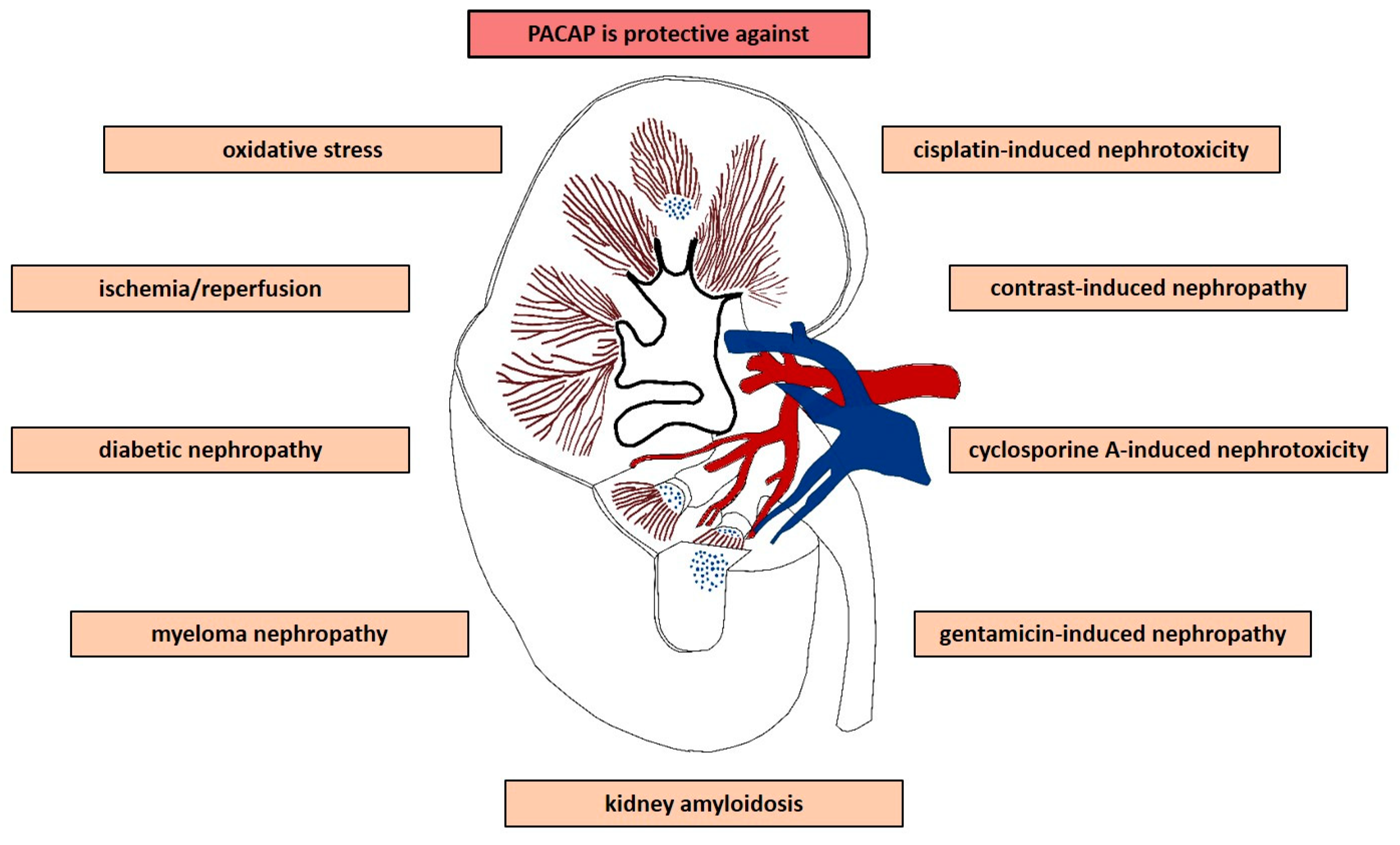
The Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Is Protective in Inflammation and Oxidative Stress-Induced Damage in the Kidney
Abstract
:1. Introduction
2. Effects of PACAP In Vitro
2.1. Oxidative Stress and Hypoxia
2.2. Effect of PACAP against Drug-Induced Nephropathy
2.2.1. Effects of Exogenous PACAP against Gentamicin-Induced Nephrotoxicity In Vitro
2.2.2. Cisplatin Nephrotoxicity
2.2.3. Cyclosporine A-Induced Nephrotoxicity
2.2.4. Contrast-Induced Nephrotoxicity
2.3. Myeloma Kidney Injury
2.4. Diabetic Nephropathy
3. In Vivo Protective Effects of PACAP
3.1. Ischemia/Reperfusion Kidney Injury
3.2. Diabetic Nephropathy
3.3. Myeloma Kidney Injury
3.4. Drug-Induced Nephropathy
3.4.1. Gentamicin-Induced Nephropathy
3.4.2. Cisplatin-Induced Nephropathy
3.4.3. Contrast Agent-Induced Nephropathy
3.4.4. Cyclosporine A-Induced Nephrotoxicity
3.5. Kidney Amyloidosis
4. Concluding Remarks
Funding
Conflicts of Interest
References
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharm. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Horvath, G.; Lubics, A.; Laszlo, E.; Tamas, A.; Racz, B.; Szakaly, P. Effects of pituitary adenylate cyclase activating polypeptide in the urinary system, with special emphasis on its protective effects in the kidney. Neuropeptides 2012, 46, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ojala, J.; Tooke, K.; Hsiang, H.; Girard, B.M.; May, V.; Vizzard, M.A. PACAP/PAC1 expression and function in micturition pathways. J. Mol. Neurosci. 2019, 68, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, M.; Friis, U.G.; Desch, M.; Todorov, V.; Castrop, H.; Segerer, F.; Otto, C.; Schütz, G.; Schweda, F. Pituitary adenylate cyclase-activating polypeptide stimulates renin secretion via activation of PAC1 receptors. J. Am. Soc. Nephrol. 2007, 18, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.M.; Rakhit, T.; Kemp, P.A.; March, J.E.; Bennett, T. Regional haemodynamic responses to pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide in conscious rats. Br. J. Pharm. 1994, 111, 589–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, S.F. PACAP-27 and PACAP-38: Vascular effects in the eye and some other tissues in the rabbit. Eur. J. Pharm. 1994, 253, 17–25. [Google Scholar] [CrossRef]
- Girard, B.M.; Wolf-Johnston, A.; Braas, K.M.; Birder, L.A.; May, V.; Vizzard, M.A. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J. Mol. Neurosci. 2008, 36, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Heppner, T.J.; Hennig, G.W.; Nelson, M.T.; May, V.; Vizzard, M.A. PACAP38-mediated bladder afferent nerve activity hyperexcitability and Ca2+ activity in urothelial cells from mice. J. Mol. Neurosci. 2019, 68, 348–356. [Google Scholar] [CrossRef]
- Gasz, B.; Racz, B.; Roth, E.; Borsiczky, B.; Ferencz, A.; Tamas, A.; Cserepes, B.; Lubics, A.; Gallyas, F., Jr.; Toth, G.; et al. Pituitary adenylate cyclase activating polypeptide protects cardiomyocytes against oxidative stress-induced apoptosis. Peptides 2006, 27, 87–94. [Google Scholar] [CrossRef]
