A Novel Panel of 80 RNA Biomarkers with Differential Expression in Multiple Human Solid Tumors against Healthy Blood Samples
Abstract
:1. Introduction
2. Results
2.1. Discovery Set
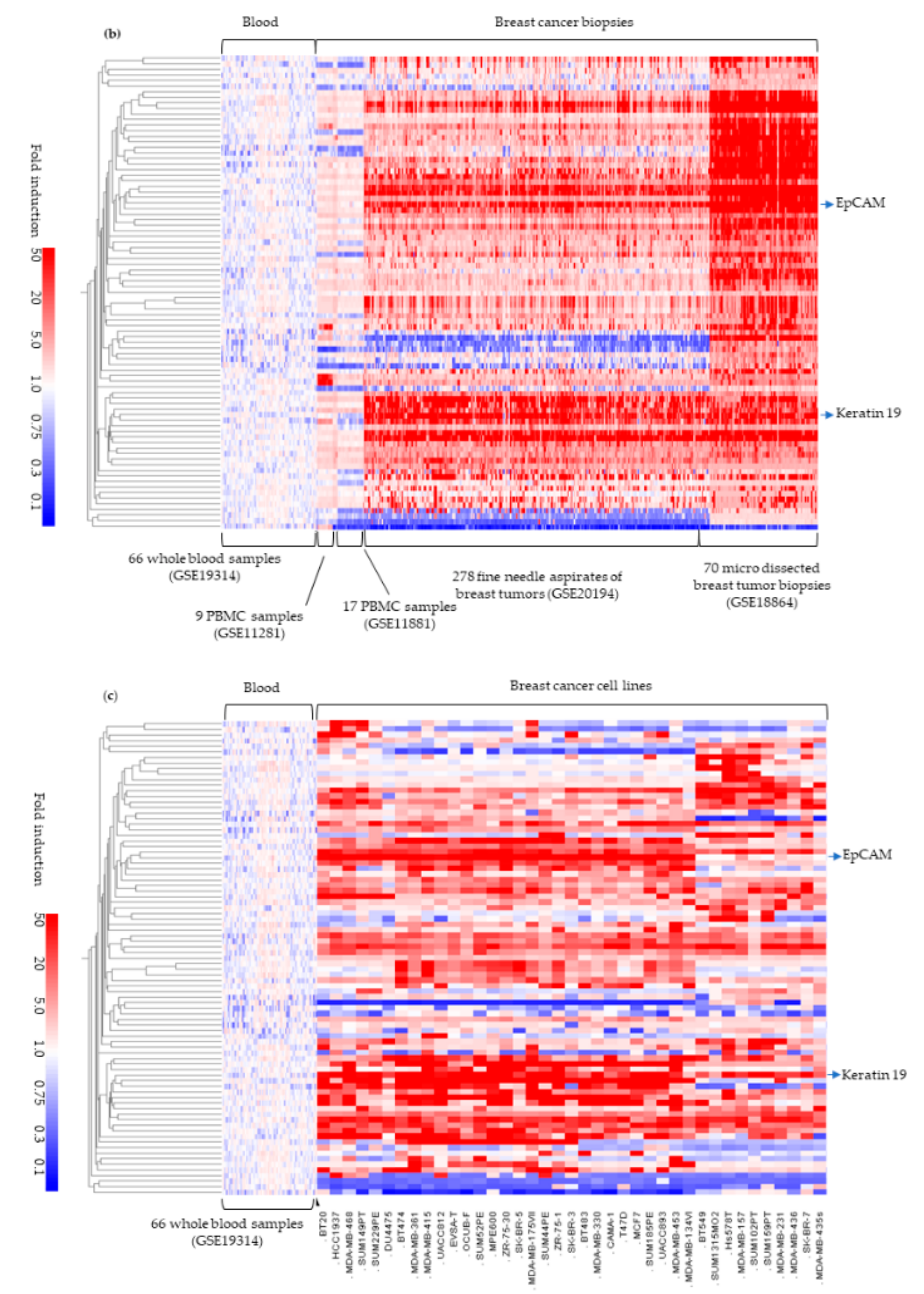
2.2. External Validation in Breast Tumor Samples
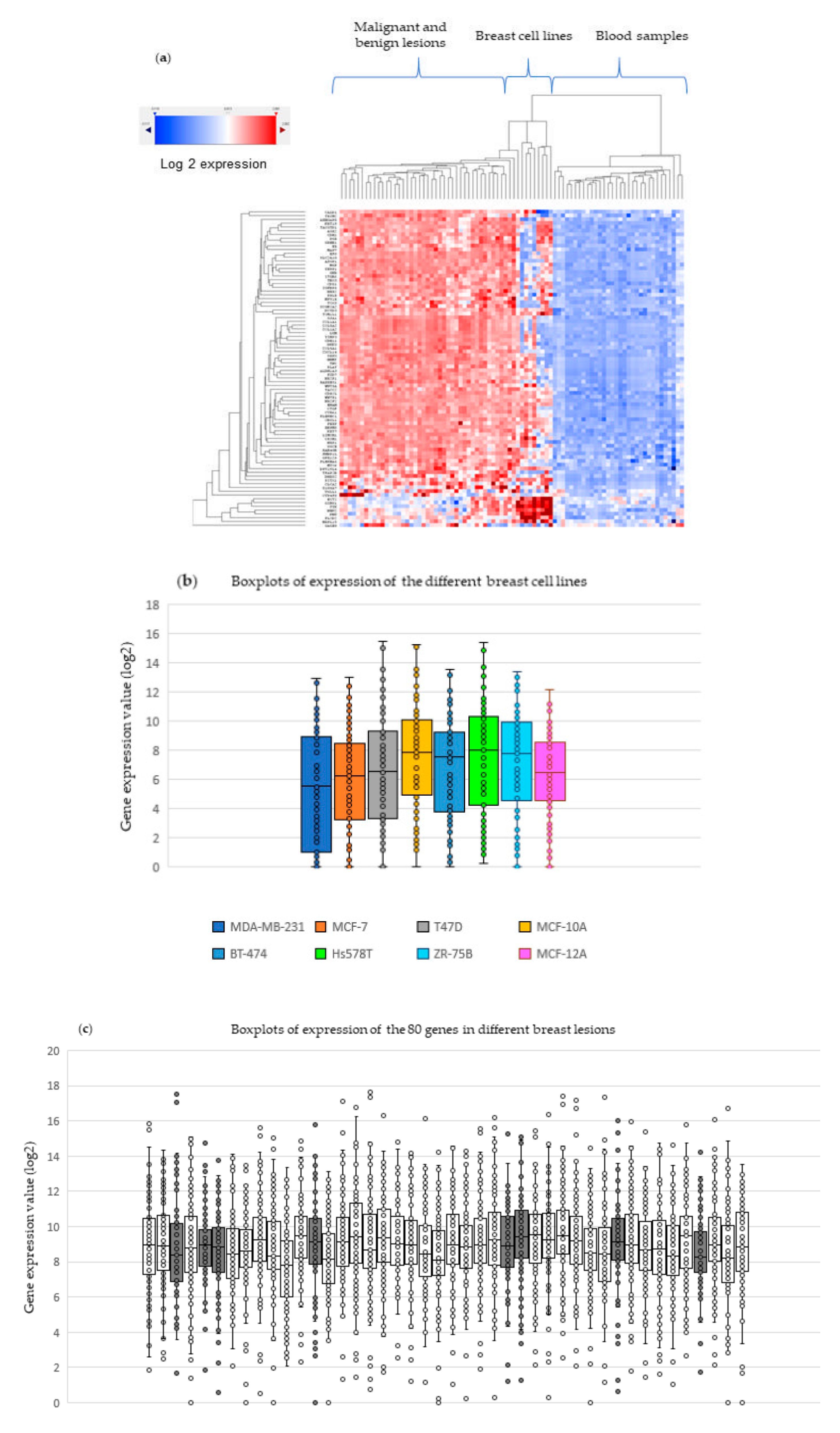
2.3. External Validation in 39 Breast Cell Lines
2.4. Experimental Validation in Breast Tissue Samples and Blood
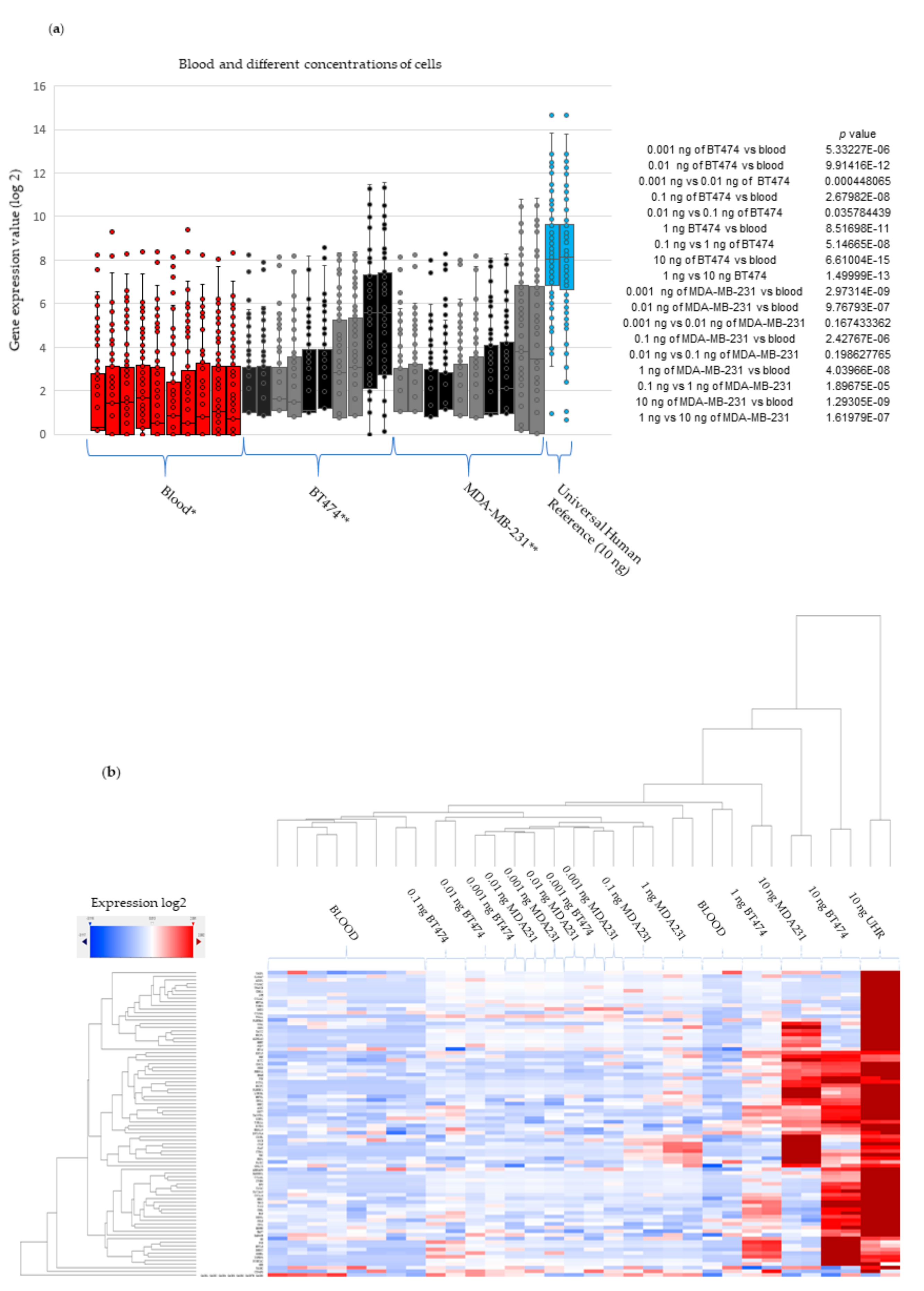
2.5. Experimental Validation-Spiking Experiments
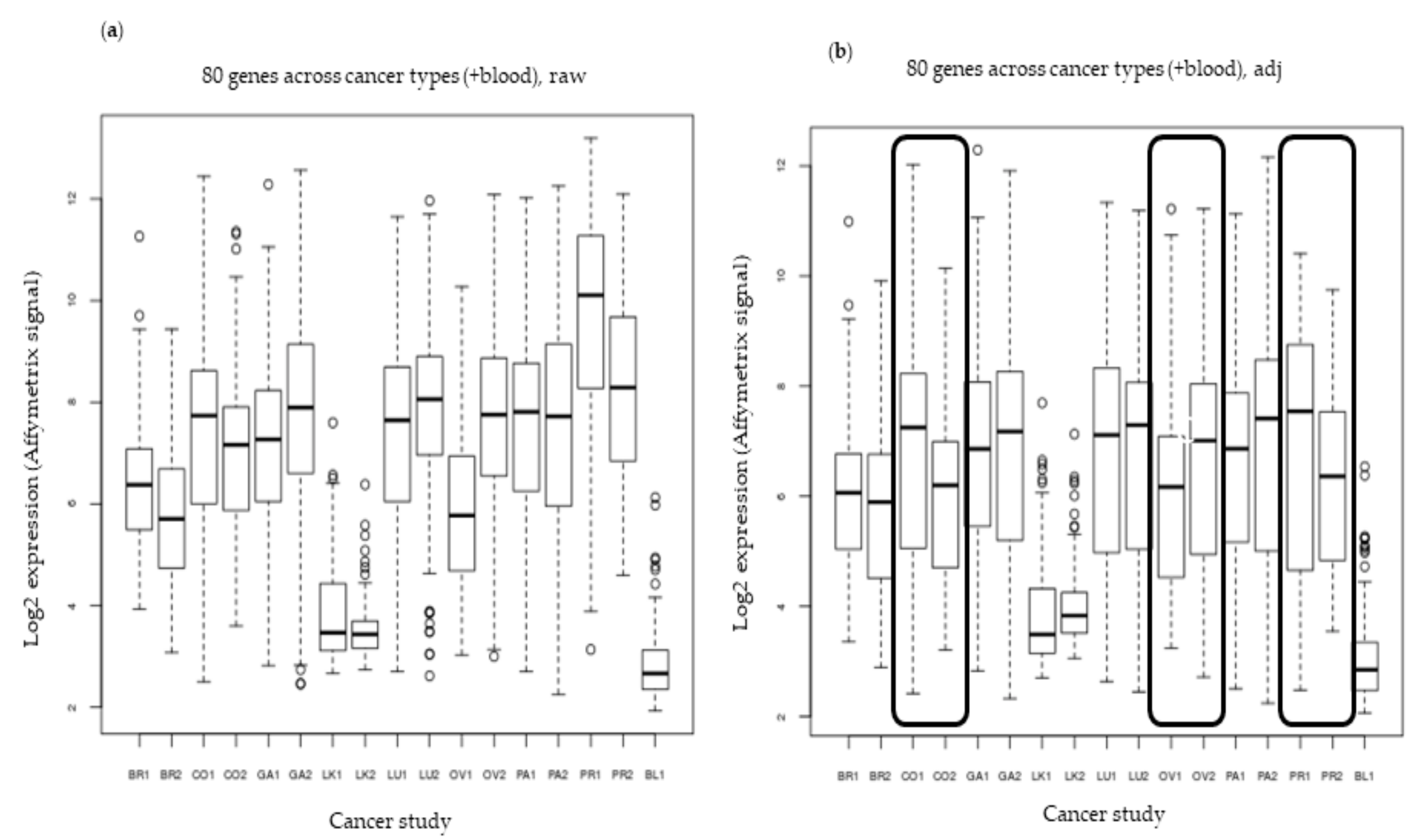
2.6. External Validation in Eight Cancer Types
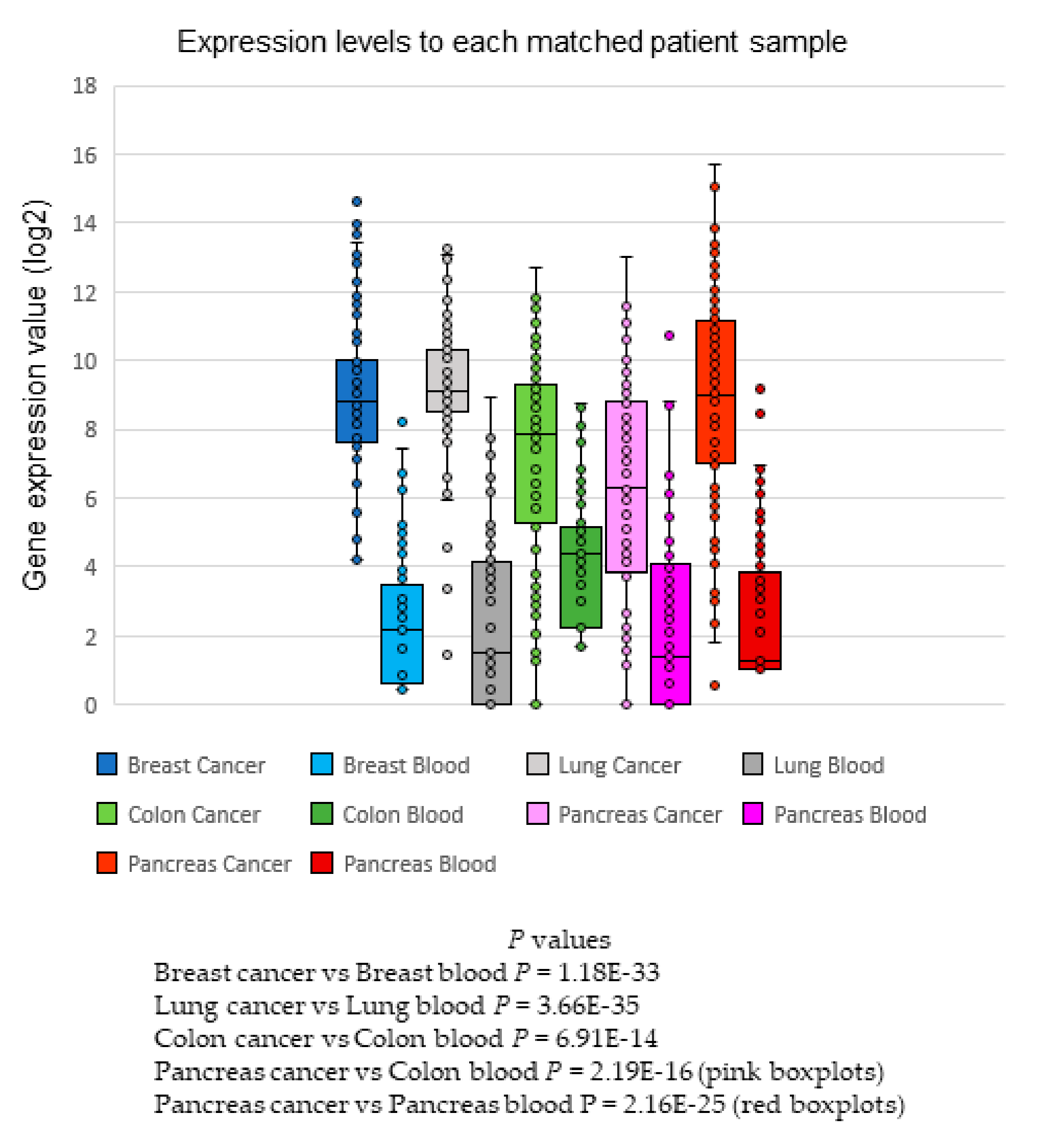
2.7. Matched Samples—Tumor Versus Blood
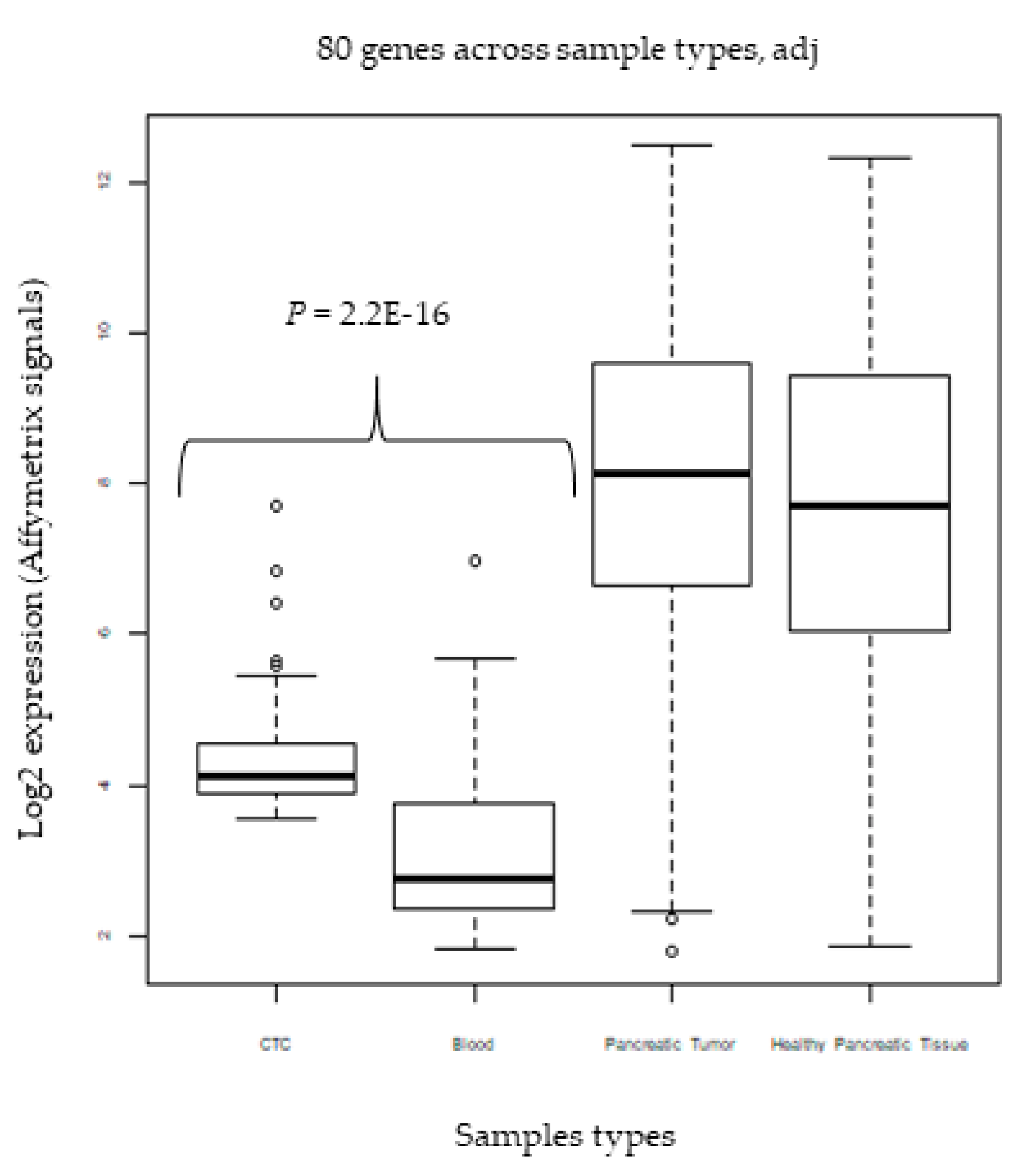
2.8. External Validation in CTCs
3. Discussion
4. Methods
4.1. Discovery Set
4.2. External Validation 1
4.3. External Validation 2
4.4. External Validation 3
4.5. External Validation 4
4.6. Affymetrix Data analysis
4.7. Experimental Validation
4.7.1. NanoString Assays
4.7.2. Breast Tissue Samples
4.7.3. Blood Samples
4.7.4. Breast Cell Lines
4.7.5. Spiking Experiments
4.7.6. Matched Samples—Tumor versus Blood
4.7.7. Quantification and Quality (QC) Metrics
4.8. NanoString Protocol
4.8.1. Sample Preparation and Hybridization
4.8.2. Preparation Station and Digital Analyzer
4.8.3. NanoString Custom-Designed Assay Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2018. [Google Scholar]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based cancer biomarkers in liquid biopsy: A promising non-invasive alternative to tissue biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef] [PubMed]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid biopsy of cancer: A multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018, 29, 1758835918794630. [Google Scholar] [CrossRef] [PubMed]
