Mildew Resistance Locus O Genes CsMLO1 and CsMLO2 Are Negative Modulators of the Cucumis sativus Defense Response to Corynespora cassiicola
Abstract
:1. Introduction
2. Results
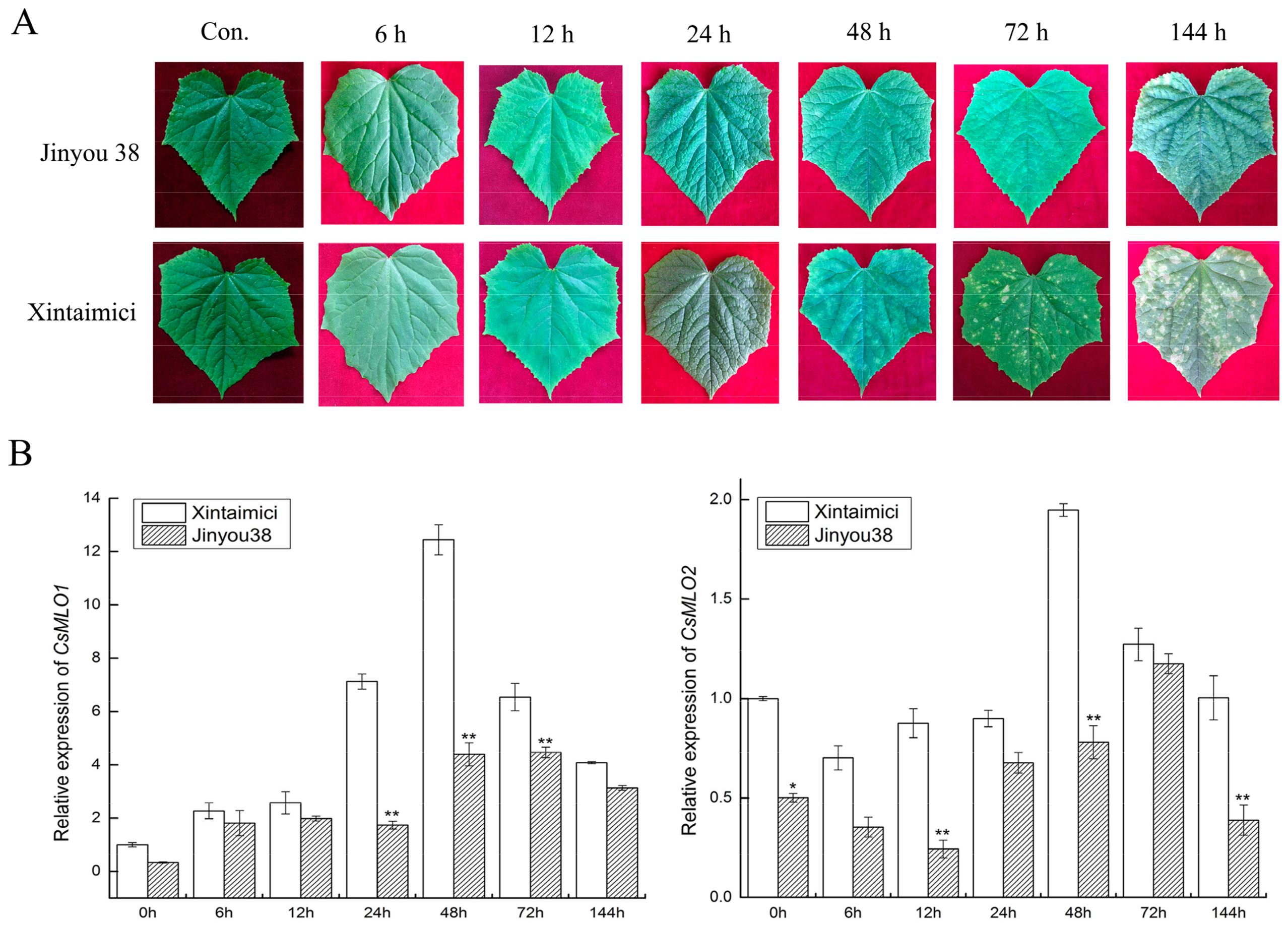
2.1. Phenotypic Characterization of Cucumber Cultivars after C. cassiicola Infection
2.2. Isolation and Phylogenetic Analysis of CsMLO1 and CsMLO2
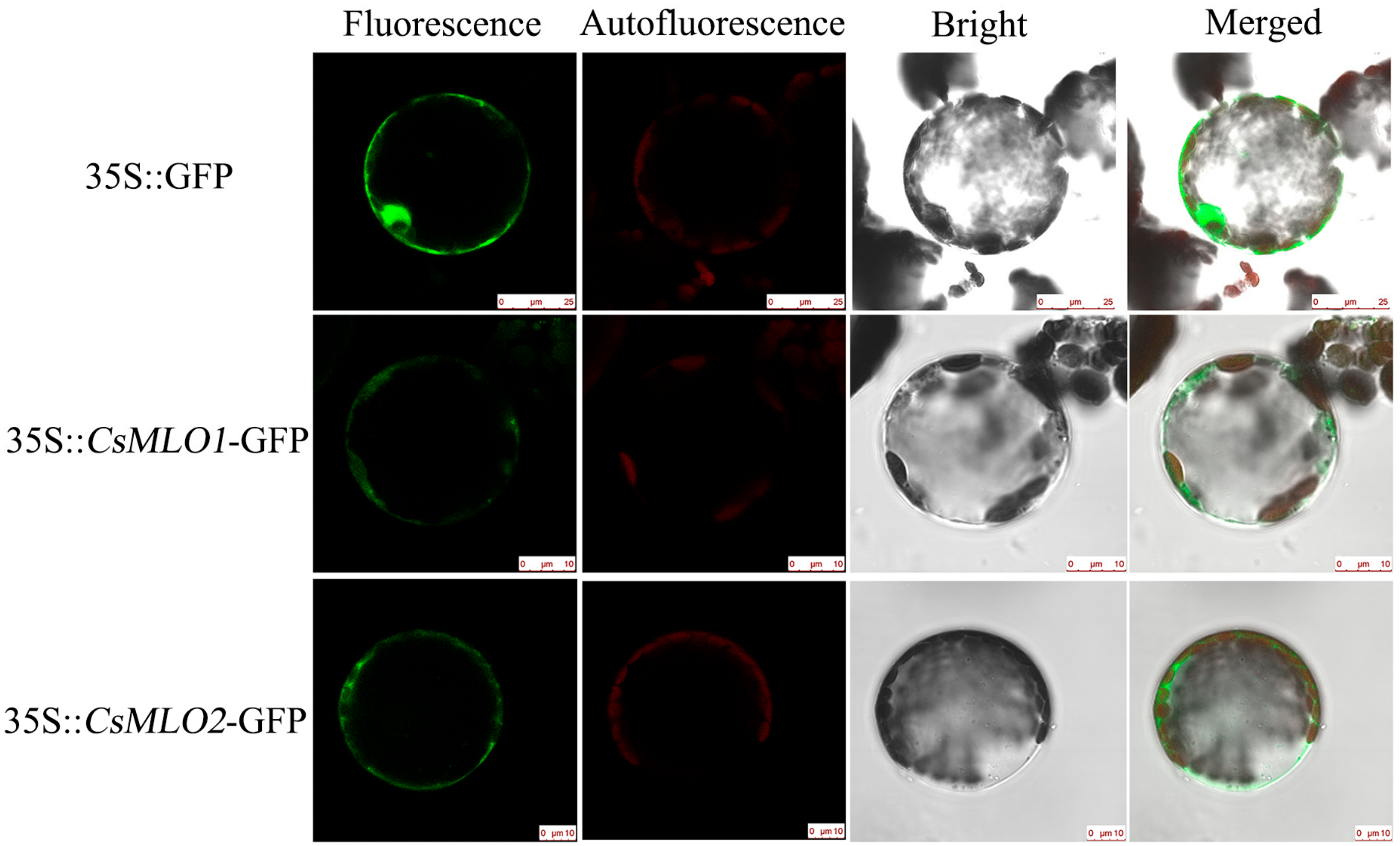
2.3. Subcellular Localization and Expression Patterns of CsMLO1 and CsMLO2
2.4. Response to Abiotic Stresses
2.5. Expression Patterns of CsMLO1 and CsMLO2 in the Resistant/Susceptible Cucumber Cultivars after C. cassiicola Infection
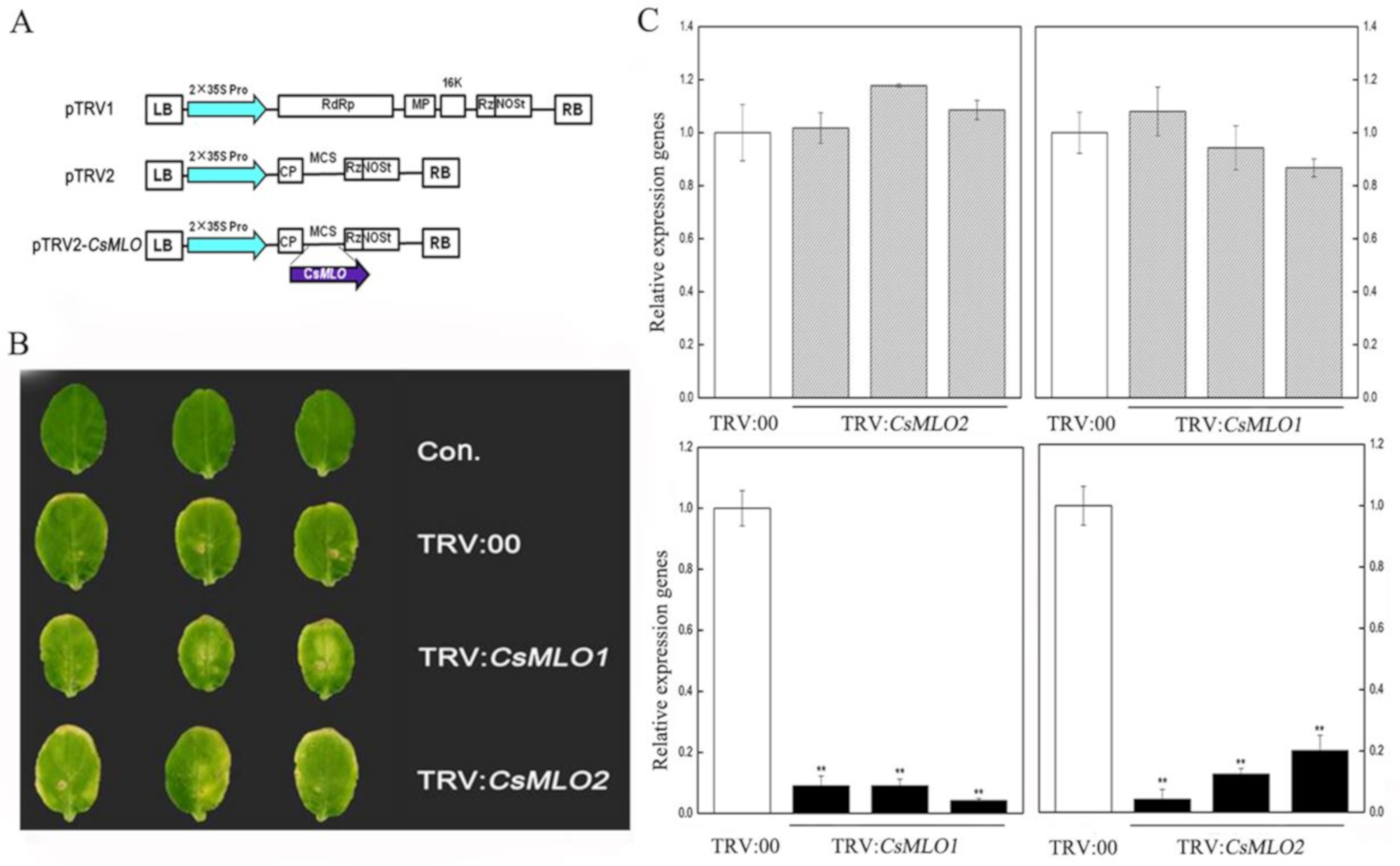
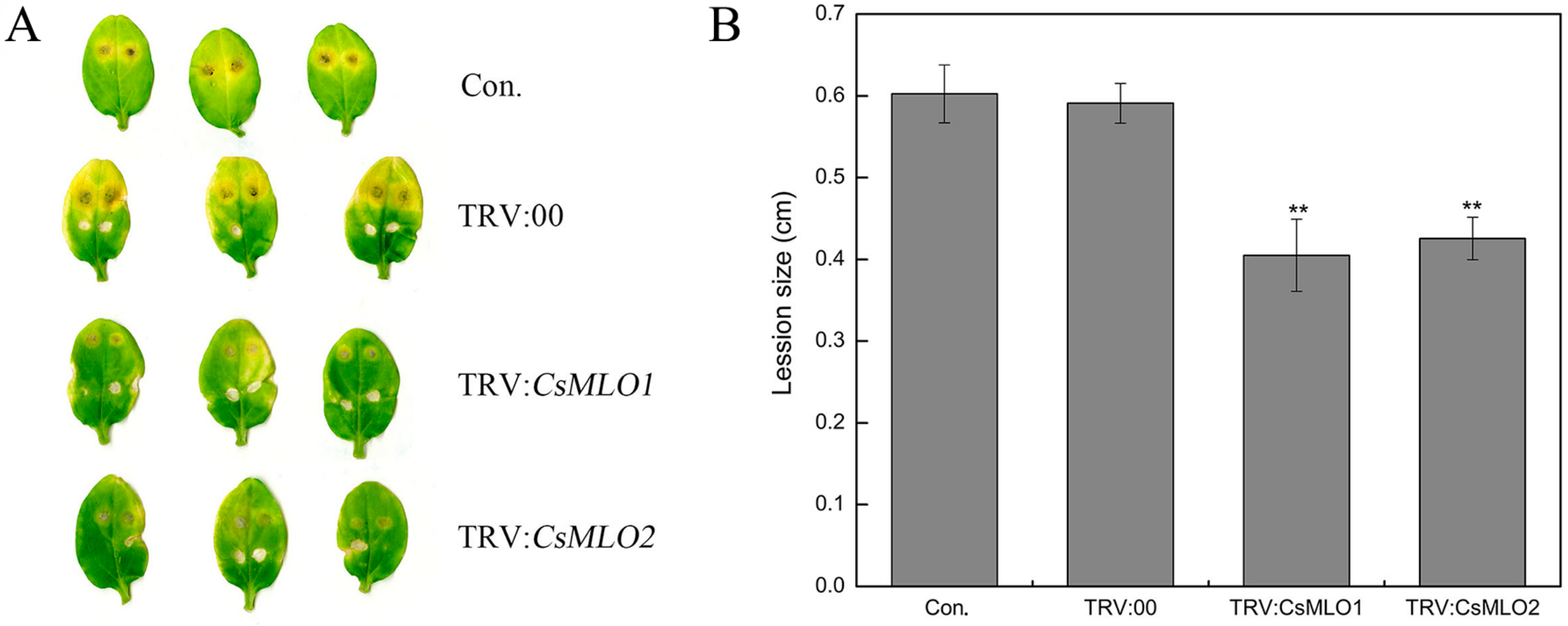
2.6. CsMLO1 or CsMLO2 Silencing Improves Resistance to C. cassiicola in Cucumber Cotyledon
2.7. Transient Overexpression of CsMLO1 or CsMLO2 in Cucumber Cotyledons Impairs Resistance to C. cassiicola
2.8. Transgenic Plants Show a Defense Response after C. cassiicola Challenge
2.9. CsMLO1 or CsMLO2 Transgenic Plants Modulate the Expression Levels of Defense-Related Genes
2.10. CsMLO1 Transient Transgenic Plants Modulate the Expression Levels of Reactive Oxygen Species (ROS)-Related Genes and Abscisic Acid (ABA) Signaling-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Pathogen Growth and Inoculation
4.3. Analysis of Sequence and Phylogenetic Tree
4.4. Subcellular Localization
4.5. Application of Plant Abiotic and Biotic Stress
4.6. Quantification of Genes Expression using Reverse-Transcription Quantitative PCR (RT-qPCR)
4.7. Histochemical Analysis
4.8. Enzyme Extraction and Antioxidant Enzyme Assays
4.9. Virus-Induced Gene Silencing (VIGS)
4.10. CsMLO-Luciferase (LUC) Fusion Overexpression Vector
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Li, S.; Guan, W. Identification Technology for Resistance to Target Leaf Spot of Cucumber and Identification for Variety Resistance. China Veg. 2008, 1, 26–27. [Google Scholar]
- Cavatorta, J.; Moriarty, G.; Henning, M.; Glos, M.; Kreitinger, M.; Munger, H.M.; Jahn, M. ‘Marketmore 97’: A Monoecious Slicing Cucumber Inbred with Multiple Disease and Insect Resistances. Hort Sci. 2007, 42, 707–709. [Google Scholar] [CrossRef]
- Wehner, T.C. Gene List 2010 for Cucumber. Cucurbit Genet. Coop. Rpt. 2010, 28, 105–141. [Google Scholar]
- Staub, J.E.; Crubaugh, L.K. Cucumber Inbred Line USDA 6632E. Cucurbit Gen. Coop. Rep. 2001, 24, 6–7. [Google Scholar]