- Racz, B.; Gasz, B.; Borsiczky, B.; Gallyas, F., Jr.; Tamas, A.; Jozsa, R.; Lubics, A.; Kiss, P.; Roth, E.; Ferencz, A.; et al. Protective effects of pituitary adenylate cyclase activating polypeptide in endothelial cells against oxidative stress-induced apoptosis. Gen. Comp. Endocrinol. 2007, 153, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Racz, B.; Gasz, B.; Gallyas, F., Jr.; Kiss, P.; Tamas, A.; Jozsa, R.; Lubics, A.; Lengvari, I.; Toth, G.; Hegyi, O.; et al. PKA-Bad-14–3-3 and Akt-Bad-14–3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul. Pept. 2008, 145, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Racz, B.; Reglodi, D.; Horvath, G.; Szigeti, A.; Balatonyi, B.; Roth, E.; Weber, G.; Alotti, N.; Toth, G.; Gasz, B. Protective effect of PACAP against doxorubicin-induced cell death in cardiomyocyte culture. J. Mol. Neurosci 2010, 42, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Reglodi, D.; Opper, B.; Brubel, R.; Tamas, A.; Kiss, P.; Toth, G.; Csernus, V.; Matkovits, A.; Racz, B. Effects of PACAP on the oxidative stress-induced cell death in chicken pinealocytes is influenced by the phase of the circadian clock. Neurosci. Lett. 2010, 484, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Koshimura, K.; Murakami, Y.; Sohmiya, M.; Yanaihara, N.; Kato, Y. Neuronal protection from apoptosis by pituitary adenylate cyclase activating polypeptide. Regul. Pept. 1997, 72, 1–8. [Google Scholar] [CrossRef]
- Vaudry, D.; Rousselle, C.; Basille, M.; Falluel-Morel, A.; Pamantung, T.F.; Fontaine, M.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc. Natl. Acad. Sci. USA 2002, 99, 6398–6403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaudry, D.; Pamantung, T.F.; Basille, M.; Rousselle, C.; Fournier, A.; Vaudry, H.; Beauvillain, J.C.; Gonzalez, B.J. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur. J. Neurosci. 2002, 15, 1451–1460. [Google Scholar] [CrossRef] [Green Version]
- Vaudry, D.; Falluel-Morel, A.; Basille, M.; Pamantung, T.F.; Fontaine, M.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. Pituitary adenylate cyclase-activating polypeptide prevents C2-ceramide-induced apoptosis of cerebellar granule cells. J. Neurosci. Res. 2003, 72, 303–316. [Google Scholar] [CrossRef]
- Reglodi, D.; Somogyvari-Vigh, A.; Vigh, S.; Maderdrut, J.L.; Arimura, A. Neuroprotective effects of PACAP38 in a rat model of transient focal ischemia under various experimental conditions. Ann. N. Y. Acad. Sci. 2000, 921, 119–128. [Google Scholar] [CrossRef]
- Chen, Y.; Samal, B.; Hamelink, C.R.; Xiang, C.C.; Chen, Y.; Chen, M.; Vaudry, D.; Brownstein, M.J.; Hallenbeck, J.M.; Eiden, L.E. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul. Pept. 2006, 137, 4–19. [Google Scholar] [CrossRef] [Green Version]
- Reglodi, D.; Lubics, A.; Tamas, A.; Szalontay, L.; Lengvari, I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson’s disease. Behav. Brain Res. 2004, 151, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, M.; Nakamachi, T.; Sugiyama, K.; Tsuchikawa, D.; Watanabe, J.; Hori, M.; Yoshikawa, A.; Imai, N.; Kagami, N.; Matkovits, A.; et al. PACAP stimulates functional recovery after spinal cord injury through axonal regeneration. J. Mol. Neurosci. 2014, 54, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Tang, Z.; Yin, J.; Maalouf, M.; Beach, T.G.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol. Aging 2014, 35, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ito, A.; Kawanokuchi, J.; Jin, S.; Mizuno, T.; Ojika, K.; Ueda, R.; Suzumura, A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult. Scler. J. 2004, 10, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Vaczy, A.; Werling, D.; Kiss, P.; Tamas, A.; Kovacs, K.; Fabian, E.; Kvarik, T.; Mammel, B.; Danyadi, B.; et al. Neuroprotective effects of PACAP in the retina. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; pp. 501–527. [Google Scholar] [CrossRef]