- Sokolenko, A.P.; Imyanitov, E.N. Molecular diagnostics in clinical oncology. Front. Mol. Biosci. 2018, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Hanahan, D. Switch to the angiogenic phenotype during tumorigenesis. Princess Takamatsu Symp. 1991, 22, 339–347. [Google Scholar] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L. Isolation of rare circulating tumor cells in patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O’Rourke, M.A.; Lew, D.L.; et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 2014, 32, 3483–3489. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Cristofanilli, M.; Singh, B.; Reuben, J.; Gao, H.; Cohen, E.N.; Andreopoulou, E.; Hall, C.S.; Lodhi, A.; Jackson, S.; et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer 2010, 116, 3330–3337. [Google Scholar] [CrossRef]
- Bidard, F.C.; Mathiot, C.; Delaloge, S.; Brain, E.; Giachetti, S.; de Cremoux, P.; Marty, M.; Pierga, J.Y. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann. Oncol. 2010, 21, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.S.; Karhade, M.G.; Bowman Bauldry, J.B.; Valad, L.M.; Kuerer, H.M.; DeSnyder, S.M.; Lucci, A. Prognostic value of circulating tumor cells identified before surgical resection in nonmetastatic breast cancer patients. J. Am. Coll. Surg. 2016, 223, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Naume, B.; Borgen, E.; Beiske, K.; Herstad, T.K.; Ravnås, G.; Renolen, A.; Trachsel, S.; Thrane-Steen, K.; Funderud, S.; Kvalheim, G. Immunomagnetic techniques for the enrichment and detection of isolated breast carcinoma cells in bone marrow and peripheral blood. J. Hematother. 1997, 6, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [Green Version]
