10, 1070–1073. [Google Scholar] [CrossRef] [Green Version]
- Burks, A.W.; Harthoorn, L.F.; Van Ampting, M.T.; Oude Nijhuis, M.M.; Langford, J.E.; Wopereis, H.; Goldberg, S.B.; Ong, P.Y.; Essink, B.J.; Scott, R.B. Synbiotics-supplemented amino acid-based formula supports adequate growth in cow’s milk allergic infants. Pediatr. Allergy Immunol. 2015, 26, 316–322. [Google Scholar] [CrossRef]
- Van der Aa, L.; Heymans, H.; Van Aalderen, W.; Sillevis Smitt, J.; Knol, J.; Ben Amor, K.; Goossens, D.; Sprikkelman, A.; Group, S.S. Effect of a new synbiotic mixture on atopic dermatitis in infants: A randomized-controlled trial. Clin. Exp. Allergy 2010, 40, 795–804. [Google Scholar] [CrossRef]
- Van der Aa, L.; Van Aalderen, W.; Heymans, H.; Henk Sillevis Smitt, J.; Nauta, A.; Knippels, L.; Ben Amor, K.; Sprikkelman, A.; Group, S.S. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef]
- Chua, M.C.; Ben-Amor, K.; Lay, C.; Goh, A.E.; Chiang, W.C.; Rao, R.; Chew, C.; Chaithongwongwatthana, S.; Khemapech, N.; Knol, J. Effect of synbiotic on the gut microbiota of cesarean delivered infants: A randomized, double-blind, multicenter study. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, E.; Khalesi, S.; Singh, I.; Williams, L.T.; West, N.P.; Colson, N. Effect of probiotics and synbiotics on blood glucose: A systematic review and meta-analysis of controlled trials. Eur. J. Nutr. 2018, 57, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Ouwehand, A.C.; Ibarra, A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: Systematic review and meta-analysis of randomized controlled trials. Ann. Gastroenterol. 2017, 30, 629. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Lau, C.S.; Chamberlain, R.S. Probiotics and synbiotics decrease postoperative sepsis in elective gastrointestinal surgical patients: A meta-analysis. J. Gastrointest. Surg. 2016, 20, 1123–1131. [Google Scholar] [CrossRef]
- Umu, O.C.O.; Rudi, K.; Diep, D.B. Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microb. Ecol. Health. Dis. 2017, 28, 1348886. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review article: Dietary fibre in the era of microbiome science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Kuipers, E.J.; Peppelenbosch, M.P. Functional genomic analyses of the gut microbiota for crc screening. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 741. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Agostoni, C.; Goulet, O.; Kolacek, S.; Koletzko, B.; Moreno, L.; Puntis, J.; Rigo, J.; Shamir, R.; Szajewska, H.; Turck, D. Fermented infant formulae without live bacteria. J. Pediatric Gastroenterol. Nutr. 2007, 44, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Skórka, A.; Pieścik-Lech, M. Fermented infant formulas without live bacteria: A systematic review. Eur. J. Pediatr. 2015, 174, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.; Tölkkö, S.; Kulmala, J.; Salminen, S.; Salminen, E. Adhesion of inactivated probiotic strains to intestinal mucus. Lett. Appl. Microbiol. 2000, 31, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Kataria, J.; Li, N.; Wynn, J.L.; Neu, J. Probiotic microbes: Do they need to be alive to be beneficial? Nutr. Rev. 2009, 67, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-probiotics for preterm neonates-the next frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Olle, B. Medicines from microbiota. Nat. Biotechnol. 2013, 31, 309. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J. Probiotic viability–does it matter? Microb. Ecol. Health Dis. 2012, 23, 18567. [Google Scholar] [CrossRef]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Gosálbez, L.; Ramón, D. Probiotics in transition: Novel strategies. Trends Biotechnol. 2015, 33, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, J.; Wang, N.; Li, Y.; Sun, X.; Zhang, Y.; Zhang, H. Lactobacillus casei zhang modulate cytokine and toll-like receptor expression and beneficially regulate poly i: C-induced immune responses in raw264. 7 macrophages. Microbiol. Immunol. 