An Improved Prediction Model for Ovarian Cancer Using Urinary Biomarkers and a Novel Validation Strategy
Abstract
1. Introduction
2. Results
2.1. Urinary Protein Biomarkers in Patients with Ovarian Cancer
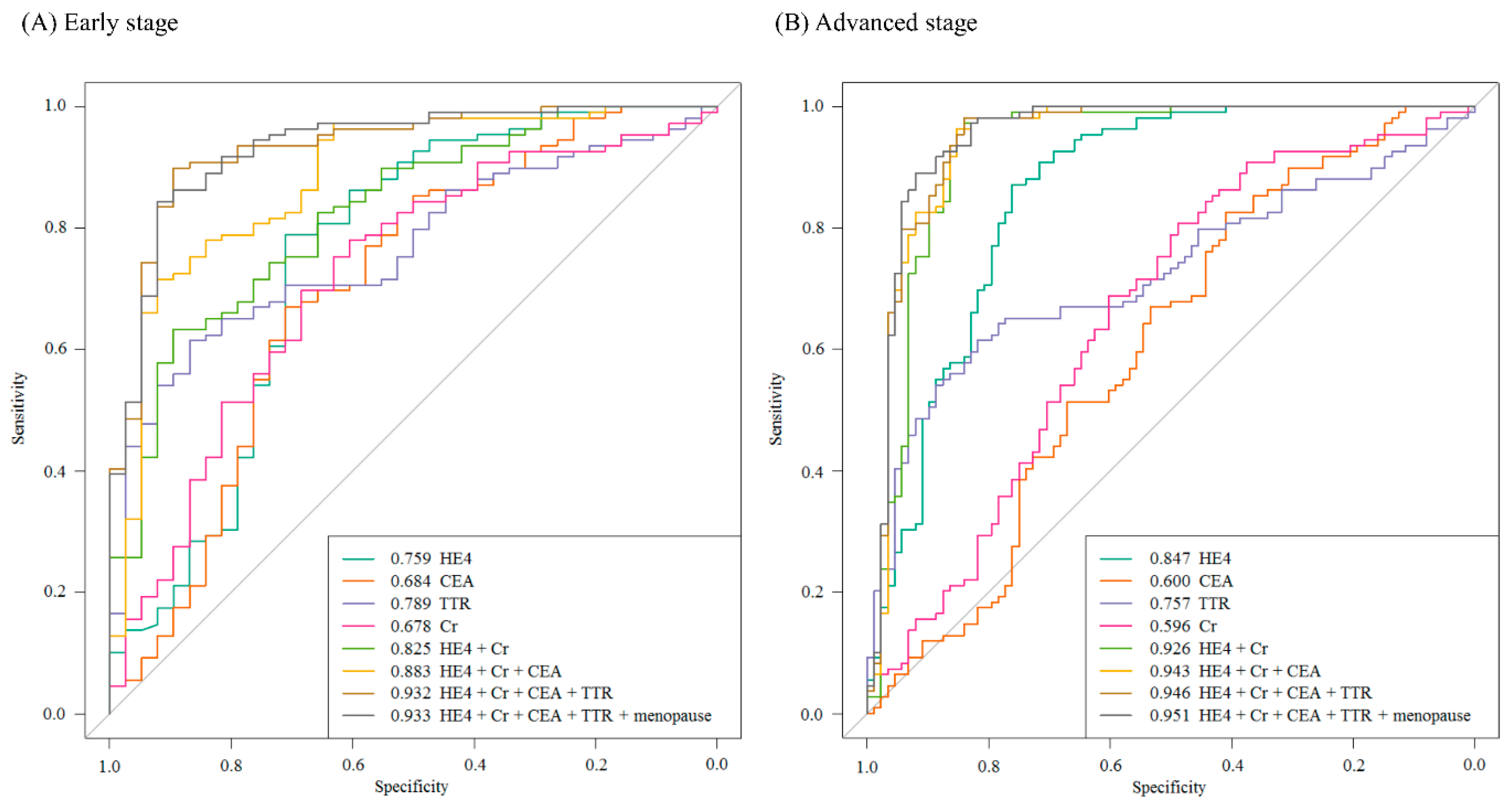
2.2. Urinary Multimarker Panel Analysis in Patients with Ovarian Cancer
2.3. Clinical Characteristics of Difficult Samples to Predict
3. Discussion
4. Materials and Methods
4.1. Patient Populations and Urine Sample Collections
4.2. Multiplexed Urinary Biomarker Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OC | ovarian cancer |
| EOC | epithelial ovarian cancer |
| SN | sensitivity |
| SP | specificity |
| RMI | Risk of Malignancy Index |
| ROMA | Risk of Malignancy Algorithm |
| FIGO | the International Federation of Gynecology and Obstetrics |
| HE4 | human epididymis protein 4 |
| VCAM | vascular cell adhesion molecule |
| TTR | transthyretin |
| CEA | carcinoembryonic antigen |
| NCAM | neural cell adhesion molecule |
| CA-125 | cancer antigen 125 |
| CRP | C-reactive protein |
| PDGF | platelet-derived growth factor |
| MPO | myeloperoxidase |
| IL | interleukin |
| MIF | macrophage migration inhibitory factor |
| ApoAI | apolipoprotein A1 |
| ApoCIII | apolipoprotein C3 |
| PAI-1 | plasminogen activator inhibitor-1 |
| OPN | osteopontin |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| PPV | positive predictive value |
| NPV | negative predictive value |
| AUC | area under the receiver operating characteristic curve |
| CIs | confidence intervals |
| IVDMIA | in vitro diagnostic multivariate index assay |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.G.; White, M.; Cruz, A.; Farias-Eisner, R. In 2014, can we do better than CA125 in the early detection of ovarian cancer? World J. Biol. Chem. 2014, 5, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Garshell, J.; Neyman, N.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975–2010. Available online: https://seer.cancer.gov/archive/csr/1975_2010/ (accessed on 1 December 2017).