- Somogyvari-Vigh, A.; Reglodi, D. Pituitary adenylate cyclase activating polypeptide: A potential neuroprotective peptide. Curr. Pharm. Des. 2004, 10, 2861–2889. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Tamas, A.; Jungling, A.; Vaczy, A.; Rivnyak, A.; Fulop, B.D.; Szabo, E.; Lubics, A.; Atlasz, T. Protective effects of pituitary adenylate cyclase activating polypeptide against neurotoxic agents. Neurotoxicology 2018, 66, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 2002, 24, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Seo, S.R. Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 2014, 47, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lu, T.; Zhang, C.; Xue, Z.; Xu, J.; Busuttil, R.W.; Xia, Q.; Xu, N.; Kupiec-Weglinski, J.W.; Ji, H. Pituitary adenylate cyclase-activating polypeptides prevent hepatocyte damage by promoting Yes-associated protein in liver ischemia-reperfusion injury. Transplantation 2019, 103, 1639–1648. [Google Scholar] [CrossRef]
- Ferencz, A.; Racz, B.; Tamás, A.; Nedvig, K.; Nemeth, J.; Kalmar-Nagy, K.; Horvath, O.P.; Weber, G.; Roth, E.; Reglodi, D. Changes and effect of PACAP-38 on intestinal ischemia-reperfusion and autotransplantation. Transpl. Proc. 2009, 41, 57–59. [Google Scholar] [CrossRef]
- Elekes, K.; Sandor, K.; Moricz, A.; Kereskai, L.; Kemeny, A.; Szoke, E.; Perkecz, A.; Reglodi, D.; Hashimoto, H.; Pinter, E.; et al. Pituitary adenylate cyclase-activating polypeptide plays an anti-inflammatory role in endotoxin-induced airway inflammation: In vivo study with gene-deleted mice. Peptides 2011, 32, 1439–1446. [Google Scholar] [CrossRef]
- Kemeny, A.; Reglodi, D.; Cseharovszky, R.; Hashimoto, H.; Baba, A.; Szolcsanyi, J.; Pinter, E.; Helyes, Z. Pituitary adenylate cyclase-activating polypeptide deficiency enhances oxazolone-induced allergic contact dermatitis in mice. J. Mol. Neurosci. 2010, 42, 443–449. [Google Scholar] [CrossRef]
- Roth, E.; Weber, G.; Kiss, P.; Horvath, G.; Toth, G.; Gasz, B.; Ferencz, A.; Gallyas, F., Jr.; Reglodi, D.; Racz, B. Effects of PACAP and preconditioning against ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro. Ann. N. Y. Acad. Sci. 2009, 1163, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Nakamachi, T.; Ohtaki, H.; Yofu, S.; Sato, A.; Endo, K.; Iso, Y.; Suzuki, H.; Takeyama, Y.; Shintani, N.; et al. Cardioprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on Doxorubicin-induced cardiomyopathy in mice. Circ. J. 2010, 74, 1183–1190. [Google Scholar] [CrossRef]
- Nakata, M.; Shintani, N.; Hashimoto, H.; Baba, A.; Yada, T. Intra-islet PACAP protects pancreatic β-cells against glucotoxicity and lipotoxicity. J. Mol. Neurosci. 2010, 42, 404–410. [Google Scholar] [CrossRef]
- Khan, A.M.; Batuman, V. Renoprotective effects of pituitary adenylate cyclase activating polypeptide 38 (PACAP38). In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; pp. 289–312. [Google Scholar] [CrossRef]