2013, 57, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Wang, L.; Forsythe, P.; Goettsche, G.; Mao, Y.; Wang, Y.; Tougas, G.; Bienenstock, J. Inhibitory effects of lactobacillus reuteri on visceral pain induced by colorectal distension in sprague-dawley rats. Gut 2006, 55, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, A.; Shima, T.; Kato, K.; Mizuno, S.; Uehara, T.; Matsumoto, S.; Setoyama, H.; Hara, T.; Umesaki, Y. Anti-inflammatory activity of probiotic bifidobacterium: Enhancement of il-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of il-8 secretion in ht-29 cells. World J. Gastroenterol. 2008, 14, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, C.; Lagaraine, C.; Martin, L.; Velge-Roussel, F.; Lebranchu, Y. Supernatant of bifidobacterium breve induces dendritic cell maturation, activation, and survival through a toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 2006, 117, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Menard, S.; Laharie, D.; Asensio, C.; Vidal-Martinez, T.; Candalh, C.; Rullier, A.; Zerbib, F.; Megraud, F.; Matysiak-Budnik, T.; Heyman, M. Bifidobacterium breve and streptococcus thermophilus secretion products enhance t helper 1 immune response and intestinal barrier in mice. Exp. Biol. Med. 2005, 230, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus paracasei cba l74 metabolic products and fermented milk for infant formula have anti-inflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLoS ONE 2014, 9, e87615. [Google Scholar] [CrossRef] [PubMed]
- Korcz, E.; Kerenyi, Z.; Varga, L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef]
- Ahmadi Badi, S.; Moshiri, A.; Fateh, A.; Rahimi Jamnani, F.; Sarshar, M.; Vaziri, F.; Siadat, S.D. Microbiota-derived extracellular vesicles as new systemic regulators. Front. Microbiol. 2017, 8, 1610. [Google Scholar] [CrossRef]
- Ryan, P.M.; Ross, R.P.; Fitzgerald, G.F.; Caplice, N.M.; Stanton, C. Sugar-coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Ruas-Madiedo, P. Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Crit. Rev. Food Sci. Nutr. 2016, 56, 1440–1453. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Oregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, 168–200. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Baruah, R.; Goyal, A. A food additive with prebiotic properties of an alpha-d-glucan from lactobacillus plantarum dm5. Int. J. Biol. Macromol. 2014, 69, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hongpattarakere, T.; Cherntong, N.; Wichienchot, S.; Kolida, S.; Rastall, R.A. In vitro prebiotic evaluation of exopolysaccharides produced by marine isolated lactic acid bacteria. Carbohyd. Polym. 2012, 87, 846–852. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a novel exopolysaccharide with antitumor activity from lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, M.J.; Aguilera, L.; Giménez, R.; Varela, E.; Alexandra Cañas, M.; Antolín, M.; Badía, J.; Baldomà, L. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic escherichia coli strains. Front. Microbiol. 2016, 7, 705. [Google Scholar] [CrossRef]
- Kang, C.-s.; Ban, M.; Choi, E.-J.; Moon, H.-G.; Jeon, J.-S.; Kim, D.-K.; Park, S.-K.; Jeon, S.G.; Roh, T.-Y.; Myung, S.-J. Extracellular vesicles derived from gut microbiota, especially akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef]
- Van de Heijning, B.; Berton, A.; Bouritius, H.; Goulet, O. GI symptoms in infants are a potential target for fermented infant milk formulae: A review. Nutrients 2014, 6, 3942–3967. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Ludwig, T.; Bouritius, H.; Alliet, P.; Forde, D.; Peeters, S.; Huet, F.; Hourihane, J. Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides reduces the incidence of infantile colic. Acta Paediatrica 2017, 106, 1150–1158. [Google Scholar] [CrossRef]