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet. Gynecol. 2011, 117, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Kapp, D.S.; Shin, J.Y.; Husain, A.; Teng, N.N.; Berek, J.S.; Osann, K.; Leiserowitz, G.S.; Cress, R.D.; O'Malley, C. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet. Gynecol. 2007, 109, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet. 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br. J. Obstet. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C., Jr.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bast, R.C., Jr.; Yu, Y.; Li, J.; Sokoll, L.J.; Rai, A.J.; Rosenzweig, J.M.; Cameron, B.; Wang, Y.Y.; Meng, X.Y. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Cancer Res. 2004, 64, 5882–5890. [Google Scholar] [CrossRef]
- Ueland, F.R.; Desimone, C.P.; Seamon, L.G.; Miller, R.A.; Goodrich, S.; Podzielinski, I.; Sokoll, L.; Smith, A.; van Nagell, J.R., Jr.; Zhang, Z. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet. Gynecol. 2011, 117, 1289–1297. [Google Scholar] [CrossRef]
- Ye, B.; Skates, S.; Mok, S.C.; Horick, N.K.; Rosenberg, H.F.; Vitonis, A.; Edwards, D.; Sluss, P.; Han, W.K.; Berkowitz, R.S.; et al. Proteomic-based discovery and characterization of glycosylated eosinophil-derived neurotoxin and COOH-terminal osteopontin fragments for ovarian cancer in urine. Clin. Cancer Res. 2006, 12, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Badgwell, D.; Lu, Z.; Cole, L.; Fritsche, H.; Atkinson, E.N.; Somers, E.; Allard, J.; Moore, R.G.; Lu, K.H.; Bast, R.C., Jr. Urinary mesothelin provides greater sensitivity for early stage ovarian cancer than serum mesothelin, urinary hCG free beta subunit and urinary hCG beta core fragment. Gynecol. Oncol. 2007, 106, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.S.; Bermudez, Y.; Badgwell, D.; Chen, R.; Nicosia, S.V.; Bast, R.C., Jr.; Kruk, P.A. Urinary levels of Bcl-2 are elevated in ovarian cancer patients. Gynecol. Oncol. 2009, 112, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.B.; Yip, Y.Y.; Swisher, E.M.; Agnew, K.; Hellstrom, K.E.; Hellstrom, I. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms: Clinical correlates. Gynecol. Oncol. 