- Horvath, G.; Brubel, R.; Kovacs, K.; Reglodi, D.; Opper, B.; Ferencz, A.; Szakaly, P.; Laszlo, E.; Hau, L.; Kiss, P.; et al. Effects of PACAP on oxidative stress-induced cell death in rat kidney and human hepatocyte cells. J. Mol. Neurosci. 2011, 43, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Li, M.; Brant, E.; Maderdrut, J.L.; Majid, D.S.; Simon, E.E.; Batuman, V. Renoprotection with pituitary adenylate cyclase-activating polypeptide in cyclosporine A-induced nephrotoxicity. J. Investig. Med. 2011, 59, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Reglodi, D.; Czetany, P.; Illes, A.; Reman, G.; Fekete, A.; Toth, G.; Laszlo, E.; Opper, B. Effects of pituitary adenylate cyclase activating polypeptide in human proximal tubule cells against gentamicin toxicity. Int. J. Pept. Res. Ther. 2019, 25, 257–264. [Google Scholar] [CrossRef]
- Li, M.; Balamuthusamy, S.; Khan, A.M.; Maderdrut, J.L.; Simon, E.E.; Batuman, V. Pituitary adenylate cyclase-activating polypeptide prevents cisplatin-induced renal failure. J. Mol. Neurosci. 2011, 43, 58–66. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kuno, K.; Takemoto, M.; He, P.; Ishikawa, T.; Onishi, S.; Ishibashi, R.; Okabe, E.; Shoji, M.; Hattori, A.; et al. Pituitary adenylate cyclase-activating polypeptide protects glomerular podocytes from inflammatory injuries. J. Diabetes Res. 2015, 727152. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Khan, A.M.; Maderdrut, J.L.; Simon, E.E.; Batuman, V. The effect of PACAP38 on MyD88-mediated signal transduction in ischemia-/hypoxia-induced acute kidney injury. Am. J. Nephrol. 2010, 32, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A.; Li, M.; Batuman, V. Potential protective action of pituitary adenylate cyclase-activating polypeptide (PACAP38) on in vitro and in vivo models of myeloma kidney injury. Blood 2006, 107, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Torras, J.; Cruzado, J.M.; Lloberas, N.; Liron, J.; Herrero, I.; Navarro, M.A.; Grinyo, J.M. The enhancement of endogenous cAMP with pituitary adenylate cyclase-activating polypeptide protects rat kidney against ischemia through the modulation of inflammatory response. Transplantation 2001, 72, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Szakaly, P.; Kiss, P.; Lubics, A.; Magyarlaki, T.; Tamas, A.; Racz, B.; Lengvari, I.; Toth, G.; Reglodi, D. Effects of PACAP on survival and renal morphology in rats subjected to renal ischemia/reperfusion. J. Mol. Neurosci. 2008, 36, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Racz, B.; Reglodi, D.; Kovacs, K.; Kiss, P.; Gallyas, F., Jr.; Bognar, Z.; Szabo, A.; Magyarlaki, T.; Laszlo, E.; et al. Effects of PACAP on mitochondrial apoptotic pathways and cytokine expression in rats subjected to renal ischemia/reperfusion. J. Mol. Neurosci. 2010, 42, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, E.; Juhasz, T.; Varga, A.; Czibere, B.; Kovacs, K.; Degrell, P.; Horvath, G.; Jancso, G.; Szakaly, P.; Tamas, A.; et al. Protective effect of PACAP on ischemia/reperfusion-induced kidney injury of male and female rats: Gender differences. J. Mol. Neurosci. 2019, 68, 408–419. [Google Scholar] [CrossRef]