- Ligthart, S.T.; Bidard, F.C.; Decraene, C.; Bachelot, T.; Delaloge, S.; Brain, E.; Campone, M.; Viens, P.; Pierga, J.Y.; Terstappen, L.W. Unbiased quantitative assessment of Her-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann. Oncol. 2013, 24, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Müller, V.; Tewes, M.; Zeitz, J.; Kasimir-Bauer, S.; Loehberg, C.R.; Rack, B.; Schneeweiss, A.; Fehm, T. Comparison of estrogen and progesterone receptor status of circulating tumor cells and the primary tumor in metastatic breast cancer patients. Gynecol. Oncol. 2011, 122, 356–360. [Google Scholar] [CrossRef]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Soysal, S.D.; Muenst, S.; Barbie, T.; Fleming, T.; Gao, F.; Spizzo, G.; Oertli, D.; Viehl, C.T.; Obermann, E.C.; Gillanders, W.E. EpCAM expression varies significantly and is differentially associated with prognosis in the luminal B HER2+, basal-like, and HER2 intrinsic subtypes of breast cancer. Br. J. Cancer 2013, 108, 1480–1487. [Google Scholar] [CrossRef]
- De Wit, S.; van Dalum, G.; Lenferink, A.T.; Tibbe, A.G.; Hiltermann, T.J.; Groen, H.J.; van Rijn, C.J.; Terstappen, L.W. The detection of EpCAM(+) and EpCAM(-) circulating tumor cells. Sci. Rep. 2015, 5, 12270. [Google Scholar] [CrossRef]
- Hong, B.; Zu, Y. Detecting circulating tumor cells: Current challenges and new trends. Theranostics 2013, 3, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Lu, D.; Schreiber, N.A.; Louw, J.; Graf, R.P.; Vargas, H.A.; Johnson, A.; Jendrisak, A.; Bambury, R.; Danila, D.; et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-specific