- Huet, F.; Abrahamse-Berkeveld, M.; Tims, S.; Simeoni, U.; Beley, G.; Savagner, C.; Vandenplas, Y.; Hourihane, J.O.B. Partly fermented infant formulae with specific oligosaccharides support adequate infant growth and are well-tolerated. J. Pediatric Gastroenterol. Nutr. 2016, 63, e43. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.R.; Ludwig, T.; Bouritius, H.; Rubio, R.P.; Munoz, A.; Agosti, M.; Lista, G.; Corvaglia, L.T.; Navero, J.L. Op-18 the combination of scgos/lcfos and fermented infant formula softens stools of infants compared to unfermented infant formula without scgos/lcfos. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 516–517. [Google Scholar] [CrossRef]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.-J.; Soulaines, P.; Leroux, B.; Kalach, N. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory iga. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Morisset, M.; Aubert-Jacquin, C.; Soulaines, P.; Moneret-Vautrin, D.; Dupont, C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 2011, 65, 175. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Ladisa, G.; Mautone, A.; Montagna, O. Effect of a fermented formula on thymus size and stool ph in healthy term infants. Pediatr. Res. 2007, 62, 98. [Google Scholar] [CrossRef] [PubMed]
- Mullié, C.; Yazourh, A.; Thibault, H.; Odou, M.-F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.-B. Increased poliovirus-specific intestinal antibody response coincides with promotion of bifidobacterium longum-infantis and bifidobacterium breve in infants: A randomized, double-blind, placebo-controlled trial. Pediatric Res. 2004, 56, 791. [Google Scholar] [CrossRef] [PubMed]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of long-term consumption of a fermented infant formula (with bifidobacterium breve c50 and streptococcus thermophilus 065) on acute diarrhea in healthy infants. J. Pediatric Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Campeotto, F.; Butel, M.; Kalach, N.; Derrieux, S.; Aubert-Jacquin, C.; Barbot, L.; Francoual, C.; Dupont, C.; Kapel, N. High faecal calprotectin concentrations in newborn infants. Arch. Dis. Child. -Fetal Neonatal Ed. 2004, 89, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Boudraa, G.; Boukhrelda, M.; de Lempdes, J.R.; Blareau, J.; Touhami, M. 41 effect of fermented infant formula on incidence of diarrhea at early weaning. J. Pediatric Gastroenterol. Nutr. 1994, 19, 339. [Google Scholar] [CrossRef]
- Thapar, N.; Sanderson, I.R. Diarrhoea in children: An interface between developing and developed countries. Lancet 2004, 363, 641–653. [Google Scholar] [CrossRef]
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Vecchio, A.L.; Shamir, R.; Szajewska, H. European society for pediatric gastroenterology, hepatology, and nutrition/european society for pediatric infectious diseases evidence-based guidelines for the management of acute gastroenteritis in children in europe: Update 2014. J. Pediatric Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Lievin-Le Moal, V.; Sarrazin-Davila, L.E.; Servin, A.L. An experimental study and a randomized, double-blind, placebo-controlled clinical trial to evaluate the antisecretory activity of lactobacillus acidophilus strain lb against nonrotavirus diarrhea. Pediatrics 2007, 120, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Kaila, M.; Isolauri, E.; Saxelin, M.; Arvilommi, H.; Vesikari, T. Viable versus inactivated lactobacillus strain gg in acute rotavirus diarrhoea. Arch. Dis. Child. 1995, 72, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Sharieff, W.; Bhutta, Z.; Schauer, C.; Tomlinson, G.; Zlotkin, S. Micronutrients (including zinc) reduce diarrhoea in children: The pakistan sprinkles diarrhoea study. Arch. Dis. Child. 2006, 91, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Kirjavainen, P.V.; Salminen, S.J.; Isolauri, E. Probiotic bacteria in the management of atopic disease: Underscoring the importance of viability. J. Pediatric Gastroenterol. Nutr. 2003, 36, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P. Preventive effect of cow’s milk fermented with lactobacillus paracasei cba l74 on common infectious diseases in children: A multicenter randomized controlled trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Laiola, M.; Paparo, L.; Calignano, A.; De Caro, C.; Coretti, L.; Chiariotti, L.; Gilbert, J.A.; et al. Specific signatures of the gut microbiota and increased levels of butyrate in children treated with fermented cow’s milk containing heat-killed lactobacillus paracasei cba l74. Appl. Environ. Microbiol. 2017, 83, e01206-17. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.C.; Hsu, C.H. The efficacy and safety of heat-killed lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatric Allergy Immunol. 2005, 16, 433–438. [Google Scholar] [CrossRef]