- Laszlo, E.; Varga, A.; Kovacs, K.; Jancso, G.; Kiss, P.; Tamas, A.; Szakaly, P.; Fulop, B.; Reglodi, D. Ischemia/reperfusion-induced kidney injury in heterozygous PACAP-deficient mice. Transpl. Proc. 2015, 47, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Szakaly, P.; Laszlo, E.; Kovacs, K.; Racz, B.; Horvath, G.; Ferencz, A.; Lubics, A.; Kiss, P.; Tamas, A.; Brubel, R.; et al. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) show increased susceptibility to in vivo renal ischemia/reperfusion injury. Neuropeptides 2011, 45, 113–121. [Google Scholar] [CrossRef]
- Banki, E.; Degrell, P.; Kiss, P.; Kovacs, K.; Kemeny, A.; Csanaky, K.; Duh, A.; Nagy, D.; Toth, G.; Tamas, A.; et al. Effect of PACAP treatment on kidney morphology and cytokine expression in rat diabetic nephropathy. Peptides 2013, 42, 125–130. [Google Scholar] [CrossRef]
- Li, M.; Maderdrut, J.L.; Lertora, J.J.; Arimura, A.; Batuman, V. Renoprotection by pituitary adenylate cyclase-activating polypeptide in multiple myeloma and other kidney diseases. Regul. Pept. 2008, 145, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Banki, E.; Kovacs, K.; Nagy, D.; Juhasz, T.; Degrell, P.; Csanaky, K.; Kiss, P.; Jancso, G.; Toth, G.; Tamas, A.; et al. Molecular mechanisms underlying the nephroprotective effects of PACAP in diabetes. J. Mol. Neurosci. 2014, 54, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Atlasz, T.; Szabo, E.; Jungling, A.; Tamas, A.; Juhasz, T.; Fulop, B.D.; Bardosi, A. PACAP deficiency as a model of aging. Geroscience 2018, 40, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Jungling, A.; Longuespée, R.; Kriegsmann, J.; Casadonte, R.; Kriegsmann, M.; Juhasz, T.; Bardosi, S.; Tamas, A.; Fulop, B.D.; et al. Accelerated pre-senile systemic amyloidosis in PACAP knockout mice—A protective role of PACAP in age-related degenerative processes. J. Pathol. 2018, 245, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar] [CrossRef] [PubMed]
- Csipo, T.; Fulop, G.A.; Lipecz, A.; Tarantini, S.; Kiss, T.; Balasubramanian, P.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. Geroscience 2018, 40, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Sandor, B.; Tamas, A.; Kiss, P.; Hashimoto, H.; Nagy, A.D.; Fulop, B.D.; Juhasz, T.; Manavalan, S.; Reglodi, D. Early neurobehavioral development of mice lacking endogenous PACAP. J. Mol. Neurosci. 2017, 61, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Tan, Y.V. Immunomodulatory roles of PACAP and VIP: Lessons from knockout mice. J. Mol. Neurosci. 2018, 66, 102–113. [Google Scholar] [CrossRef]
- Fulop, B.D.; Sandor, B.; Szentleleky, E.; Karanyicz, E.; Reglodi, D.; Gaszner, B.; Zakany, R.; Hashimoto, H.; Juhasz, T.; Tamas, A. Altered Notch Signaling in Developing Molar Teeth of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)-Deficient Mice. J. Mol. Neurosci. 2019, 68, 377–388. [Google Scholar] [CrossRef]
- Vaudry, D.; Hamelink, C.; Damadzic, R.; Eskay, R.L.; Gonzalez, B.; Eiden, L.E. Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides 2005, 26, 2518–2524. [Google Scholar] [CrossRef] [Green Version]
- Reglodi, D.; Kiss, P.; Szabadfi, K.; Atlasz, T.; Gabriel, R.; Horvath, G.; Szakaly, P.; Sandor, B.; Lubics, A.; Laszlo, E.; et al. PACAP is an endogenous protective factor-insights from PACAP-deficient mice. J. Mol. Neurosci. 