Low-Energy Extracorporeal Shock Wave Ameliorates Streptozotocin Induced Diabetes and Promotes Pancreatic Beta Cells Regeneration in a Rat Model
Abstract
:1. Introduction
2. Results
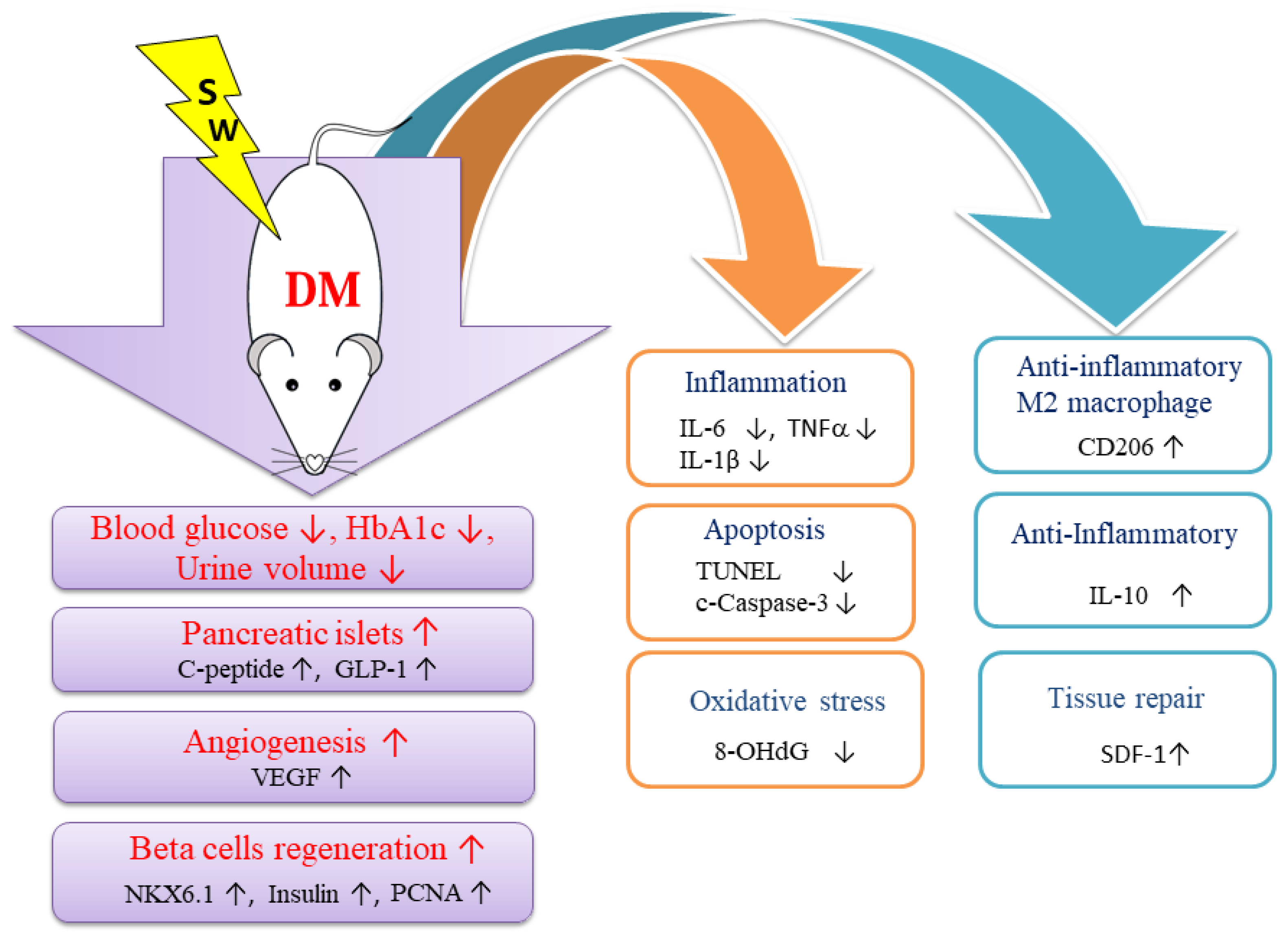
2.1. Low-Energy SW Significantly Reduced Blood Glucose, Hemoglobin A1c and Urine Volume in DM
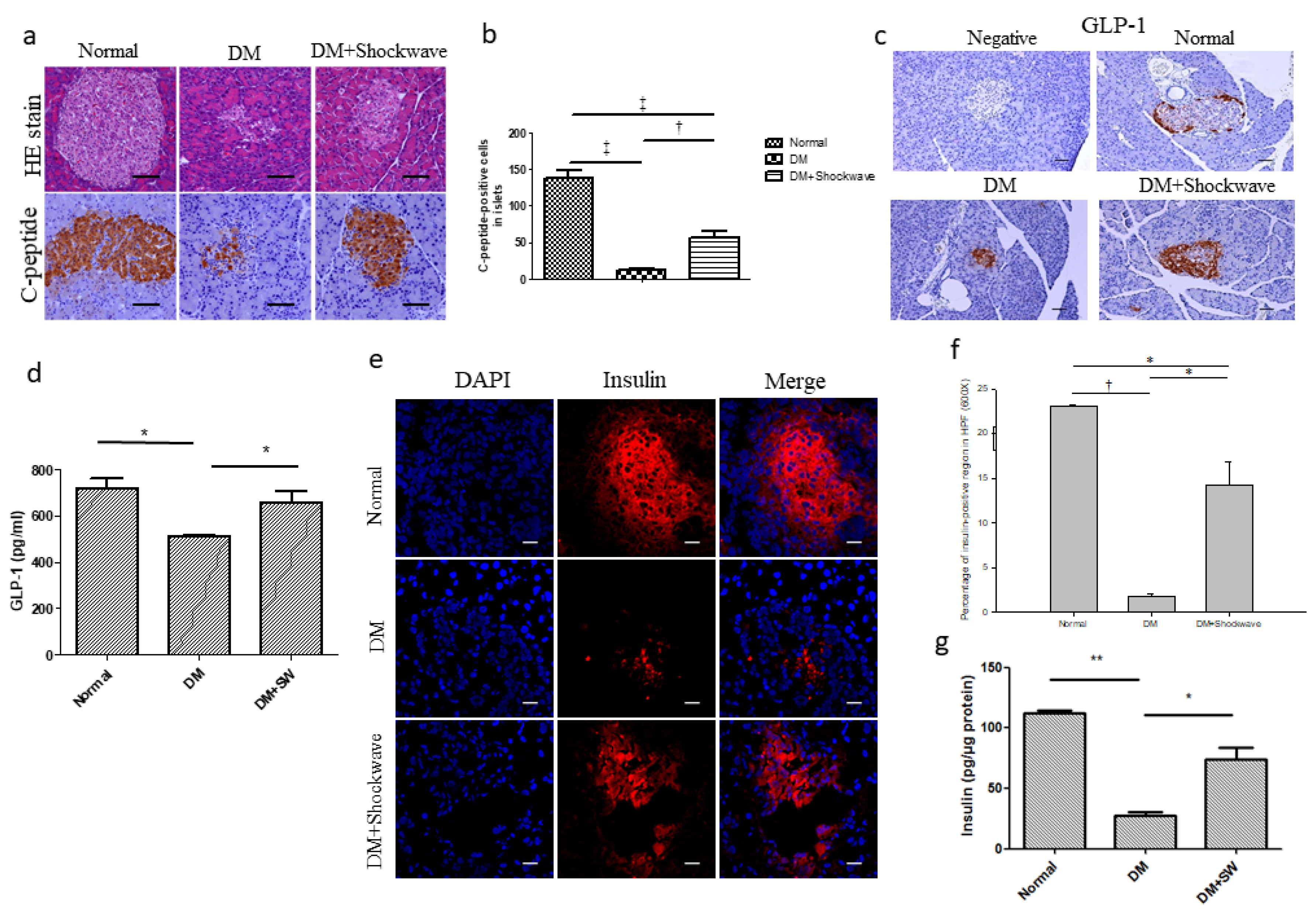