- Rampengan, N.H.; Manoppo, J.; Warouw, S.M. Comparison of efficacies between live and killed probiotics in children with lactose malabsorption. Southeast Asian J. Trop. Med. Public Health 2010, 41, 474–481. [Google Scholar]
- Sawada, D.; Sugawara, T.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of continuous ingestion of a beverage prepared with lactobacillus gasseri cp2305 inactivated by heat treatment on the regulation of intestinal function. Food Res. Int. 2016, 79, 33–39. [Google Scholar] [CrossRef]
- Tarrerias, A.; Costil, V.; Vicari, F.; Letard, J.; Adenis-Lamarre, P.; Aisene, A.; Batistelli, D.; Bonnaud, G.; Carpentier, S.; Dalbies, P. The effect of inactivated lactobacillus lb fermented culture medium on symptom severity: Observational investigation in 297 patients with diarrhea-predominant irritable bowel syndrome. Dig. Dis. 2011, 29, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.D.; Zhang, D.Z.; Lu, H.; JIANG, S.H.; LIU, H.Y.; Wang, G.S.; XU, G.M.; Zhang, Z.B.; LIN, G.J.; WANG, G.L. Multicenter randomized controlled trial of heat-killed lactobacillus acidophilus lb in patients with chronic diarrhea. Chin. J. Dig. Dis. 2002, 3, 167–171. [Google Scholar] [CrossRef]
- Moroi, M.; Uchi, S.; Nakamura, K.; Sato, S.; Shimizu, N.; Fujii, M.; Kumagai, T.; Saito, M.; Uchiyama, K.; Watanabe, T. Beneficial effect of a diet containing heat-killed lactobacillus paracasei k71 on adult type atopic dermatitis. J. Dermatol. 2011, 38, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, S.; Toba, M.; Saito, T.; Sato, I.; Tsubouchi, M.; Taira, K.; Kakumoto, K.; Inamatsu, T.; Yoshida, H.; Fujiwara, Y. Immunoprotective effects of oral intake of heat-killed lactobacillus pentosus strain b240 in elderly adults: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2013, 109, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Arimori, Y.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Shidara, O.; Ichikawa, H.; Yoshikai, Y. Daily intake of heat-killed lactobacillus plantarum l-137 enhances type i interferon production in healthy humans and pigs. Immunopharmacol. Immunotoxicol. 2012, 34, 937–943. [Google Scholar] [CrossRef]
- Tapiovaara, L.; Kumpu, M.; Mäkivuokko, H.; Waris, M.; Korpela, R.; Pitkäranta, A.; Winther, B. Human rhinovirus in experimental infection after peroral lactobacillus rhamnosus gg consumption, a pilot study. In International Forum of Allergy & Rhinology; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 848–853. [Google Scholar]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, 35–38. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, 900–910. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; He, L.; Wu, L.; Wang, X.; Liu, Z. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J. Cancer 2018, 9, 2510–2517. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W. Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002, 1, 287. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Lambert, N.A.; Boettger, T.; Offermanns, S.; Ganapathy, V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (slc5a8)-dependent inhibition of histone deacetylases. J. Biol. Chem. 2010, 285, 27601–27608. [Google Scholar] [CrossRef] [PubMed]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential modulation by akkermansia muciniphila and faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. MBio 2014, 5, e01438. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; van Zelm, M.; Muir, J.; Gibson, P. Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Campbell, G.; Mayer, C.-D.; Flint, H.J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J. Bacteriol. 2006, 188, 4340–4349. [Google Scholar] [CrossRef]
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Rodionov, D.A.; Arzamasov, A.A.; Khoroshkin, M.S.; Iablokov, S.N.; Leyn, S.A.; Peterson, S.N.; Novichkov, P.S.; Osterman, A.L. Micronutrient requirements and sharing capabilities of the human gut microbiome. Front. Microbiol. 2019, 10, 1316. [Google Scholar] [CrossRef]