2012, 48, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Mark, L.; Brubel, R.; Szakaly, P.; Racz, B.; Kiss, P.; Tamas, A.; Helyes, Z.; Lubics, A.; Hashimoto, H.; et al. Mice deficient in pituitary adenylate cyclase activating polypeptide display increased sensitivity to renal oxidative stress in vitro. Neurosci. Lett. 2010, 469, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Racz, B.; Szakaly, P.; Kiss, P.; Laszlo, E.; Hau, L.; Tamas, A.; Helyes, Z.; Lubics, A.; Hashimoto, H.; et al. Mice deficient in neuropeptide PACAP demonstrate increased sensitivity to in vitro kidney hypoxia. Transpl. Proc. 2010, 42, 2293–2295. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte. Blood Press. 2014, 12, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Balamuthusamy, S.; Khan, A.M.; Maderdrut, J.L.; Simon, E.E.; Batuman, V. Pituitary adenylate cyclase-activating polypeptide ameliorates cisplatin-induced acute kidney injury. Peptides 2010, 31, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Rinner, B.; Cerullo, V.; Yliperttula, M.; Mazzaferro, V.; Ciana, P. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J. Control. Release 2019, 294, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Busauschina, A.; Schnuelle, P.; van der Woude, F. Cyclosporine nephrotoxicity. Transpl. Proc. 2004, 36, S229–S233. [Google Scholar] [CrossRef]
- Young, E.W.; Ellis, C.N.; Messana, J.M.; Johnson, K.J.; Leichtman, A.B.; Mihatsch, M.J.; Hamilton, T.A.; Groisser, D.S.; Fradin, M.S.; Voorhees, J.J. A prospective study of renal structure and function in psoriasis patients treated with cyclosporin. Kidney Int. 1994, 46, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, H.S.; Morcos, S.K. Radiographic contrast media. BJU Int. 2000, 86, 1–10. [Google Scholar] [CrossRef]
- Khan, A.M.; Maderdrut, J.L.; Li, M.; Toliver, H.L.; Coy, D.H.; Simon, E.E.; Batuman, V. Pituitary adenylate cyclase-activating polypeptide prevents contrast-induced nephropathy in a novel mouse model. Physiol. Rep. 2013, 1, e00163. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; de Zeeuw, D. Need for better diabetes treatment for improved renal outcome. Kidney Int. Suppl. 2011, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Arimura, A.; Somogyvári-Vigh, A.; Shioda, S.; Banks, W.A. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 1996, 736, 280–286. [Google Scholar] [CrossRef]
- Reglodi, D.; Vaczy, A.; Rubio-Beltran, E.; MaassenVanDenBrink, A. Protective effects of PACAP in ischemia. J. Headache Pain 2018, 19, 19. [Google Scholar] [CrossRef]
- Atlasz, T.; Babai, N.; Kiss, P.; Reglodi, D.; Tamás, A.; Szabadfi, K.; Toth, G.; Hegyi, O.; Lubics, A.; Gabriel, R. Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. Gen. Comp. Endocrinol. 2007, 153, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Illes, A.; Heimessaat, M.M.; Bardosi, A.; Bardosi, S.; Tamas, A.; Fulop, B.D.; Opper, B.; Nemeth, J.; Ferencz, A.; et al. Protective intestinal effects of pituitary adenylate cyclase activating polypeptide. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; pp. 271–288. [Google Scholar] [CrossRef]
- Laszlo, E.; Kiss, P.; Horvath, G.; Szakaly, P.; Tamas, A.; Reglodi, D. The effects of pituitary adenylate cyclase activating polypeptide in renal ischemia/reperfusion. Acta Biol. Hung. 2014, 65, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.M.; Li, M.; Abdulnour-Nakhoul, S.; Maderdrut, J.L.; Simon, E.E.; Batuman, V. Delayed administration of pituitary adenylate cyclase-activating polypeptide 38 ameliorates renal ischemia/reperfusion injury in mice by modulating Toll-like receptors. Peptides 2012, 38, 395–403. [Google Scholar] [CrossRef]