- Abul-Hayja, Z.; Williams, P.H.; Peterson, C.E. Inheritance of resistance to anthracnose and target leaf spot in cucumbers. Plant Dis. Rep. 1978, 62, 43–45. [Google Scholar]
- Yang, L.; Koo, D.H.; Li, Y.; Zhang, X.; Luan, F.; Havey, M.J.; Jiang, J.; Weng, Y. Chromosome rearrangements during domestication of cucumber as revealed by high-density genetic mapping and draft genome assembly. Plant J. 2012, 71, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Mao, A.; Dong, C.; Liu, H.; Yu, S.; Guo, Y.D.; Weng, Y.; Xu, Y. Fine genetic mapping of target leaf spot resistance gene cca-3 in cucumber, Cucumis sativus L. Theor. Appl. Genet. 2015, 128, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Strugala, R.; Delventhal, R.; Schaffrath, U. An organ-specific view on non-host resistance. Front. Plant Sci. 2015, 6, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphry, M.; Consonni, C.; Panstruga, R. Mlo-based powdery mildew immunity: Silver bullet or simply non-host resistance? Mol. Plant Pathol. 2006, 7, 605–610. [Google Scholar] [CrossRef]
- Lipka, U.; Fuchs, R.; Lipka, V. Arabidopsis non-host resistance to powdery mildews. Curr. Opin. Plant Biol. 2008, 11, 404–411. [Google Scholar] [CrossRef]
- Thordal-Christensen, H. Fresh insights into processes of nonhost resistance. Curr. Opin. Plant Biol. 2003, 6, 351–357. [Google Scholar] [CrossRef]
- Nürnberger, T.; Brunner, F. Innate immunity in plants and animals: Emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr. Opin. Plant Biol. 2002, 5, 318–324. [Google Scholar] [CrossRef]
- Panstruga, R. Serpentine plant MLO proteins as entry portals for powdery mildew fungi. Biochem. Soc. Trans. 2005, 33, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Devoto, A.; Hartmann, H.A.; Piffanelli, P.; Elliott, C.; Simmons, C.; Taramino, G.; Goh, C.S.; Cohen, F.E.; Emerson, B.C.; Schulze-Lefert, P. Molecular phylogeny and evolution of the plant-specific seven-transmembrane MLO family. J. Mol. Evol. 2003, 56, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kusch, S.; Pesch, L.; Panstruga, R. Comprehensive phylogenetic analysis sheds light on the diversity and origin of the MLO family of integral membrane proteins. Genome Bio. Evol. 2016, 8, 878–895. [Google Scholar] [CrossRef] [PubMed]