biomarker with outcomes and in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016, 2, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, D.A.; Zweitzig, D.R.; Foulk, B.W.; Miller, M.C.; Doyle, G.V.; Pienta, K.J.; Meropol, N.J.; Weiner, L.M.; Cohen, S.J.; Moreno, J.G.; et al. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005, 65, 4993–4997. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Kuske, A.; Röck, K.; Mauermann, O.; Müller, V.; Peine, S.; Verpoort, K.; Novosadova, V.; Kubista, M.; Riethdorf, S.; et al. Accession of tumor heterogeneity by multiplex transcriptome profiling of single circulating tumor cells. Clin. Chem. 2016, 62, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.A.; Koo, G.B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.I.; Jung, H.I.; Kim, Y.S. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 2016, 7, 24677–24687. [Google Scholar] [CrossRef]
- Sergeant, G.; van Eijsden, R.; Roskams, T.; van Duppen, V.; Topal, B. Pancreatic cancer circulating tumour cells express a cell motility gene signature that predicts survival after surgery. BMC Cancer 2012, 12, 527. [Google Scholar] [CrossRef]
- Hüsemann, Y.; Geigl, J.B.; Schubert, F.; Musiani, P.; Meyer, M.; Burghart, E.; Forni, G.; Eils, R.; Fehm, T.; Riethmüller, G. Systemic spread is an early step in breast cancer. Cancer Cell 2008, 13, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Stoecklein, N.H.; Klein, C.A. Genetic disparity between primary tumors, disseminated tumor cells, and manifest metastasis. Int. J. Cancer 2010, 126, 589–598. [Google Scholar] [CrossRef]
