Expression, Cellular and Subcellular Localisation of Kv4.2 and Kv4.3 Channels in the Rodent Hippocampus
Abstract
:1. Introduction
2. Results
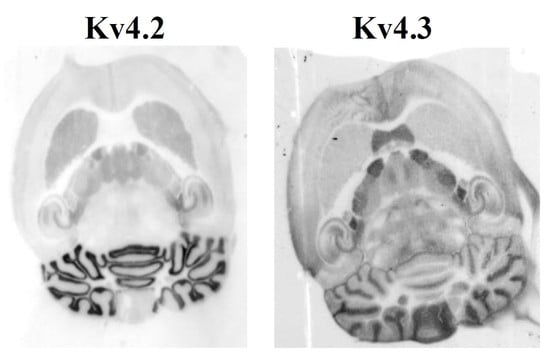
2.1. Differential Regional Expression of Kv4.2 and Kv4.3 Channels in the Adult Brain
2.2. Expression Patterns of Kv4.2 and Kv4.3 Channels during Postnatal Development
2.3. Distribution of Immunoreactivity for Kv4.2 and Kv4.3 as Detected by Light Microscopy
2.3.1. Immunoreactivity for Kv4.2.
2.3.2. Immunoreactivity for Kv4.3.
2.4. Distribution of Immunoreactivity for Kv4.2 and Kv4.3 as Detected by Electron Microscopy
2.4.1. Subcellular localisation of Kv4.2.
2.4.2. Subcellular localisation of Kv4.3.
2.5. Co-Localisation of Kv4.2 and Kv4.3 as Detected by Electron Microscopy
3. Discussion
3.1. Differential Expression of Kv4.2 and Kv4.3 Channels in the Adult and Developing Brain
3.2. Cellular and Subcellular Localisation of Kv4.2 and Kv4.3 Channels in the Hippocampus
3.3. Co-Localisation and Non-Uniform Distribution of Kv4.2 and Kv4.3 Channels in Granule Cells
4. Material and Methods
4.1. Animals
4.2. Antibodies and Chemicals
4.3. Histoblotting
4.4. Immunohistochemistry for Light Microscopy
4.5. Immunohistochemistry for Electron Microscopy
4.6. Quantification of Kv4 Channel Immunoreactivities
4.7. Controls
4.8. Data Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Anderson, P.; Morris, R.; Amaral, D.; Bliss, T.; O’Kefefe, J. The Hippocampus Book; Oxford University Press Inc.: New York, NY, USA, 2007. [Google Scholar]
- Poolos, N.P.; Johnston, D. Dendritic ion channelopathy in acquired epilepsy. Epilepsia 2012, 53 (Suppl. 9), 32–40. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.F.; Belrose, J.C.; Xie, Y.F.; Jackson, M.F. Nonselective cation channels and links to hippocampal ischemia, aging, and dementia. Adv. Exp. Med. Biol. 2013, 961, 433–447. [Google Scholar] [PubMed]
- Trimmer, J.S. Subcellular localization of K+ channels in mammalian brain neurons: Remarkable precision in the midst of extraordinary complexity. Neuron 2015, 85, 238–256. [Google Scholar] [CrossRef]
- Hille, B. Ionic Channels of Excitable Membranes; Sinauer Associates Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Birnbaum, S.G.; Varga, A.W.; Yuan, L.L.; Anderson, A.E.; Sweatt, J.D.; Schrader, L.A. Structure and function of Kv4-family transient potassium channels. Physiol. Rev. 2004, 84, 803–833. [Google Scholar] [CrossRef] [PubMed]
- Jerng, H.H.; Pfaffinger, P.J.; Covarrubias, M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol. Cell. Neurosci. 2004, 27, 343–369. [Google Scholar] [CrossRef] [PubMed]
- An, W.F.; Bowlby, M.R.; Betty, M.; Cao, J.; Ling, H.P.; Mendoza, G.; Hinson, J.W.; Mattsson, K.I.; Strassle, B.W.; Trimmer, J.S.; et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature 2000, 403, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Nadal, M.S.; Ozaita, A.; Amarillo, Y.; de Miera, E.V.; Ma, Y.; Mo, W.; Goldberg, E.M.; Misumi, Y.; Ikehara, Y.; Neubert, T.A.; et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 2003, 37, 449–461. [Google Scholar] [CrossRef]
- Holmqvist, M.H.; Cao, J.; Hernández-Pineda, R.; Jacobson, M.D.; Carroll, K.I.; Sung, M.A.; Betty, M.; Ge, P.; Gilbride, K.J.; Brown, M.E.; et al. Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain. Proc. Natl. Acad. Sci. USA 2002, 99, 1035–1040. [Google Scholar] [CrossRef] [Green Version]
- Buxbaum, J.D.; Choi, E.K.; Luo, Y.; Lilliehook, C.; Crowley, A.C.; Merriam, D.E.; Wasco, W. Calsenilin: A calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat. Med. 1998, 4, 1177–1181. [Google Scholar] [CrossRef]