2.2. Low-Energy SW Significantly Enhanced Pancreatic Islets Area, c-Peptide, GLP-1 and Insulin Production
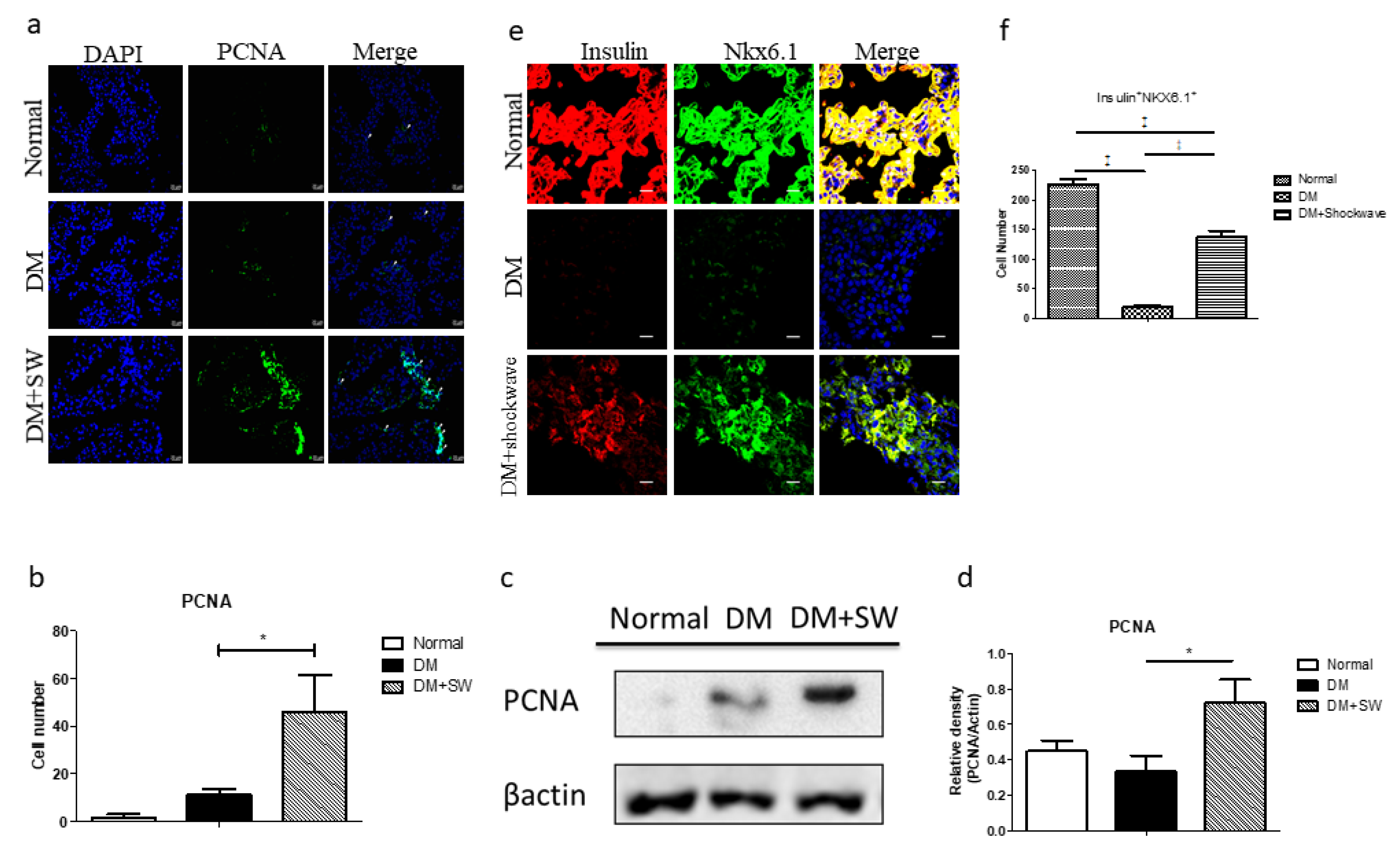
2.3. Low-Energy SW Enhanced Beta Cells Regeneration