2015, 137, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetsky, Z.; Skates, S.; Lomakin, A.; Nolen, B.; Pulsipher, T.; Modugno, F.; Marks, J.; Godwin, A.; Gorelik, E.; Jacobs, I.; et al. Development of a multimarker assay for early detection of ovarian cancer. J. Clin. Oncol. 2010, 28, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Amonkar, S.D.; Bertenshaw, G.P.; Chen, T.H.; Bergstrom, K.J.; Zhao, J.; Seshaiah, P.; Yip, P.; Mansfield, B.C. Development and preliminary evaluation of a multivariate index assay for ovarian cancer. PLoS ONE 2009, 4, e4599. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Visintin, I.; Alvero, A.B.; Mor, G. Development and validation of a protein-based signature for the detection of ovarian cancer. Clin. Lab. Med. 2009, 29, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Bae, S.M.; Lim, H.; Kim, Y.J.; Ahn, W.S. Development of multiplexed bead-based immunoassays for the detection of early stage ovarian cancer using a combination of serum biomarkers. PLoS ONE 2012, 7, e44960. [Google Scholar] [CrossRef] [PubMed]
- Nolen, B.M.; Lokshin, A.E. Biomarker testing for ovarian cancer: Clinical utility of multiplex assays. Mol. Diagn. Ther. 2013, 17, 139–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chambers, A.F.; Vanderhyden, B.C. Ovarian cancer biomarkers in urine. Clin. Cancer Res. 2006, 12, 323–327. [Google Scholar] [CrossRef]
- Hellstrom, I.; Heagerty, P.J.; Swisher, E.M.; Liu, P.; Jaffar, J.; Agnew, K.; Hellstrom, K.E. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms. Cancer Lett. 2010, 296, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Nolen, B.M.; Orlichenko, L.S.; Marrangoni, A.; Velikokhatnaya, L.; Prosser, D.; Grizzle, W.E.; Ho, K.; Jenkins, F.J.; Bovbjerg, D.H.; Lokshin, A.E. An extensive targeted proteomic analysis of disease-related protein biomarkers in urine from healthy donors. PLoS ONE 2013, 8, e63368. [Google Scholar] [CrossRef] [PubMed]
- Nolen, B.M.; Lomakin, A.; Marrangoni, A.; Velikokhatnaya, L.; Prosser, D.; Lokshin, A.E. Urinary protein biomarkers in the early detection of lung cancer. Cancer Prev. Res (Phila). 2015, 8, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Jacobs, I.J. Ovarian cancer screening in the general population. Curr. Opin. Obstet. Gynecol. 