- Wandell, P.; Carlsson, A.C.; Sundquist, J.; Sundquist, K. The association between relevant comorbidities and dementia in patients with atrial fibrillation. Geroscience 2018, 40, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.T.; Wang, J.; Wu, H.Y.; Huang, J.W.; Chien, K.L. Age modifies the risk factor profiles for acute kidney injury among recently diagnosed type 2 diabetic patients: A population-based study. Geroscience 2018, 40, 201–217. [Google Scholar] [CrossRef]
- Sakul, A.; Arı, N.; Sotnikova, R.; Ozansoy, G.; Karasu, C. A pyridoindole antioxidant SMe1EC2 regulates contractility, relaxation ability, cation channel activity, and protein-carbonyl modifications in the aorta of young and old rats with or without diabetes mellitus. Geroscience 2018, 40, 377–392. [Google Scholar] [CrossRef]
- Li, M.; Cortez, S.; Nakamachi, T.; Batuman, V.; Arimura, A. Pituitary adenylate cyclase-activating polypeptide is a potent inhibitor of the growth of light chain-secreting human multiple myeloma cells. Cancer Res. 2006, 66, 8796–8803. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Maderdrut, J.L.; Lertora, J.J.; Batuman, V. Intravenous infusion of pituitary adenylate cyclase-activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney: A case study. Peptides 2007, 28, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Aubert, N.; Vaudry, D.; Falluel-Morel, A.; Desfeux, A.; Fisch, C.; Ancian, P.; de Jouffrey, S.; Le Bigot, J.F.; Couvineau, A.; Laburthe, M.; et al. PACAP prevents toxicity induced by cisplatin in rat and primate neurons but not in proliferating ovary cells: Involvement of the mitochondrial apoptotic pathway. Neurobiol. Dis. 2008, 32, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Reglodi, D.; Horvath, G.; Opper, B.; Toth, G.; Fazakas, C.; Vegh, A.G.; Wilhelm, I.; Krizbai, I.A. Pituitary adenylate cyclase activating polypeptide acts against neovascularization in retinal pigment epithelial cells. Ann. N. Y. Acad. Sci. 2019, (in press). [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, I.; Krizbai, I.A. Effects of PACAP on biological barriers. Pituitary Adenylate Cyclase Activating Polypeptide—PACAP. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; pp. 433–448. [Google Scholar] [CrossRef]
- Ohtaki, H.; Satoh, A.; Nakamachi, T.; Yofu, S.; Dohi, K.; Mori, H.; Ohara, K.; Miyamoto, K.; Hashimoto, H.; Shintani, N.; et al. Regulation of oxidative stress by pituitary adenylate cyclase-activating polypeptide (PACAP) mediated by PACAP receptor. J. Mol. Neurosci. 2010, 42, 397–403. [Google Scholar] [CrossRef]
- Kovacs-Valasek, A.; Szabadfi, K.; Denes, V.; Szalontai, B.; Tamas, A.; Kiss, P.; Szabo, A.; Setalo, G., Jr.; Reglodi, D.; Gabriel, R. Accelerated retinal aging in PACAP knock-out mice. Neuroscience 2017, 348, 1–10. [Google Scholar] [CrossRef]
- Eneman, B.; van den Heuvel, L.; Freson, K.; Van Geet, C.; Willemsen, B.; Dijkman, H.; Levtchenko, E. Distribution and function of PACAP and its receptors in the healthy and nephrotic kidney. Nephron 2016, 132, 301–311. [Google Scholar] [CrossRef]
- Tamas, A.; Javorhazy, A.; Reglodi, D.; Sarlos, D.P.; Banyai, D.; Semjen, D.; Nemeth, J.; Lelesz, B.; Fulop, D.B.; Szanto, Z. Examination of PACAP-like immunoreactivity in urogenital tumor samples. J. Mol. Neurosci. 2016, 59, 177–183. [Google Scholar] [CrossRef]