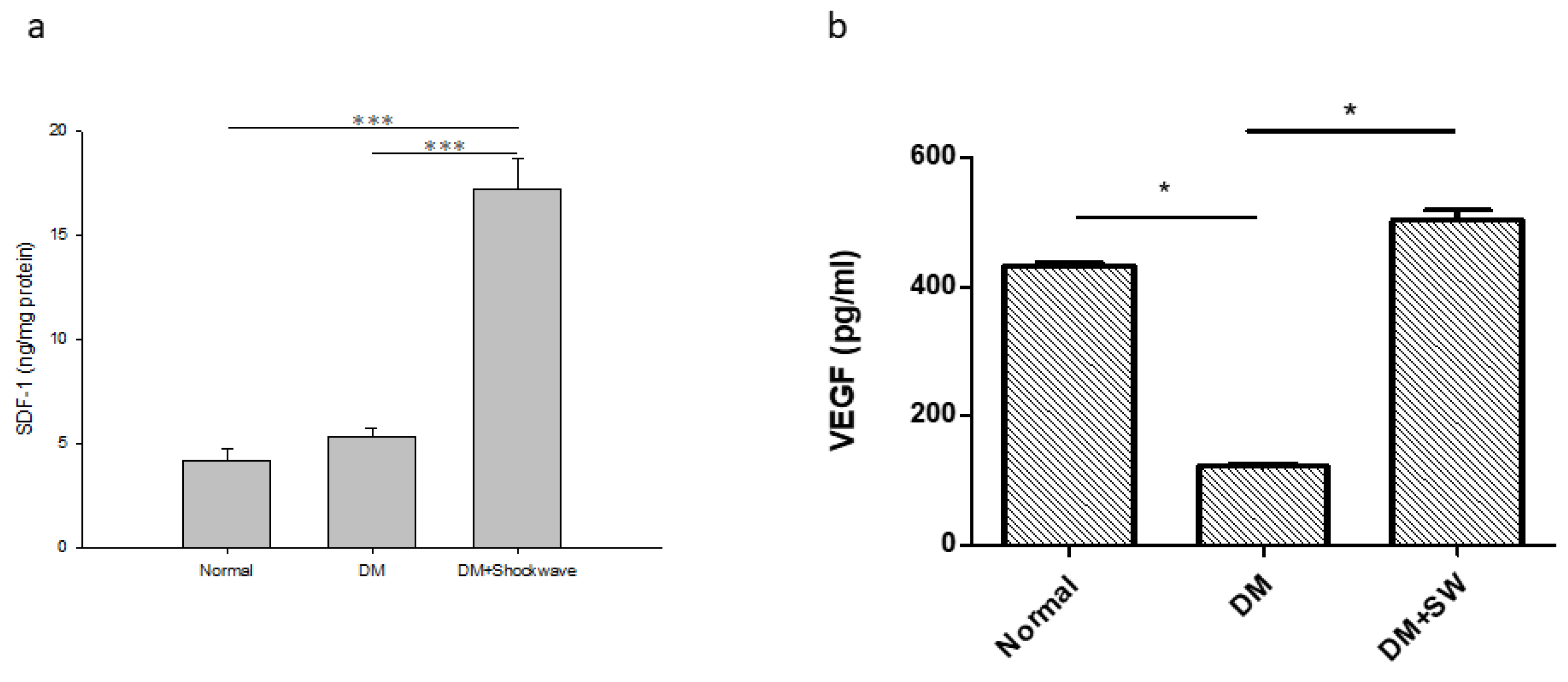
2.4. Low-Energy SW Enhanced Tissue Repair and Angiogenesis
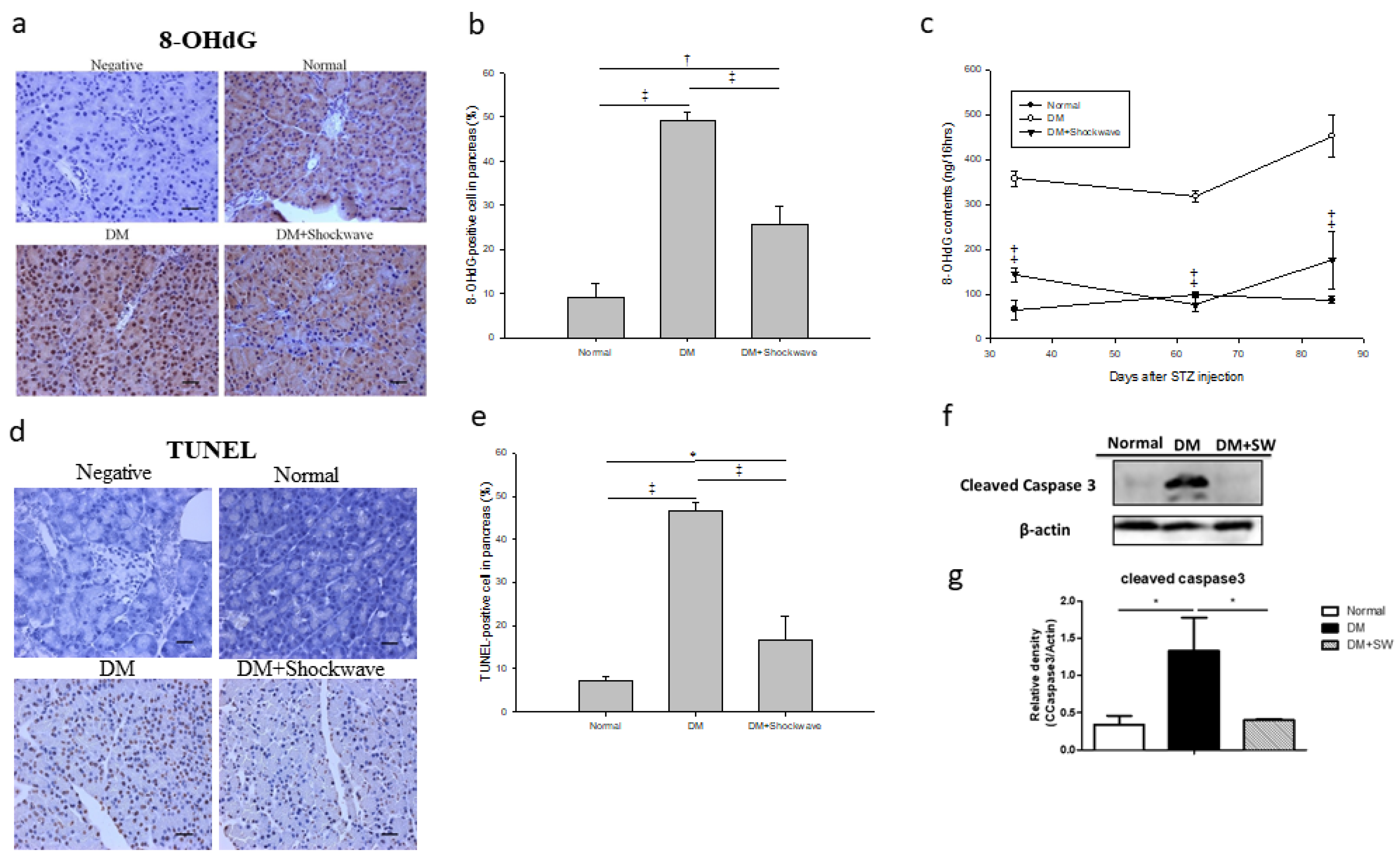
2.5. Low-Energy SW Alleviated Oxidative Stress and Cell Apoptosis
2.6. Low-Energy SW Prevented Diabetes-Induced Pancreatic Tissue Inflammation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Establishment of the DM Rat Model