2001, 13, 61–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacobs, I.J.; Menon, U. Progress and challenges in screening for early detection of ovarian cancer. Mol. Cell. Proteomics 2004, 3, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Jabre-Raughley, M.; Brown, A.K.; Robison, K.M.; Miller, M.C.; Allard, W.J.; Kurman, R.J.; Bast, R.C.; Skates, S.J. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am. J. Obstet. Gynecol. 2010, 203, 228.e1–228.e6. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.A.; Sandhu, N.; Hogdall, C.; Christensen, I.J.; Nedergaard, L.; Lundvall, L.; Engelholm, S.A.; Pedersen, A.T.; Hartwell, D.; Lydolph, M.; et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2012, 127, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Grenache, D.G.; Heichman, K.A.; Werner, T.L.; Vucetic, Z. Clinical performance of two multi-marker blood tests for predicting malignancy in women with an adnexal mass. Clin. Chim. Acta 2015, 438, 358–363. [Google Scholar] [CrossRef]
- Bristow, R.E.; Smith, A.; Zhang, Z.; Chan, D.W.; Crutcher, G.; Fung, E.T.; Munroe, D.G. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol. Oncol. 2013, 128, 252–259. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. An Introduction to the Bootstrap; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Efron, B. Second Thoughts on the Bootstrap. Statist. Sci. 2003, 18, 135–140. [Google Scholar] [CrossRef]
- Henderson, A.R. The bootstrap: A technique for data-driven statistics. Using computer-intensive analyses to explore experimental data. Clin. Chim. Acta 2005, 359, 1–26. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Musoro, J.Z.; Zwinderman, A.H.; Puhan, M.A.; ter Riet, G.; Geskus, R.B. Validation of prediction models based on lasso regression with multiply imputed data. BMC Med. Res. Methodol. 2014, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- UPCI Luminex Core Facility. Available online: http://www.upci.upmc.edu/luminex/index.cfm (accessed on 1 December 2017).