- Morohashi, Y.; Hatano, N.; Ohya, S.; Takikawa, R.; Watabiki, T.; Takasugi, N.; Imaizumi, Y.; Tomita, T.; Iwatsubo, T. Molecular cloning and characterization of CALP/KChIP4, a novel EF-hand protein interacting with presenilin 2 and voltage-gated potassium channel subunit Kv4. J. Biol. Chem. 2002, 277, 14965–14975. [Google Scholar] [CrossRef]
- Pruunsild, P.; Timmusk, T. Structure, alternative splicing, and expression of the human and mouse KCNIP gene family. Genomics 2005, 86, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tsaur, M.L.; Jan, Y.N.; Jan, L.Y. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron 1992, 9, 271–284. [Google Scholar] [CrossRef]
- Serôdio, P.; Rudy, B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J. Neurophysiol. 1998, 79, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.W.; Anderson, A.E.; Adams, J.P.; Vogel, H.; Sweatt, J.D. Input specific immunolocalization of differentially phosphorylated Kv4.2 in the mouse brain. Learn. Mem. 2000, 7, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, K.J.; Carroll, K.I.; Sung, M.A.; Doliveira, L.C.; Monaghan, M.M.; Burke, S.L.; Strassle, B.W.; Buchwalder, L.; Menegola, M.; Cao, J.; et al. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J. Neurosci. 2004, 24, 7903–7915. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, L.L.; Zhao, C.; Birnbaum, S.G.; Frick, A.; Jung, W.E.; Schwarz, T.L.; Sweatt, J.D.; Johnston, D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J. Neurosci. 2006, 26, 12143–12151. [Google Scholar] [CrossRef]
- Tönnes, J.; Stierli, B.; Cerletti, C.; Behrmann, J.T.; Molnar, E.; Streit, P. Regional distribution and developmental changes of GluR1-flop protein revealed by monoclonal antibody in rat brain. J. Neurochem. 1999, 73, 2195–2205. [Google Scholar]
- Fernández-Alacid, L.; Watanabe, M.; Molnár, E.; Wickman, K.; Luján, R. Developmental regulation of G protein-gated inwardly-rectifying K+ (GIRK/Kir3) channel subunits in the brain. Eur. J. Neurosci. 2011, 34, 1724–1736. [Google Scholar] [CrossRef]
- Frick, A.; Johnston, D. Plasticity of dendritic excitability. J. Neurobiol. 2005, 64, 100–115. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, D.A.; Magee, J.C.; Colbert, C.M.; Johnston, D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 1997, 387, 869–875. [Google Scholar] [CrossRef]
- Mitterdorfer, J.; Bean, B.P. Potassium currents during the action potential of hippocampal CA3 neurons. J. Neurosci. 2002, 22, 10106–10115. [Google Scholar] [CrossRef]
- Cai, X.; Liang, C.W.; Muralidharan, S.; Kao, J.P.; Tang, C.M.; Thompson, S.M. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 2004, 44, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Magee, J.; Johnston, D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat. Neurosci. 2004, 7, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Tkatch, T.; Baranauskas, G.; Ichinohe, N.; Kitai, S.T.; Surmeier, D.J. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J. Neurosci. 1998, 18, 3124–3137. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Nakahira, K.; Shibasaki, K.; Wakazono, Y.; Imoto, K.; Ikenaka, K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J. Neurosci. 2000, 20, 4145–4155. [Google Scholar] [CrossRef] [PubMed]
- Luján, R. Organisation of potassium channels on the neuronal surface. J. Chem. Neuroanat. 2010, 40, 1–20. [Google Scholar] [CrossRef]
- Ballesteros-Merino, C.; Lin, M.; Wu, W.W.; Ferrandiz-Huertas, C.; Cabañero, M.J.; Watanabe, M.; Fukazawa, Y.; Shigemoto, R.; Maylie, J.; Adelman, J.P.; et al. Developmental profile of SK2 channel expression and function in CA1 neurons. Hippocampus 2012, 22, 1467–1480. [Google Scholar] [CrossRef]