| Harmful Stimulus/Disease | Observed Effect | Reference(s) |
| In Vitro | ||
| oxidative stress | ↓ oxidative stress-induced cell death in rat renal cells | [39] |
| cyclosporine A | ↓ morphological changes ↓ cyclosporine a-induced TGF-β1 ↓ apoptosis | [40] |
| gentamicin | ↓ gentamicin-induced cell death in HK-2 cells | [41] |
| cisplatin | ↓ cisplatin-induced apoptosis in mouse renal proximal tubule cells | [42] |
| LPS exposure | ↓ proinflammatory cytokines in podocytes | [43] |
| hypoxia | ↓ cytokine activation ↓ cell death | [44] |
| kappa light chains | ↓ IL-6, TNF-α ↓ p38 phosphorylation | [45] |
| In Vivo | ||
| Ischemia/reperfusion | ↑ renal function and kidney morphology in warm ischemia | [46] |
| ↓ tubular degeneration and mortality | [47] | |
| ↓ I/R-induced increase of CNTF, fractalkine, sICAM-1, RANTES, TIMP-1 and MIP-3α ↓ Bcl-2 | [48] | |
| ↓ SOD and glutathione | [39] | |
| ↓renal failure, histological damage, neutrophil influx and tubule cell apoptosis | [44] | |
| gender-different protective effect ↓ tubular damage ↓ fractalkine, L-selectin, RANTES, sICAM-1, thymus chemokine, CNTF ↑ SOD in females influences BMP signaling | [49] | |
| in PACAP deficiency after I/R: ↓ SOD level more severe histological damage elevated expression of: BLC, G-CSF, IL-1ra, IL-6, KC, MCP-1, MIP-2, TIMP-1, TREM-1 decreased expression of: sICAM-1, interferon-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-7, IL-10, IL-13, IL-16, IL-17, IL-23, IL-27, IP-10, M-CSF, MCP-5, MIG, SDF-1 | [50,51] | |
| cyclosporine A | ↓ apoptosis ↓ renal injury and fibrosis ↓ extracellular matrix mRNA expression reverse activation of epithelial mesenchymal transition markers ↓ ROS | [40] |
| diabetic nephropathy | ↓ histological damage ↓ cytokine activation | [52,53] |
| ↓ TNF-α | [53] | |
| ↑ pAkt, pERK1/2 ↓ collagen IV, TGF-β-1 ↓ pp38MAPK, cleaved caspase-3 ↓ p60 subunit of NFκB ↑ GSH no focal segmental glomerular basement membrane thickening ↓ podocyte injury | [54] | |
| gentamicin | ↓ TNF-α production | [53] |
| cisplatin | ↓tubular damage ↑renal function ↑ tubular cell regeneration | [42] |
| amyloidosis | pre-senile amyloidosis in PACAP deficiency | [55,56] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvath, G.; Opper, B.; Reglodi, D. The Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Is Protective in Inflammation and Oxidative Stress-Induced Damage in the Kidney. Int. J. Mol. Sci. 2019, 20, 4944. https://doi.org/10.3390/ijms20194944
Horvath G, Opper B, Reglodi D. The Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Is Protective in Inflammation and Oxidative Stress-Induced Damage in the Kidney. International Journal of Molecular Sciences. 2019; 20(19):4944. https://doi.org/10.3390/ijms20194944
Chicago/Turabian StyleHorvath, Gabriella, Balazs Opper, and Dora Reglodi. 2019. "The Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Is Protective in Inflammation and Oxidative Stress-Induced Damage in the Kidney" International Journal of Molecular Sciences 20, no. 19: 4944. https://doi.org/10.3390/ijms20194944
APA StyleHorvath, G., Opper, B., & Reglodi, D. (2019). The Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Is Protective in Inflammation and Oxidative Stress-Induced Damage in the Kidney. International Journal of Molecular Sciences, 20(19), 4944. https://doi.org/10.3390/ijms20194944