- Nolen, B.M.; Marks, J.R.; Ta'san, S.; Rand, A.; Luong, T.M.; Wang, Y.; Blackwell, K.; Lokshin, A.E. Serum biomarker profiles and response to neoadjuvant chemotherapy for locally advanced breast cancer. Breast Cancer Res. 2008, 10, R45. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.W.; Collins, C.D.; Shimizu-Motohashi, Y.; Holm, I.A.; Campbell, M.G.; Lee, I.H.; Brewster, S.J.; Hanson, E.; Harris, H.K.; Lowe, K.R.; et al. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PLoS ONE 2012, 7, e49475. [Google Scholar] [CrossRef] [PubMed]

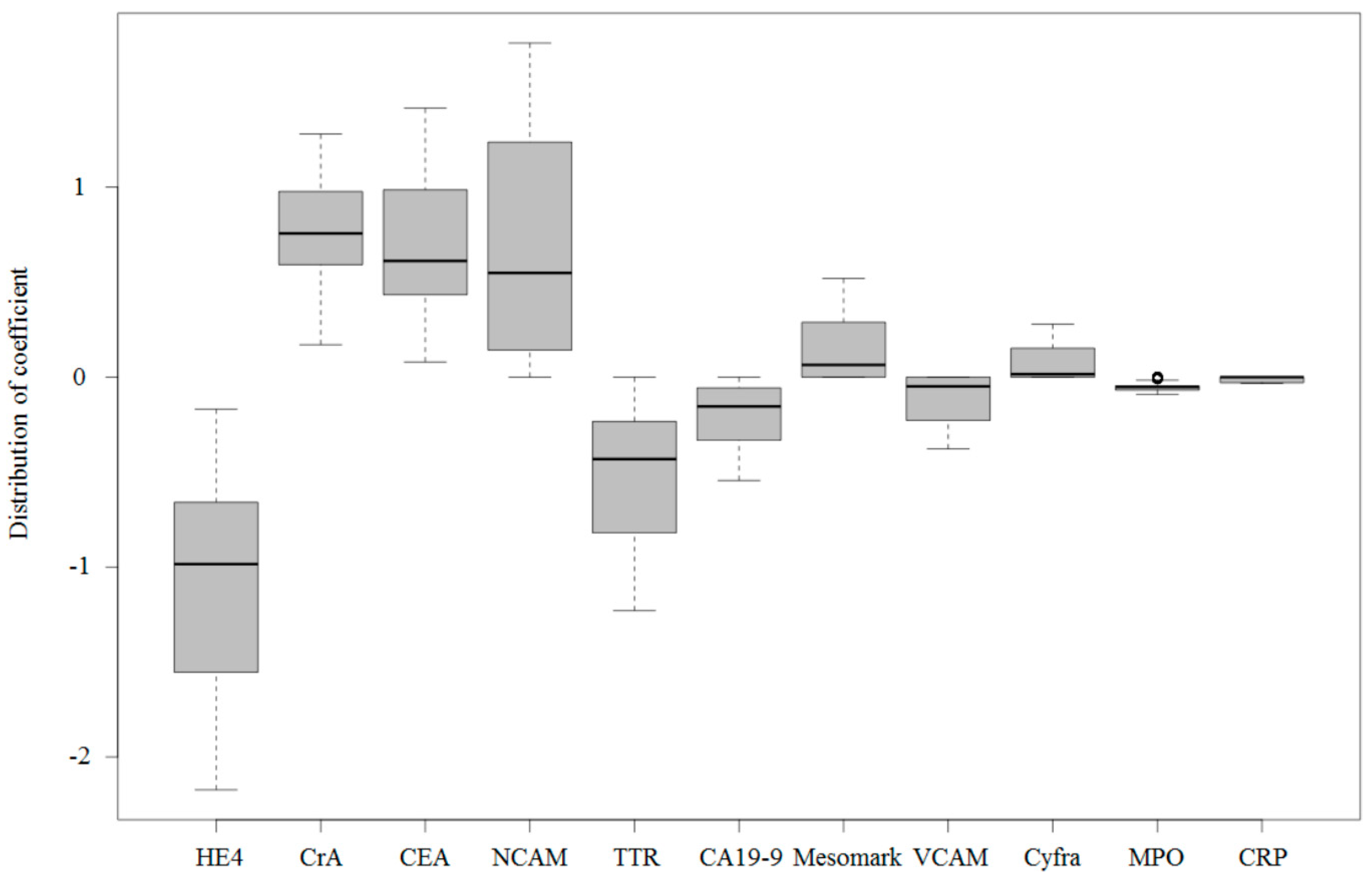

| Number of Totals | Premenopausal | Postmenopausal | |
|---|---|---|---|
| Total | 295 | 175 | 119 |
| Age (years), median and range | 48 (20–82) | ||
| Benign tumor | 125 (42.4%) | 99 | 25 |
| Endometriosis | 44 | 41 | 3 |
| Teratoma | 30 * | 27 | 2 |
| Mucinous cystadenoma | 16 | 13 | 3 |
| Serous cystadenoma | 9 | 5 | 4 |
| Inflammation 1 | 5 | 1 | 4 |
| Others 2 | 21 | 12 | 9 |
| Borderline tumor | 12 (4.1%) | 10 | 2 |
| Malignant tumor | 158 (53.5%) | 66 | 92 |
| Serous adenocarcinoma | 111 | 40 | 71 |
| Mucinous adenocarcinoma | 12 | 6 | 6 |
| Endometrioid adenocarcinoma | 15 | 11 | 4 |
| Clear cell carcinoma | 12 | 4 | 8 |
| Other EOC 3 | 3 | 1 | 2 |
| Granulosa cell tumor | 2 | 2 | - |
| Dysgerminoma | 1 | 1 | - |
| Other non-EOC 4 | 2 | 1 | 1 |
| FIGO stage of malignancy | |||
| I | 36 | 23 | 13 |
| II | 12 | 5 | 7 |
| III | 91 | 32 | 59 |
| IV | 19 | 6 | 13 |
| Markers | All Samples | Stage I and II | Stage III and IV | |||
|---|---|---|---|---|---|---|
| AUC | (95% CIs) | AUC | (95% CIs) | AUC | (95% CIs) | |
| HE4 | 0.822 | (0.772–0.869) | 0.759 | (0.659–0.852) | 0.847 | (0.791–0.898) |
| VCAM | 0.776 | (0.717–0.829) | 0.744 | (0.650–0.830) | 0.788 | (0.725–0.847) |
| Leptin | 0.771 | (0.701–0.837) | 0.779 | (0.657–0.889) | 0.772 | (0.689–0.848) |
| TTR | 0.767 | (0.706–0.824) | 0.789 | (0.714–0.856) | 0.757 | (0.693–0.819) |
| Prolactin | 0.713 | (0.624–0.793) | 0.732 | (0.573–0.870) | 0.701 | (0.600–0.794) |
| CRP | 0.710 | (0.644–0.772) | 0.644 | (0.541–0.743) | 0.734 | (0.662–0.800) |
| PDGF-AA | 0.697 | (0.632–0.758) | 0.734 | (0.644–0.820) | 0.677 | (0.607–0.747) |
| NCAM | 0.678 | (0.613–0.741) | 0.672 | (0.576–0.761) | 0.678 | (0.609–0.742) |
| Mesomark | 0.670 | (0.578–0.756) | 0.648 | (0.520–0.769) | 0.680 | (0.583–0.769) |