- Consonni, C.; Humphry, M.E.; Hartmann, H.A.; Livaja, M.; Durner, J.; Westphal, L.; Vogel, J.; Lipka, V.; Kemmerling, B.; Schulzelefert, P. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 2006, 38, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Consonni, C.; Bednarek, P.; Humphry, M.; Francocci, F.; Ferrari, S.; Harzen, A.; Themaat, E.V.L.; Panstruga, R. Tryptophan-derived metabolites are required for antifungal defense in the Arabidopsis mlo2 mutant. Plant Physiol. 2010, 152, 1544–1561. [Google Scholar] [CrossRef] [PubMed]
- Gruner, K.; Zeier, T.; Aretz, C.; Zeier, J. A critical role for Arabidopsis MILDEW RESISTANCE LOCUS O2 in systemic acquired resistance. Plant J. 2018, 94, 1064–1082. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Hückelhoven, R.; Beckhove, U.; Nagarajan, S.; Kogel, K.H. A compromised Mlo pathway affects the response of barley to the necrotrophic fungus bipolaris sorokiniana (teleomorph: Cochliobolus sativus) and its toxins. Phytopathology 2001, 91, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, F.; Tilmony, R.; He, S.Y. The Arabidopsis thaliana-Pseudomonas syringae interaction. The Arabidopsis Book 2002, 1, e0039. [Google Scholar] [CrossRef] [PubMed]
- Slusarenko, A.J.; Schlaich, N.L. Downy mildew of Arabidopsis thaliana caused by Hyaloperonospora parasitica (formerly Peronospora parasitica). Mol. Plant Pathol. 2003, 4, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Tines, M.; Choi, Y.J.; Kemen, E.; Ploch, S.; Holub, E.B.; Shin, H.D.; Jones, J.D.G. A new species of Albugo parasitic to Arabidopsis thaliana reveals new evolutionary patterns in white blister rusts (Albuginaceae). Persoonia 2009, 22, 123–128. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.; Herbert, C.; Sreenivasaprasad, S.; Khatib, M.; Esquerré-Tugayé, M.; Dumas, B. A novel Arabidopsis-Colletotrichum pathosystem for the molecular dissection of plant-fungal interactions. Mol. Plant-Microbe Interact. 2004, 17, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Garcia, J.; Gruner, K.; Reinstädler, A.; Kemen, A.; Kemen, E.; Cao, L.; Takken, F.L.W.; Reitz, M.U.; Schäfer, P.; O’Connell, R.J.; et al. The powdery mildew-resistant Arabidopsis mlo2 mlo6 mlo12 triple mutant displays altered infection phenotypes with diverse types of phytopathogens. Sci. Rep. 2017, 7, 3913. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, D.; Cui, N.; Yu, Y.; Yu, G.; Fan, H. Transcriptome and miRNA analyses of the response to Corynespora cassiicolain cucumber. Sci. Rep. 2018, 8, 7798. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Yu, Y.; Fan, H.; Zhang, D.; Cui, N.; Wang, X.; Jia, S.; Yang, Y.; Zhao, J. Analysis of protein regulation in the leaves of resistant cucumber after inoculation with Corynespora cassiicola: A proteomic approach. Biochemistry (Moscow) 2019, 84, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, S.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Reactive oxygen species signaling and stomatal movement: Current updates and future perspectives. Redox Biol. 2017, 11, 213–218. [Google Scholar] [CrossRef]
- Cui, F.; Wu, H.; Safronov, O.; Zhang, P.; Kumar, R.; Kollist, H.; Salojärvi, J.; Panstruga, R.; Overmyer, K. Arabidopsis MLO2 is a negative regulator of sensitivity to extracellular ROS. Plant Cell Environ. 2018, 41, 782–796. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Bio. 2017, 38, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.Y.; Yoshioka, K.; Desveaux, D. The roles of ABA in plant-pathogen interactions. J. Plant Res. 2011, 124, 489–499. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.N.; Luo, X.Z.; Shah, J.; Schlauch, K.; et al. Temporal-Spatial Interaction between Reactive Oxygen Species and Abscisic Acid Regulates Rapid Systemic Acclimation in Plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, Y.; Duan, C.; Chen, P.; Li, Q.; Dai, S.; Sun, L.; Ji, K.; Sun, Y.; Xu, W.; et al. The expression profiling of the CsPYL, CsPP2C and CsSnRK2 gene families during fruit development and drought stress in cucumber. J. Plant Physiol. 2012, 169, 1874–1882. [Google Scholar] [CrossRef]
- Lopez-Molina, L.; Mongrand, S.; Mclachlin, D.T.; Chait, B.T.; Chua, N.H. ABI5 acts downstream of abi3 to execute an aba-dependent growth arrest during germination. Plant J. 2010, 32, 317–328. [Google Scholar] [CrossRef]
- Mahesh, H.M.; Murali, M.; Anup, C.P.M.; Melvin, P.; Sharada, M.S. Salicylic acid seed priming instigates defense mechanism by inducing PR-Proteins in Solanum melongena L. upon infection with Verticillium dahliae Kleb. Plant Physiol. Bioch. 2017, 117, 12–23. [Google Scholar] [CrossRef]
- Soliman, M.H.; Elmohamedy, R.S.R. Induction of Defense-Related Physiological and Antioxidant Enzyme Response against Powdery Mildew Disease in Okra (Abelmoschus esculentus L.) Plant by Using Chitosan and Potassium Salts. Mycobiology 2017, 45, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Analyses of transcriptome and microRNAs in response to cucumber infection by Corynespora cassiicola. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2018. [Google Scholar]