- Sharma, V.; Rodionov, D.A.; Leyn, S.A.; Tran, D.; Iablokov, S.N.; Ding, H.; Peterson, D.A.; Osterman, A.L.; Peterson, S.N. B vitamin sharing promotes stability of gut microbial communities. Front. Microbiol. 2019, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from bifidobacterium and lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Gueimonde, M.; Hernandez-Barranco, A.M.; Ruas-Madiedo, P.; de los Reyes-Gavilan, C.G. Exopolysaccharides produced by intestinal bifidobacterium strains act as fermentable substrates for human intestinal bacteria. Appl. Environ. Microbiol. 2008, 74, 4737–4745. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.Y.; Hooi Ling, F.; Teck Chwen, L.; May Foong, O.; Anjas Asmara, S. Inhibitory activity of postbiotic produced by strains of lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.; Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocin production: A probiotic trait? Appl. Environ. Microbiol. 2012, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]

| Study | Type of study | Intervention | Comparison | N (age at start) | Population | Duration | Outcome |
|---|---|---|---|---|---|---|---|
| Berni Canani et al. 2017 [90] | Double-blind, randomized, controlled trial | Cow’s milk powder Fermented with L. paracasei CBA L74 | Cow’s milk powder with maltodextrin | 20 (12–48 months) | Healthy, term children | 3 months | Based on 16S rRNA gene amplicon sequencing of fecal samples, the relative abundance of Lactobacillus was increased and individual Blautia, Roseburia and Faecalibacterium oligotypes were associated with the intervention but not the placebo, showing correlative associations with immune biomarkers. The intervention, not placebo, showed an increase in the relative abundance of predicted genes involved in butyrate synthesis by PICRUSt-predicted metagenomes. |
| Corsello et al. 2017 [89] | Double-blind, randomized, controlled trial | Cow’s Milk Fermented with L. paracasei CBA L74 | Cow’s milk with maltodextrin | 146 (12–48 months) | Healthy, term children | 3 months | Children presenting common infectious diseases were significantly lower in the intervention group compared to the placebo group in both intention to treat (60% vs. 83%, absolute risk difference of 23%, p < 0.01) and per protocol (18% vs. 40%, absolute risk difference of 22%, p < 0.01). Moreover, significant changes in innate and acquired immune biomarkers were only observed in the intervention group. |
| Vandenplas et al. 2017 [73] | Double-blind, randomized, controlled trial | Lactofidus 50%FERM, scGOS/lcFOS+ 15%FERM or scGOS/lcFOS+ 50%FERM | scGOS/lcFOS | 432 (0–28 days) | Healthy, term infants | 17 weeks | All formulas were well tolerated, infant colic was significant lower (8%) with scGOS/lcFOS+50% FERM than scGOS/lcFOS (20%, p = 0.034) or 50% FERM (20%, p = 0.036) at week 4. Daily crying duration was lower in the scGOS/lcFOS + 50%FERM and stools were softer compared to 50% FERM. |
| Huet et al. 2016 [74] | Double-blind, randomized, controlled trial | Lactofidus 50% FERM, scGOS/lcFOS+ 15% FERM or scGOS/lcFOS+ 50% FERM | scGOS/lcFOS | 432 (0–28 days) | Healthy, term infants | 17 weeks | Equivalence of weight gain (SD) per day in all groups, scGOS/lcFOS 29.7 (6.1), scGOS/lcFOS+ 15% FERM 28.5 (4.8), scGOS/lcFOS + 50% FERM 28.5 (5.0) and 50% FERM 28.7 (5.9) g/day. No differences in other growth parameters, formula intake or number/severity of AEs. All scGOS/lcFOS-containing formula; lower stool pH and Clostridium difficile levels and higher IgA levels. |
| Herrera et al. 2015 [75] | Double-blind, randomized, controlled, trial Meeting abstract | Lactofidus scGOS/lcFOS+ 30%FERM | scGOS/lcFOS and N = 100 breast-fed children as reference group | 200 (0–28 days) 100 reference group | Healthy, term infants | 17 weeks | The scGOS/lcFOS + 30% FERM was well tolerated. Stool consistency for the scGOS/lcFOS + 30% FERM group was closer to the breast-fed reference group, and a significantly softer median from 4 weeks onwards compared to the scGOS/lcFOS group (p ≤ 0.005). From week 9 onwards scGOS/lcFOS + 30% FERM had a significant higher median stool frequency compared to scGOS/lcFOS only (p ≤ 0.05). |