- Mego, M.; Gao, H.; Cohen, E.N.; Anfossi, S.; Giordano, A.; Sanda, T.; Fouad, T.M.; De Giorgi, U.; Giuliano, M.; Woodward, W.A.; et al. Circulating tumor cells (CTC) are associate with defects in adaptive immunity in patients with inflammatory breast cancer. J. Cancer 2016, 7, 1095–1104. [Google Scholar] [CrossRef]
- Markiewicz, A.; Książkiewicz, M.; Wełnicka-Jaśkiewicz, M.; Seroczyńska, B.; Skokowski, J.; Żaczek, A.J. Mesenchymal phenotype of CTC-enriched blood fraction and lymph node metastasis formation potential. PLoS ONE 2014, 9, e93901. [Google Scholar] [CrossRef]
- Danila, D.C.; Samoila, A.; Patel, C.; Schreiber, N.; Herkal, A.; Anand, A.; Bastos, D.; Heller, G.; Fleisher, M.; Scher, H.I. Clinical validity of detecting circulating tumor cells by AdnaTest assay compared with direct detection of tumor mRNA in stabilized whole blood, as biomarker predicting overall survival for metastatic castration-resistant prostate cancer patients. Cancer J. 2016, 22, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Mikulová, V.; Cabiňaková, M.; Janatková, I.; Mestek, O.; Zima, T.; Tesařová, P. Detection of circulating tumor cells during follow-up of patients with early breast cancer: Clinical utility for monitoring of therapy efficacy. Scand. J. Clin. Lab. Investig. 2014, 74, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Zhang, D.; Bing, Z.; Tian, J.; Ni, M.; Zhang, X.; Meng, Z.; Liu, S. Identification of potential key genes associated with the pathogenesis and prognosis of gastric cancer based on integrated bioinformatics analysis. Front. Genet. 