- Kim, D.S.; Hwang, B.K. The pepper MLO gene, CaMLO2, is involved in the susceptibility cell-death response and bacterial and oomycete proliferation. Plant J. Cell Mol. Bio. 2012, 72, 843–855. [Google Scholar] [CrossRef]
- Panstruga, R. Discovery of novel conserved peptide domains by ortholog comparison within plant multi-protein families. Plant Mol. Biol. 2005, 59, 485–500. [Google Scholar] [CrossRef]
- Büschges, R.; Hollricher, K.; Panstruga, R.; Simons, G.; Wolter, M.; Frijters, A.; Van, D.R.; Van, D.L.T.; Diergaarde, P.; Groenendijk, J. The barley Mlo gene: A novel control element of plant pathogen resistance. Cell 1997, 88, 695–705. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Chen, Q.; Cui, N.; Yu, Y.; Fan, H. Cucumber Mildew Resistance Locus O Interacts with Calmodulin and Regulates Plant Cell Death Associated with Plant Immunity. Int. J. Mol. Sci. 2019, 20, 2995. [Google Scholar] [CrossRef] [PubMed]
- Veronese, P.; Nakagami, H.; Bluhm, B.; Abuqamar, S.; Chen, X.; Salmeron, J.; Dietrich, R.A.; Hirt, H.; Mengiste, T. The membrane-anchored botrytis-induced kinase1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 2006, 18, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Freialdenhoven, A.; Peterhänsel, C.; Kurth, J.; Kreuzaler, F.; Schulze-Lefert, P. Identification of Genes Required for the Function of Non-Race-Specific mlo Resistance to Powdery Mildew in Barley. Plant Cell 1996, 8, 5–14. [Google Scholar] [CrossRef]
- Chen, Z.; Hartmann, H.A.; Wu, M.J.; Friedman, E.J.; Chen, J.G.; Pulley, M.; Schulze-Lefert, P.; Panstruga, R.; Jones, A.M. Expression analysis of the ATMLO, gene family encoding plant-specific seven-transmembrane domain proteins. Plant Mol. Biol. 2006, 60, 583–597. [Google Scholar] [CrossRef]
- Berg, J.A.; Appiano, M.; Martínez, M.S.; Hermans, F.W.K.; Vriezen, W.H.; Visser, R.G.F.; Bai, Y.; Schouten, H.J. A transposable element insertion in the susceptibility gene CsaMLO8 results in hypocotyl resistance to powdery mildew in cucumber. BMC Plant Biol. 2015, 15, 243. [Google Scholar] [CrossRef]