| MPO | 0.668 | (0.598–0.737) | 0.640 | (0.554–0.723) | 0.684 | (0.610–0.756) |
| Cyfra21-1 | 0.660 | (0.591–0.726) | 0.728 | (0.630–0.821) | 0.628 | (0.549–0.701) |
| CEA | 0.627 | (0.558–0.692) | 0.684 | (0.583–0.778) | 0.600 | (0.524–0.676) |
| Creatinine | 0.622 | (0.554–0.687) | 0.678 | (0.585–0.770) | 0.596 | (0.518–0.668) |
| CA19-9 | 0.598 | (0.529–0.666) | 0.578 | (0.470–0.678) | 0.604 | (0.528–0.677) |
| IL6 | 0.576 | (0.450–0.701) | 0.490 | (0.323–0.657) | 0.599 | (0.459–0.730) |
| MIF | 0.572 | (0.500–0.642) | 0.640 | (0.531–0.743) | 0.537 | (0.456–0.618) |
| ApoAI | 0.557 | (0.485–0.623) | 0.517 | (0.422–0.612) | 0.569 | (0.493–0.644) |
| ApoCIII | 0.524 | (0.448–0.600) | 0.580 | (0.466–0.687) | 0.562 | (0.479–0.644) |
| PAI-1 | 0.523 | (0.446–0.598) | 0.514 | (0.417–0.616) | 0.533 | (0.451–0.611) |
| CA125 | 0.523 | (0.453–0.591) | 0.486 | (0.370–0.597) | 0.533 | (0.456–0.611) |
| OPN | 0.521 | (0.45–0.591) | 0.540 | (0.443–0.635) | 0.514 | (0.435–0.594) |
| IL8 | 0.488 | (0.416–0.563) | 0.541 | (0.449–0.633) | 0.531 | (0.453–0.607) |
| CA15-3 | 0.486 | (0.417–0.552) | 0.540 | (0.439–0.638) | 0.536 | (0.460–0.613) |
| Markers | All Samples | Stage I and II | Stage III and IV | |||
|---|---|---|---|---|---|---|
| AUC | (95% CIs) | AUC | (95% CIs) | AUC | (95% CIs) | |
| HE4 | 0.822 | (0.772–0.869) | 0.759 | (0.659–0.852) | 0.847 | (0.791–0.898) |
| CEA | 0.627 | (0.558–0.692) | 0.684 | (0.583–0.778) | 0.600 | (0.524–0.676) |
| TTR | 0.767 | (0.706–0.824) | 0.789 | (0.714–0.856) | 0.757 | (0.693–0.819) |
| Creatinine (Cr) | 0.622 | (0.554–0.687) | 0.678 | (0.585–0.770) | 0.596 | (0.518–0.668) |
| HE4+Cr | 0.904 | (0.854–0.938) | 0.825 | (0.740–0.910) | 0.926 | (0.888–0.972) |
| HE4+Cr+CEA | 0.923 | (0.878–0.954) | 0.883 | (0.772–0.934) | 0.943 | (0.907–0.982) |
| HE4+Cr+CEA+TTR | 0.938 | (0.900–0.964) | 0.932 | (0.844–0.970) | 0.946 | (0.911–0.983) |
| Algorithm or Assay | N | SN (%) | SP (%) | PPV | NPV | AUC | |
|---|---|---|---|---|---|---|---|
| Serum Multimarker Panels | |||||||
| Moore, 2010 [27] | RMI | 457 | 84.6 | 75.0 | - | - | 0.870 |
| ROMA | 457 | 94.3 | 75.0 | - | - | 0.953 | |
| Karlsen, 2012 [28] | RMI | 1218 | 96.0 | 75.0 | - | - | 0.958 |