2018, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei, H.; Gao, Z.; Chen, G.; Liu, Y.; Gao, X.; Bai, G.; He, S.; Liu, T.; Xu, W.; et al. COL5A1 may contribute the metastasis of lung adenocarcinoma. Gene 2018, 30, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Naume, B.; Zhao, X.; Synnestvedt, M.; Borgen, E.; Russnes, H.G.; Lingjaerde, O.C.; Strømberg, M.; Wiedswang, G.; Kvalheim, G.; Kåresen, R.; et al. Presence of bone marrow micrometastasis is associated with different recurrence risk within molecular subtypes of breast cancer. Mol. Oncol. 2007, 1, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, U.; Cloos, J.; Sauter, G.; Riethdorf, L.; Jänicke, F.; van Diest, P.; Brakenhoff, R.; Pantel, K. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res. 2003, 63, 5679–5684. [Google Scholar]
- Lang, J.E.; Scott, J.H.; Wolf, D.M.; Novak, P.; Punj, V.; Magbanua, M.J.; Zhu, W.; Mineyev, N.; Haqq, C.M.; Crothers, J.R.; et al. Expression profiling of circulating tumor cells in metastatic breast cancer. Breast Cancer Res. Treat. 2015, 149, 121–131. [Google Scholar] [CrossRef]
- Finotti, A.; Allegretti, M.; Gasparello, J.; Giacomini, P.; Spandidos, D.A.; Spoto, G.; Gambari, R. Liquid biopsy and PCR-free ultrasensitive detection systems in oncology (Review). Int. J. Oncol. 2018, 53, 1395–1434. [Google Scholar] [CrossRef]
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004, 10, 8152–8162. [Google Scholar] [CrossRef]
- El Rassy, E.; Klaled, H.; Pavlidis, N. Liquid biopsy: A new diagnostic, predictive and prognostic window in cancers of unknown primary. Eur. J. Cancer 2018, 105, 28–32. [Google Scholar] [CrossRef]
- Delmonico, L.; Goodwin, E.C.; Fournier, M.V. Isolation and detection of breast cancer cells in blood samples using antibody cocktails selected by large-scale genome-wide screening. In Cancer Research, Proceedings of the American Association for Cancer Research Annual Meeting, Chicago, IL, USA, 14–18 April 2018; AACR: Philadelphia, PA, USA, 2018; Volume 78. [Google Scholar]