- Shang, Y.; Ma, Y.; Zhou, Y.; Zhang, H.; Duan, L.; Chen, H.; Zeng, J.; Zhou, Q.; Wang, S.; Gu, W.; et al. Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 2014, 346, 1084–1088. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L.; Jones, J.D. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fones, H.; Preston, G.M. Reactive oxygen and oxidative stress tolerance in plant pathogenic pseudomonas. FEMS Microbiol. Lett. 2012, 327, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zurbriggen, M.D.; Carrillo, N.; Hajirezaei, M.R. ROS signaling in the hypersensitive response: When, where and what for? Plant Signal. Behav. 2010, 5, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Huang, Y.; Sun, S.; Sun, J.; Cao, H.; Shabala, S.; Bie, Z. Root respiratory burst oxidase homologue-dependent H2O2 production confers salt tolerance on a grafted cucumber by controlling Na+ exclusion and stomatal closure. J. Expl. Bot. 2018, 69, 3465–3476. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Haswell, E.S. A force of nature: Molecular mechanisms of mechanoperception in plants. J. Exp. Bot. 2013, 64, 4663–4680. [Google Scholar] [CrossRef] [PubMed]
- Hafizur, R.; You-Ping, X.; Xuan-Rui, Z.; Xin-Zhong, C. Brassica napus Genome Possesses Extraordinary High Number of CAMTA Genes and CAMTA3 Contributes to PAMP Triggered Immunity and Resistance to Sclerotinia sclerotiorum. Front. Plant Sci. 2016, 7, 581. [Google Scholar]
- Michie, K.; Ikuko, O.; Kazuhito, K.; Naohiko, Y.; Masayuki, F.; Ko, S.; Noriyuki, D.; Hirofumi, Y. Calcium-dependent protein kina-ses regulate the production of reactive oxygen species by potato NADPHoxi-dase. Plant Cell 2007, 19, 1065–1080. [Google Scholar]
- Beffagna, N.; Buffoli, B.; Busi, C. Modulation of reactive oxygen speciesproduction during osmotic stress in Arabidopsis thaliana cultured cells: Involvement of the plasma membrane Ca2+-ATPase and H+-ATPase. Plant Cell Physiol. 2005, 46, 1326–1339. [Google Scholar] [CrossRef]
- Choi, H.W.; Lee, D.H.; Hwang, B.K. The pepper calmodulin gene CaCaM1 is involved in reactive oxygen species and nitric oxide generation required for cell death and the defense response. Mol. Plant-Microbe Interactions 2009, 22, 1389–1400. [Google Scholar] [CrossRef]
- Abuqamar, S.; Moustafa, K.; Tran, L.P. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 2017, 61, 1–16. [Google Scholar]
- Xue, M.; Yi, H. Enhanced Arabidopsis disease resistance against Botrytis cinerea induced by sulfur dioxide. Ecotoxicol. Environ. Saf. 2017, 147, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, A.; Ryan, C.A. Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proc. Natl. Acad. Sci. USA 2007, 104, 10732–10736. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, S.C. Functional roles of the pepper MLO protein gene, CaMLO2, in abscisic acid signaling and drought sensitivity. Plant Mol Biol. 2013, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallet, A.; López, G.; Ramos, B.; Delgado-Cerezo, M.; Riviere, M.P.; Llorente, F.; Fernández, P.V.; Miedes, E.; Estevez, J.M.; Grant, M.; et al. Disruption of abscisic acid signaling constitutively activates Arabidopsis resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant Physiol. 2012, 160, 2109–2124. [Google Scholar] [CrossRef] [PubMed]
- Ulferts, S.; Delventhal, R.; Splivallo, R.; Karlovsky, P.; Schaffrath, U. Abscisic acid negatively interferes with basal defence of barley against Magnaporthe oryzae. BMC Plant Biol. 2015, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, A.; Akinyemi, A.; Mandon, J.; Cristescu, S.M.; Hall, M.A.; Harren, F.J.; Mur, L.A. ABA suppresses Botrytis cinerea Elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Front. Plant Sci. 2016, 7, 709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. Analysis of CsTCTP regulated signal pathway and construction of yeast two-hybrid system in cucumber. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2017. [Google Scholar]
- Jiang, M.; Zhang, J. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant de-fence in leaves of maize seedlings. Plant Cell Environ. 2003, 26, 929–939. [Google Scholar] [CrossRef]
- Xu, S. Abscisic acid activates a Ca2+-calmodulin-stimulated protein kinase involved in antioxidant defense in maize leaves. Acta Biochim. Biophys. Sin. 2010, 42, 646–655. [Google Scholar] [CrossRef]
- Srinivas, A.; Raghavendra, A.S. Convergence and Divergence of Signaling Events in Guard Cells during Stomatal Closure by Plant Hormones or Microbial Elicitors. Front. Plant Sci. 2016, 7. [Google Scholar]
- Parvin, S.; Lee, O.R.; Sathiyaraj, G.; Khorolragchaa, A.; Kim, Y.J.; Devi, B.S.R.; Yang, D.C. Interrelationship between calmodulin (CaM) and H2O2 in abscisic acid-induced antioxidant defense in the seedlings of Panax ginseng. Mol. Biol. Rep. 2012, 39, 7327–7338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, H.; Yang, X.; Li, Q.; Ling, J.; Wang, H.; Gu, X.; Huang, S.; Jiang, W. CsWRKYW46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner. Plant Physiol. Bioch. 2016, 108, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Chen, F.; Cheng, Z.; Wang, Y.; Yang, P.; Zhang, Y.; Cha, Z. Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: Effect on arginine metabolism and ROS accumulation. J. Exp. Bot. 2013, 64, 1367–1379. [Google Scholar] [CrossRef]