| Campeotto et al. 2011 [76] | Double-blind, randomized, controlled trial | Preterm infant formula, heat-inactivated FERM with B. breve C50 and S. thermophilus 065 | Preterm infant formula | 58 (0–3 days) | Pre-term infants 30-35 weeks of GA | During hospital stay; 2–5 weeks | No differences between groups in anthropometrics and digestive tolerance, except abdominal distention, which was lower in the FERM group (0 FERM vs. 8 control, p = 0.016). Bacterial colonization was not different between groups. Significant lower fecal calprotectin was found in the FERM group from week 3 (p = 0.01). |
| Morisset et al. 2011 [77] | Double-blind, randomized, controlled trial | Infant formula, heat-inactivated with B. breve C50 and S. thermophilus | Standard infant formula | 129 (birth) | Infants with a high risk of atopy | 12 months, follow-up at 24 months | The fermented formula did not alter proportion of children with cow’s milk allergy, but decreased the proportion of positive skin prick tests to cow’s milk (1.7% vs. 12.5%, p = 0.03), incidence of digestive AEs (39% vs. 63%, p = 0.01) and respiratory potentially allergic AEs at 12 months (7% vs. 21%, p = 0.03) and respiratory potentially allergic AEs at 24 months (13% vs. 35%, p = 0.01) |
| Rampengan et al. 2010 [92] | Pretest–posttest single blinded randomized study | Lacidofil capsules containing heat-inactivated Lactobacillus helveticus R-52 and L. rhamnosus R-11 | Dialac sachets | 79 (10–12 years of age) | Healthy children with lactose mal-absorption | 2 weeks | Both groups showed a significant decrease before vs. after consumption of the intervention products of the BHT (from 34.51 ± 10.35 to 22.13 ± 12.41, p < 0.001 for live probiotics and from 36.00 ± 10.18 to 20.30 ± 8.68, p < 0.001 in the killed probiotics group). No differences were found between groups after intervention (p = 0.453). |
| Indrio et al. 2007 [78] | Double-blind, randomized, controlled trial | Infant formula, heat-inactivated with B. breve C50 and S. thermophilus | Standard infant formula And N = 30 breast-fed children as reference group | 60 (3 days) 30 reference group | Healthy, term infants | 4 months | Fecal pH was lower in breast-fed infants (p < 0.05), however it was similar to the FIF group from the third postnatal day onwards for the entire 4 months. The FIF group showed comparable thymus indices compared to breast-fed infants. Probiotic fermentation products have effects comparable to those of the bacteria composing the intestinal microbiota. |
| Lievin-Le Moal et al. 2007 [85] | Double-blind, randomized, controlled trial | Heat-killed L. acidophilus LB plus culture medium | Placebo sachets | 80 (10 months) | Infants with acute diarrhea of suspected infectious origin | 96 h of which 72 h with intervention products | Recovery time of infants with nonrotavirus diarrhea was shortened by 1 day when taking lyophilized, heat-killed L. acidophilus LB plus their culture medium (time until the first normal stool was passed compared to the placebo (39.5 ± 10.5 h vs. 63.4 ± 14.9, p < 0.01). |
| Sharieff et al. 2006 [87] | Triple-blind, randomized, controlled trial | Micronutrient sachets with heat-inactivated LAB | Micronutrient sachets or placebo sachets | 75 (6–12 months) | Healthy infants with high risk for diarrhea related mortality | 2 months | Prevalence of diarrhea was 26% in the micronutrient with LAB group, 15% in the micronutrient group and 26% in the placebo group; difference between the micronutrient with LAB and placebo was not significantly different. |
| Peng et al. 2005 [91] | Double-blind, randomized, controlled trial | Capsules with live or heat-killed L. paracasei 33 | Placebo capsules | 90 (<18 years) | Patients with perennial allergic rhinitis | 30 days | QOL increased in both intervention groups, compared to the placebo in frequency (9.47 ± 2.89, 6.30 ± 2.19 vs. –3.47 ± 1.53, respectively; p < 0.0001) and level of bother (5.91 ± 3.21, 6.04 ± 2.44, vs. –2.80 ± 1.64, respectively; p = 0.004). Efficacy of heat-killed L. paracasei LP33 was not inferior to the live variant. |