- Hess, K.R.; Anderson, K.; Symmans, W.F.; Valero, V.; Ibrahim, N.; Mejia, J.A.; Booser, D.; Theriault, R.L.; Buzdar, A.U.; Dempsey, P.J.; et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J. Clin. Oncol. 2006, 24, 4236–4244. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.V.; Martin, K.J.; Kenny, P.A.; Xhaja, K.; Bosch, I.; Yaswen, P.; Bissel, M.J. Gene expression signature in organized and growth-arrested mammary acini predicts good outcome in breast cancer. Cancer Res. 2006, 66, 7095–70102. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Garcia, A.; Alonso, C.; Millet, P.; Cornet, M.; Ramón y Cajal, T.; Baiget, M.; Barnadas, A. Molecular detection of peripheral blood breast cancer mRNA transcripts as a surrogate biomarker for circulating tumor cells. PLoS ONE 2013, 8, e74079. [Google Scholar] [CrossRef] [PubMed]
- Mann, P.S. Introductory Statistics, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 115–117. [Google Scholar]


| Specification | Accession | Number of Samples |
|---|---|---|
| Blood samples from non-breast cancer patients | GSE5418 | 401 |
| GSE12288 | ||
| GSE1343 | ||
| GSE3846 | ||
| GSE6269 | ||
| Breast cancer biopsies | GSE2034 | 417 |
| Hess et al. [44] | ||
| Human breast cell lines | GSE16795 | 41 |
| GSE8096 | ||
| Whole blood samples from non-breast cancer patients | GSE19314 | 66 |
| PBMC samples from non-breast cancer patients | GSE11281 | 26 |
| GSE11881 | ||
| Micro-dissected breast tumor biopsies | GSE18864 | 70 |
| Fine needle aspirates of breast tumors | GSE20194 | 278 |