- Zhong, R.; Kays, S.J.; Schroeder, B.P.; Ye, Z.H. Mutation of a chitinase-like gene causes ectopic deposition of lignin, aberrant cell shapes, and overproduction of ethylene. Plant Cell 2002, 14, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, J.; Chen, X.; Gao, Z.; Xuan, W.; Xu, S.; Ding, X.; Shen, W. Carbon monoxide alleviatescadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol. 2008, 177, 155–166. [Google Scholar] [PubMed]
- Huang, B.K.; Xu, S.; Xuan, W.; Li, M.; Cao, Z.Y.; Liu, K.L.; Ling, T.F.; Shen, W.B. Carbon monoxide alleviates salt-induced oxidative damage in wheat seedling leaves. J. Integr. Plant Biol. 2006, 48, 249–254. [Google Scholar] [CrossRef]

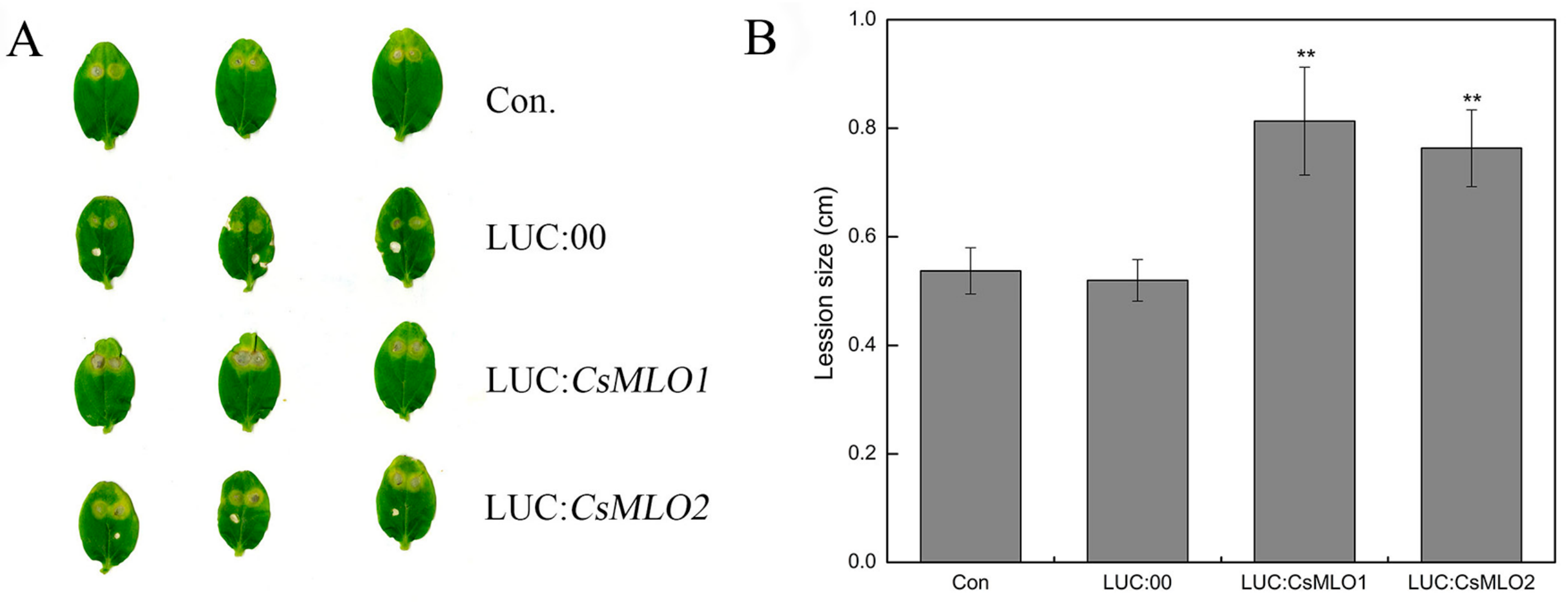
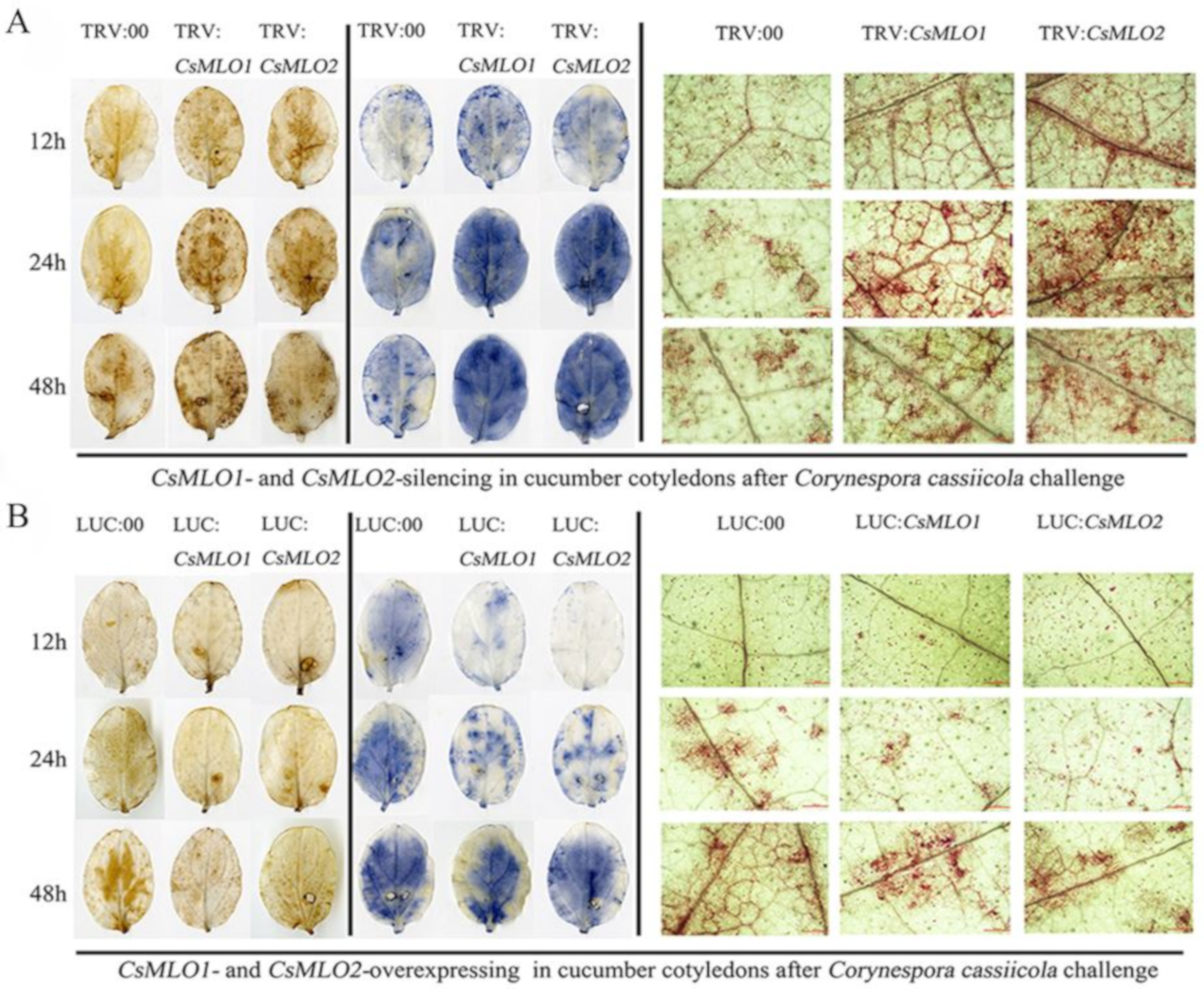


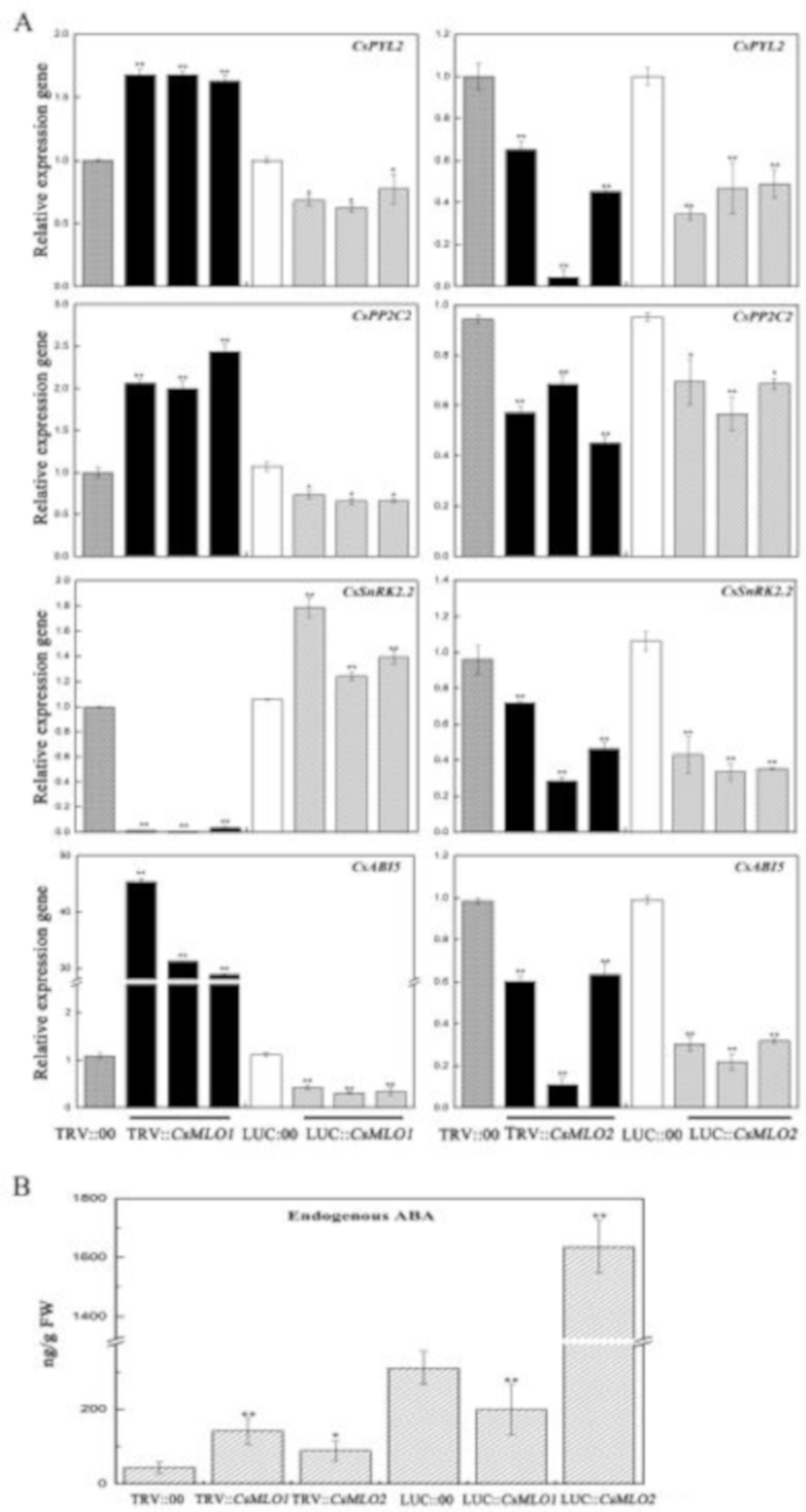
| Material Name | Disease Index (DI) | Resistance |
|---|---|---|
| Jinyou 38 | 14.38 | HR |
| B21-a-2-1-2 | 40.74 | MR |
| B21-a-2-2-2 | 67.90 | S |
| F10 | 41.50 | MR |
| 995 | 61.01 | S |
| Jinyan 4 | 60.37 | S |
| Xintaimici | 70.57 | S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Chen, Q.; Wang, X.; Meng, X.; Yu, Y.; Fan, H.; Cui, N. Mildew Resistance Locus O Genes CsMLO1 and CsMLO2 Are Negative Modulators of the Cucumis sativus Defense Response to Corynespora cassiicola. Int. J. Mol. Sci. 2019, 20, 4793. https://doi.org/10.3390/ijms20194793
Yu G, Chen Q, Wang X, Meng X, Yu Y, Fan H, Cui N. Mildew Resistance Locus O Genes CsMLO1 and CsMLO2 Are Negative Modulators of the Cucumis sativus Defense Response to Corynespora cassiicola. International Journal of Molecular Sciences. 2019; 20(19):4793. https://doi.org/10.3390/ijms20194793
Chicago/Turabian StyleYu, Guangchao, Qiumin Chen, Xiangyu Wang, Xiangnan Meng, Yang Yu, Haiyan Fan, and Na Cui. 2019. "Mildew Resistance Locus O Genes CsMLO1 and CsMLO2 Are Negative Modulators of the Cucumis sativus Defense Response to Corynespora cassiicola" International Journal of Molecular Sciences 20, no. 19: 4793. https://doi.org/10.3390/ijms20194793
APA StyleYu, G., Chen, Q., Wang, X., Meng, X., Yu, Y., Fan, H., & Cui, N. (2019). Mildew Resistance Locus O Genes CsMLO1 and CsMLO2 Are Negative Modulators of the Cucumis sativus Defense Response to Corynespora cassiicola. International Journal of Molecular Sciences, 20(19), 4793. https://doi.org/10.3390/ijms20194793