| Mullie et al. 2004 [79] | Double-blind, randomized, controlled trial | Infant formula, heat-inactivated with B. breve C50 and S. thermophilus | Standard infant formula | 30 (first days of life) | Healthy term infants | 4 months | In the FIF group at 4 months significant higher bifidobacteria levels (p = 0.0498) and Bifidobacterium longum/Bifidobacterium infantis (p = 0.0399) compared to standard formula. Antipoliovirus IgA increased after Pentacoq® challenge (p < 0.001), rise was significantly higher in the FIF group (p < 0.02). antibody titers correlated to bifidobacteria, especially with B. longum/B. infantis and B. breve (p < 0.002). |
| Thibault et al. 2004 [80] | Double-blind, randomized, controlled trial | Infant formula, heat-inactivated with B. breve C50 and S. thermophilus | Standard infant formula | 971 (4–6 months) | Healthy term infants | 5 months | Incidence and duration of diarrhea and number of hospital admissions did not differ significantly between groups. In the FIF group compared to standard formula diarrhea episodes were less severe, fewer cases of dehydration (2.5% vs. 6.1%, p = 0.01), fewer medical consultations (46% vs. 56.6%, p = 0.003), fewer ORS prescriptions (41.9% vs. 51.9%, p = 0.003) and fewer switchers to other formula (59.5% vs. 74.9%, p = 0.0001). |
| Campeotto et al. 2004 [81] | Prospective study | Infant formula, heat-inactivated with B. breve C50 and S. thermophilus | Standard infant formula N = 32 breast-fed | 37 (3–7 days) | Healthy term infants | 3 months | Fecal calprotectin concentrations did not significantly differ between groups (medians; standard formula 148 μg/g, FIF 144 μg/g and breast milk 204 μg/g), but higher (total median calprotectin 167 μg/g) than the reference value for healthy adults (50 μg/g). |
| Kirjavainen et al. 2003 [88] | Double-blind randomized | Infant formula containing live or heat inactivated L. rhamnosus GG | Hydrolyzed whey formula | 35 (mean age 5.5 months) | Infants with atopic eczema and allergy to cow’s milk | Mean of 7.5 weeks | Atopic eczema and subjective symptoms decreased in all three groups and did not differ significantly between groups. No differences were found in the bacterial numbers within the genera enumerated. However, heat inactivated L. rhamnosus GG was associated with adverse gastrointestinal symptoms and diarrhea. |
| Kaila et al. 1995 [86] | Double-blind, randomized | Heat inactivated L. casei | Viable L. casei 1010-11 colony forming units | 41 (<4 years) | Infants with acute rotavirus diarrhea | 5 days | No significant differences at the acute state for specific antibody secreting cells against rotavirus between heat inactivated L. casei vs. viable L. casei (2/8 vs. 2/9, p = 0.66) or mean antibodies (0.1 vs. 0.04, p = 0.52). In contrast to the convalescent stage, in favor of the viable L. casei for antibody secreting cells (2/13 vs. 10/12, p = 0.002) and mean antibodies (22.4 vs. 50.7, p = 0.04). Clinical recovery from rotavirus diarrhea was equal in both groups. |
| Boudraa et al. 1994 [82] | Randomized study | Infant formula, heat-inactivated with B. breve C50 and S. thermophilus | Standard infant formula | 84 (<5 months) | Healthy infants | Approx. 85 days | Rate of acceptance was similar in both groups. The FIF group had significantly less children with diarrhea compared to standard infant formula (10 vs. 19, p = 0.06), less episodes of diarrhea (49 vs. 97, p = 0.001). Respiratory tract infection was similar between groups (36 vs. 37). |
| Study | Type of study | Intervention | Comparison | N (age) | Population | Duration | Outcome |
|---|---|---|---|---|---|---|---|
| Tapiovaara et al. 2016 [99] | Double-blind, placebo controlled randomized, pilot study | Juice containing live or heat inactivated L. rhamnosus GG | Control juice without live or heat inactivated bacteria | 59 (18–65 years) | Healthy subjects | 6 weeks | A tendency towards the lowest HRV loads in the live L. rhamnosus GG group, and highest in the placebo group (on day 2: Live 6.20, heat inactivated 6.30 and placebo 7.25, p = 0.57). HRV load positively correlated with symptom scores (p = 0.034) |