4.3. Low-Energy SW Treatment.
4.4. Measurement of Urine Volume and 8-Hydroxy-2’-Deoxyguanosine (8-OHdG)
4.5. Hematoxylin and Eosin (HE) Stain, Immunohistochemistry, and Immunofluorescence
4.6. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL)
4.7. Western Blotting
4.8. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DM | streptozotocin induced diabetes |
| SW | extracorporeal shock wave |
| HbA1c | hemoglobin A1c |
| GLP-1 | glucagon-like peptide 1 |
| SDF-1 | stromal cell derived factor 1 |
| IL-1β | interleukin-1β |
| TNFα | tumor necrosis factor-α |
| Normal | normal control |
| shockwave | low-energy SW |
| HE | hematoxylin and eosin |
| IHC | immunohistochemistry |
| IF | immunofluorescence |
| PCNA | proliferating cell nuclear antigen |
| EPCs | endothelial progenitor cells |
| vWF | von Willebrand factor |
| VEGF | vascular endothelial growth factor |
| 8-OHdG | 8-hydroxy-2’ -deoxyguanosine |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| STZ | streptozotocin |
| ELISA | enzyme-linked immunosorbent assay |
References
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, D.W.; Plotnick, L. Type 1 diabetes mellitus in pediatrics. Pediatr Rev. 2008, 29, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Ortis, F.; Naamane, N.; Flamez, D.; Ladriere, L.; Moore, F.; Cunha, D.A.; Colli, M.L.; Thykjaer, T.; Thorsen, K.; Orntoft, T.F.; et al. Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes 2010, 59, 358–374. [Google Scholar] [CrossRef]
- Watson, D.; Loweth, A.C. Oxidative and nitrosative stress in beta-cell apoptosis: Their contribution to beta-cell loss in type 1 diabetes mellitus. Br. J. Biomed. Sci. 2009, 66, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lim, D.M.; Moon, C.I.; Jo, K.J.; Lee, S.K.; Baik, H.W.; Lee, K.H.; Lee, K.W.; Park, K.Y.; Kim, B.J. Exendin-4 protects oxidative stress-induced beta-cell apoptosis through reduced JNK and GSK3beta activity. J. Korean Med. Sci. 2010, 25, 1626–1632. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet 2001, 358, 221–229. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int J. Biomed. Sci 2008, 4, 89–96. [Google Scholar]
- Nathan, D.M. Finding new treatments for diabetes--how many, how fast... how good? N. Engl. J. Med. 2007, 356, 437–440. [Google Scholar] [CrossRef]
- Migliorini, A.; Bader, E.; Lickert, H. Islet cell plasticity and regeneration. Mol. Metab. 2014, 3, 268–274. [Google Scholar] [CrossRef]
- Xu, G.; Stoffers, D.A.; Habener, J.F.; Bonner-Weir, S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999, 48, 2270–2276. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, C.; Choung, J.S.; Jung, H.S.; Jun, H.S. Glucagon-Like Peptide 1 Increases beta-Cell Regeneration by Promoting alpha- to beta-Cell Transdifferentiation. Diabetes 2018, 67, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.E.; Taylor, B.L.; Benthuysen, J.R.; Liu, J.; Thorel, F.; Yuan, W.; Jiao, Y.; Kaestner, K.H.; Herrera, P.L.; Magnuson, M.A.; et al. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet. 2013, 9, e1003274. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.L.; Liu, F.F.; Sander, M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013, 4, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.A.; Toth-Kischkat, A.; Schultheiss, R. Principles of shock wave therapy. Clin. Orthop. Relat. Res. 2001, 387, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; de Prati, A.C.; Cavalieri, E.; Amelio, E.; Marlinghaus, E.; Suzuki, H. Extracorporeal shock wave therapy in inflammatory diseases: Molecular mechanism that triggers anti-inflammatory action. Curr. Med. Chem. 2009, 16, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wang, F.S.; Yang, K.D.; Weng, L.H.; Hsu, C.C.; Huang, C.S.; Yang, L.C. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J. Orthop. Res. 2003, 21, 984–989. [Google Scholar] [CrossRef]