| Eight cancer types (breast, colon, lung, ovarian, prostate, pancreatic, gastric cancers, and leukemia) and 51 different breast cell lines | GSE25055, GSE39582, GSE68468, GSE13911, GSE54129, GSE13159, GSE14471, GSE19188, GSE30219, GSE26712, GSE9891, GSE15471, GSE16515, GSE17951, GSE8218 and E-TABM-157 | 3601 |
| PBMCs from breast cancer patients, patients with benign breast abnormalities, healthy cancer-free individuals, and patients with other types of cancer | GSE27562 | |
| Circulating tumor cells, hematological cells, original tumor, and adjacent-pancreatic tissue of patients with pancreatic ductal adenocarcinoma | GSE18670 | 24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delmonico, L.; Obenauer, J.C.; Qureshi, F.; Alves, G.; Costa, M.A.S.M.; Martin, K.J.; Fournier, M.V. A Novel Panel of 80 RNA Biomarkers with Differential Expression in Multiple Human Solid Tumors against Healthy Blood Samples. Int. J. Mol. Sci. 2019, 20, 4894. https://doi.org/10.3390/ijms20194894
Delmonico L, Obenauer JC, Qureshi F, Alves G, Costa MASM, Martin KJ, Fournier MV. A Novel Panel of 80 RNA Biomarkers with Differential Expression in Multiple Human Solid Tumors against Healthy Blood Samples. International Journal of Molecular Sciences. 2019; 20(19):4894. https://doi.org/10.3390/ijms20194894
Chicago/Turabian StyleDelmonico, Lucas, John C. Obenauer, Fatir Qureshi, Gilda Alves, Mauricio Augusto Silva Magalhães Costa, Katherine J. Martin, and Marcia V. Fournier. 2019. "A Novel Panel of 80 RNA Biomarkers with Differential Expression in Multiple Human Solid Tumors against Healthy Blood Samples" International Journal of Molecular Sciences 20, no. 19: 4894. https://doi.org/10.3390/ijms20194894