| ROMA | 1218 | 94.8 | 75.0 | - | - | 0.954 | |
| Bristow, 2013 [30] | OVA1 | 494 | 92.4 | 53.5 | 31.3 | 96.8 | - |
| Grenache, 2015 [29] | ROMA | 146 | 83.9 | 83.5 | 57.8 | 95.1 | - |
| OVA1 | 146 | 96.8 | 54.8 | 36.6 | 98.4 | - | |
| Urinary Biomarker | |||||||
| Hellstrom, 2010 [22] | HE4 | 135 1 | 88.6 | 94.4 | |||
| Liao, 2015 [15] | HE4 | 279 2 | 52.2 | 95.0 | |||
| Urinary Multimarker Panel in this Study | |||||||
| HE4+Cr+CEA+TTR | 283 | 81.0 92.9 | 95.3 75.2 | 95.3 81.3 | 81.3 90.1 | 0.938 | |
| Inflammatory Mediators | IL-6 1, IL-8 1, MPO 1, MIF 1, OPN 1 |
| Tumor-associated antigens | CA19-9 1, CA15-3 1, CA-125 1, HE4 1, CEA 1 |
| Adhesion molecules | VCAM1, NCAM 1 |
| Adipokines | Leptin 1 |
| Apolipoproteins | ApoAI 1, ApoCIII 1 |
| Apoptotic proteins | Cyfra21-1 1 |
| Growth/angiogenic factors | PDGF-AA 1 |
| Carrier proteins | TTR 2 |
| Proteases/inhibitors | PAI-1 1 |
| Hormones | Prolactin 1 |
| Others | CRP1, Mesomark 3, Creatinine 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-W.; Lee, H.-Y.; Bang, H.J.; Song, H.-J.; Kong, S.W.; Kim, Y.-M. An Improved Prediction Model for Ovarian Cancer Using Urinary Biomarkers and a Novel Validation Strategy. Int. J. Mol. Sci. 2019, 20, 4938. https://doi.org/10.3390/ijms20194938
Lee S-W, Lee H-Y, Bang HJ, Song H-J, Kong SW, Kim Y-M. An Improved Prediction Model for Ovarian Cancer Using Urinary Biomarkers and a Novel Validation Strategy. International Journal of Molecular Sciences. 2019; 20(19):4938. https://doi.org/10.3390/ijms20194938
Chicago/Turabian StyleLee, Shin-Wha, Ha-Young Lee, Hyo Joo Bang, Hye-Jeong Song, Sek Won Kong, and Yong-Man Kim. 2019. "An Improved Prediction Model for Ovarian Cancer Using Urinary Biomarkers and a Novel Validation Strategy" International Journal of Molecular Sciences 20, no. 19: 4938. https://doi.org/10.3390/ijms20194938
APA StyleLee, S.-W., Lee, H.-Y., Bang, H. J., Song, H.-J., Kong, S. W., & Kim, Y.-M. (2019). An Improved Prediction Model for Ovarian Cancer Using Urinary Biomarkers and a Novel Validation Strategy. International Journal of Molecular Sciences, 20(19), 4938. https://doi.org/10.3390/ijms20194938