- Hsiao, C.-C.; Huang, W.-H.; Cheng, K.-H.; Lee, C.-T. Low-energy extracorporeal shock wave therapy ameliorates kidney function in diabetic nephropathy. Oxidative Med. Cell Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Shao, P.L.; Chiu, C.C.; Yuen, C.M.; Chua, S.; Chang, L.T.; Sheu, J.J.; Sun, C.K.; Wu, C.J.; Wang, C.J.; Yip, H.K. Shock wave therapy effectively attenuates inflammation in rat carotid artery following endothelial denudation by balloon catheter. Cardiology 2010, 115, 130–144. [Google Scholar] [CrossRef]
- Ciampa, A.R.; de Prati, A.C.; Amelio, E.; Cavalieri, E.; Persichini, T.; Colasanti, M.; Musci, G.; Marlinghaus, E.; Suzuki, H.; Mariotto, S. Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. FEBS Lett. 2005, 579, 6839–6845. [Google Scholar] [CrossRef]
- Chen, Y.T.; Yang, C.C.; Sun, C.K.; Chiang, H.J.; Chen, Y.L.; Sung, P.H.; Zhen, Y.Y.; Huang, T.H.; Chang, C.L.; Chen, H.H.; et al. Extracorporeal shock wave therapy ameliorates cyclophosphamide-induced rat acute interstitial cystitis though inhibiting inflammation and oxidative stress-in vitro and in vivo experiment studies. Am. J. Transl Res. 2014, 6, 631–648. [Google Scholar]
- Wu, K.L.; Chiu, Y.C.; Yao, C.C.; Tsai, C.E.; Hu, M.L.; Kuo, C.M.; Tai, W.C.; Chuah, S.K.; Hsiao, C.C. Effect of extracorporeal low-energy shock wave on diabetic gastroparesis in a rat model. J. Gastroenterol. Hepatol. 2019, 34, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Mense, S.; Hoheisel, U. Shock wave treatment improves nerve regeneration in the rat. Muscle Nerve 2013, 47, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Hausner, T.; Pajer, K.; Halat, G.; Hopf, R.; Schmidhammer, R.; Redl, H.; Nogradi, A. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp. Neurol. 2012, 236, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, Y.; Ito, K.; Hao, K.; Shindo, T.; Ogata, T.; Kagaya, Y.; Kurosawa, R.; Nishimiya, K.; Satoh, K.; Miyata, S.; et al. Extracorporeal low-energy shock-wave therapy exerts anti-inflammatory effects in a rat model of acute myocardial infarction. Circ. J. 2014, 78, 2915–2925. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Hills, C.E.; Brunskill, N.J.; Squires, P.E. C-peptide as a therapeutic tool in diabetic nephropathy. Am. J. Nephrol. 2010, 31, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.G.; Fleischer, N.; Shin, S.I. Insulin-dependent diabetes mellitus induced by subdiabetogenic doses of streptozotocin: Obligatory role of cell-mediated autoimmune processes. Proc. Natl. Acad. Sci. USA 1980, 77, 6129–6133. [Google Scholar] [CrossRef]
- Zhang, X.; Krier, J.D.; Amador Carrascal, C.; Greenleaf, J.F.; Ebrahimi, B.; Hedayat, A.F.; Textor, S.C.; Lerman, A.; Lerman, L.O. Low-energy shockwave therapy improves ischemic kidney microcirculation. J. Am. Soc. Nephrol. 2016, 27, 3715–3724. [Google Scholar] [CrossRef]
- Fu, M.; Sun, C.K.; Lin, Y.C.; Wang, C.J.; Wu, C.J.; Ko, S.F.; Chua, S.; Sheu, J.J.; Chiang, C.H.; Shao, P.L.; et al. Extracorporeal shock wave therapy reverses ischemia-related left ventricular dysfunction and remodeling: Molecular-cellular and functional assessment. PLoS ONE 2011, 6, e24342. [Google Scholar] [CrossRef]