| Sawada et al. 2016 [93] | Double-blind placebo controlled randomized study | Fermented milk beverage with sterilized L. gasseri CP2305 | Artificially acidified milk-based placebo beverage | 39 (20–70 years of age) | Healthy individuals with a tendency towards constipation (N = 20) or frequent bowel movements (N = 19) | 3 weeks | In the intervention group, scores on the Bristol stool scale improved significantly after 3 weeks of intervention (p < 0.05). Output and color tone were also improved, especially in subjects with a tendency towards constipation. Moreover, SCFAs (propionate, butyrate and valeric acid) were significantly increased as well as Clostridium cluster IV and a beneficial effect on the regulation of intestinal function was found. |
| Shinkai et al. 2013 [97] | Double-blind placebo controlled randomized study | Tablets containing heat killed L. pentosus b240 in a low (2 × 109) or high (2 × 1010) dose | Placebo tablets without L. pentosus b240 | 280 (>65 years) | Healthy elderly | 20 weeks | Results for high dose vs. low dose vs. placebo were, for the accumulated incidence rate of common cold: 29.0% vs. 34.8% vs. 47.3% respectively (p for trend = 0.012). General health perception, measured by SF-36, increased in both intervention groups (p for trend = 0.016). |
| Arimori et al. 2012 [98] | Randomized single-blind placebo-controlled study | Tablets containing heat-killed L. plantarum L-137 | Placebo tablets without L. plantarum L-137 | 16 (45.4 ± 8.1 years) | Healthy women | 8 weeks | No differences were found between groups in seroresponse rate and geometric mean Ab titers after the first or second dose of inactivated influenza vaccine. Levels of IFN-β were significantly higher in the intervention group than in the placebo group (p < 0.05). Moreover, type I IFN production was enhanced in the intervention group. |
| Terrerias et al. 2011 [94] | Observational study | Inactivated Lactobacillus LB and fermented culture medium (Lacteol LB) | n.a. | 297 (53.4 ± 17.3 years) | Patients with IBS-D | 1 month | Pain scores decreased from 4.46 ± 0.15 to 2.8 ± 0.14 after treatment (p < 0.0001), as well as bloating from 4.49 ± 0.18 to 2.5 ± 0.15 (p < 0.0001). The HRQOL score, inversely correlated with quality of life, decreased from 5.99 ± 0.14 to 3.92 ± 0.16 (p < 0.0001). Mean number of stools per week also decreased from 17.59 to 12.83 after treatment (p < 0.0001). |
| Moroi et al. 2011 [96] | Double-blind placebo controlled randomized study | Heat-killed L. paracasei K71 | Placebo without L. paracasei K71 | 34 (20–65 years) | Patients with mild or moderate atopic dermatitis | 12 weeks | Skin severity scores decreased significantly in the intervention group, not in the placebo group (p < 0.05). Itch scores and QOL was not significantly different between groups. Consumption of topical therapeutics was 1.9 times higher compared to the intervention group, but not significantly different. |
| Xiao et al. 2002 [95] | Prospective, randomized trial | Live or heat-killed L. acidophilus LB | n.a. | 137 (17–92 years) | Patients with chronic diarrhea | 4 weeks | Mean bowel frequency was significantly lower in the heat-killed L. acidophilus LB group compared to the live L. acidophilus LB group (1.88 ± 1.24 vs. 2.64 ± 1.12 at week 2 and 1.39 ± 0.92 vs. 2.19 ± 1.05 at week 4, p < 0.05). Improvement in stool consistency, abdominal pain, distention and feeling of incomplete evacuation were significantly higher in the heat-killed L. acidophilus LB group. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. https://doi.org/10.3390/ijms20194673
Wegh CAM, Geerlings SY, Knol J, Roeselers G, Belzer C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. International Journal of Molecular Sciences. 2019; 20(19):4673. https://doi.org/10.3390/ijms20194673
Chicago/Turabian StyleWegh, Carrie A. M., Sharon Y. Geerlings, Jan Knol, Guus Roeselers, and Clara Belzer. 2019. "Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond" International Journal of Molecular Sciences 20, no. 19: 4673. https://doi.org/10.3390/ijms20194673
APA StyleWegh, C. A. M., Geerlings, S. Y., Knol, J., Roeselers, G., & Belzer, C. (2019). Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. International Journal of Molecular Sciences, 20(19), 4673. https://doi.org/10.3390/ijms20194673