- Yeh, K.H.; Sheu, J.J.; Lin, Y.C.; Sun, C.K.; Chang, L.T.; Kao, Y.H.; Yen, C.H.; Shao, P.L.; Tsai, T.H.; Chen, Y.L.; et al. Benefit of combined extracorporeal shock wave and bone marrow-derived endothelial progenitor cells in protection against critical limb ischemia in rats. Crit. Care Med. 2012, 40, 169–177. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health. Part. C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.M.; Ferenbach, D.A.; Isenberg, J.S.; Thomson, A.W.; Hughes, J. Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat. Rev. Nephrol. 2014, 10, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, S.; Meka, A.; Dawson, D.; House, N.; Schaden, W.; Novak, M.J.; Ebersole, J.L.; Kesavalu, L. Extracorporeal shock wave therapy induces alveolar bone regeneration. J. Dent. Res. 2008, 87, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.K.; Shao, P.L.; Wang, C.J.; Yip, H.K. Study of vascular injuries using endothelial denudation model and the therapeutic application of shock wave: A review. Am. J. Transl. Res. 2011, 3, 259–268. [Google Scholar] [PubMed]
- Aicher, A.; Heeschen, C.; Sasaki, K.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: A new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 2006, 114, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wurtz, T.; Wang, C.J.; Kuo, Y.R.; Yang, K.D.; Huang, H.C.; Wang, F.S. Recruitment of mesenchymal stem cells and expression of tgf-beta 1 and vegf in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J. Orthop. Res. 2004, 22, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Nakamichi, T.; Mori, T.; Ito, K.; Shimokawa, H.; Ito, S. Low-energy extracorporeal shock wave ameliorates ischemic acute kidney injury in rats. Clin. Exp. Nephrol. 2019, 23, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Tepekoylu, C.; Wang, F.S.; Kozaryn, R.; Albrecht-Schgoer, K.; Theurl, M.; Schaden, W.; Ke, H.J.; Yang, Y.; Kirchmair, R.; Grimm, M.; et al. Shock wave treatment induces angiogenesis and mobilizes endogenous CD31/CD34-positive endothelial cells in a hindlimb ischemia model: Implications for angiogenesis and vasculogenesis. J. Thorac. Cardiovasc. Surg. 2013, 146, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Mittermayr, R.; Hartinger, J.; Antonic, V.; Meinl, A.; Pfeifer, S.; Stojadinovic, A.; Schaden, W.; Redl, H. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann. Surg. 2011, 253, 1024–1032. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, J.Y.; Ko, J.Y.; Huang, Y.T.; Kuo, Y.H.; Wang, F.S. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J. Am. Soc. Nephrol. 2010, 21, 124–135. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chiang, Y.C.; Huang, Y.T.; Chen, C.C.; Wang, F.S.; Wang, C.J.; Kuo, Y.R. Serum proteomic analysis of extracorporeal shock wave therapy-enhanced diabetic wound healing in a streptozotocin-induced diabetes model. Plast. Reconstr. Surg. 2014, 133, 59–68. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, C.-C.; Lin, C.-C.; Hou, Y.-S.; Ko, J.-Y.; Wang, C.-J. Low-Energy Extracorporeal Shock Wave Ameliorates Streptozotocin Induced Diabetes and Promotes Pancreatic Beta Cells Regeneration in a Rat Model. Int. J. Mol. Sci. 2019, 20, 4934. https://doi.org/10.3390/ijms20194934
Hsiao C-C, Lin C-C, Hou Y-S, Ko J-Y, Wang C-J. Low-Energy Extracorporeal Shock Wave Ameliorates Streptozotocin Induced Diabetes and Promotes Pancreatic Beta Cells Regeneration in a Rat Model. International Journal of Molecular Sciences. 2019; 20(19):4934. https://doi.org/10.3390/ijms20194934
Chicago/Turabian StyleHsiao, Chang-Chun, Cheng-Chan Lin, You-Syuan Hou, Jih-Yang Ko, and Ching-Jen Wang. 2019. "Low-Energy Extracorporeal Shock Wave Ameliorates Streptozotocin Induced Diabetes and Promotes Pancreatic Beta Cells Regeneration in a Rat Model" International Journal of Molecular Sciences 20, no. 19: 4934. https://doi.org/10.3390/ijms20194934