- Ballesteros-Merino, C.; Watanabe, M.; Shigemoto, R.; Fukazawa, Y.; Adelman, J.P.; Luján, R. Differential subcellular localization of SK3-containing channels in the hippocampus. Eur. J. Neurosci. 2014, 39, 883–892. [Google Scholar] [CrossRef]
- Guan, D.; Horton, L.R.; Armstrong, W.E.; Foehring, R.C. Postnatal development of A-type and Kv1- and Kv2-mediated potassium channel currents in neocortical pyramidal neurons. J. Neurophysiol. 2011, 105, 2976–2988. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.H.; Huang, H.Y.; Tsaur, M.L. Contrasting expression of Kv4.3, an A-type K+ channel, in migrating Purkinje cells and other post-migratory cerebellar neurons. Eur. J. Neurosci. 2003, 18, 601–612. [Google Scholar] [CrossRef]
- Maletic-Savatic, M.; Lenn, N.J.; Trimmer, J.S. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro. J. Neurosci. 1995, 15, 3840–3851. [Google Scholar] [CrossRef] [PubMed]
- Jinno, S.; Jeromin, A.; Kosaka, T. Postsynaptic and extrasynaptic localization of Kv4.2 channels in the mouse hippocampal region, with special reference to targeted clustering at GABAergic synapses. Neuroscience 2005, 134, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.H.; Tamas, G.; Yuste, R. Ca2+ imaging of mouse neocortical interneurone dendrites: IA-type K+ channels control action potential backpropagation. J. Physiol. 2003, 551, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Losonczy, A.; Magee, J.C. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron 2006, 50, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau, M.L.; Morin, F.; Laurent, C.E.; Azzi, M.; Lacaille, J.C. Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. J. Neurosci. 2007, 27, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.; Widmer, H. Clustering of Kv4.2 potassium channels in postsynaptic membrane of rat supraoptic neurons: An ultrastructural study. Neuroscience 1997, 77, 617–621. [Google Scholar] [PubMed]
- Shibasaki, K.; Nakahira, K.; Trimmer, J.S.; Shibata, R.; Akita, M.; Watanabe, S.; Ikenaka, K. Mossy fibre contact triggers the targeting of Kv4.2 potassium channels to dendrites and synapses in developing cerebellar granule neurons. J. Neurochem. 2004, 89, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Burkhalter, A.; Gonchar, Y.; Mellor, R.L.; Nerbonne, J.M. Differential expression of IA channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J. Neurosci. 2006, 26, 12274–12282. [Google Scholar] [CrossRef]
- Kollo, M.; Holderith, N.B.; Nusser, Z. Novel subcellular distribution pattern of A-type K+ channels on neuronal surface. J. Neurosci. 2006, 26, 2684–2691. [Google Scholar] [CrossRef]
- Kollo, M.; Holderith, N.; Antal, M.; Nusser, Z. Unique clustering of A-type potassium channels on different cell types of the main olfactory bulb. Eur. J. Neurosci. 2008, 27, 1686–1699. [Google Scholar] [CrossRef]
- Peters, A.; Palay, S.L. The morphology of synapses. J. Neurocytol. 1996, 25, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Aguado, C.; García-Madrona, S.; Gil-Minguez, M.; Luján, R. Ontogenic Changes and Differential Localization of T-type Ca2+ Channel Subunits Cav3.1 and Cav3.2 in Mouse Hippocampus and Cerebellum. Front. Neuroanat. 2016, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Jerng, H.H.; Pfaffinger, P.J. Modulatory mechanisms and multiple functions of somatodendritic A-type K+ channel auxiliary subunits. Front. Cell. Neurosci. 2014, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Luján, R.; Nusser, Z.; Roberts, J.D.; Shigemoto, R.; Somogyi, P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996, 8, 1488–1500. [Google Scholar] [CrossRef] [PubMed]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro-Ruíz, R.; Aguado, C.; Martín-Belmonte, A.; Moreno-Martínez, A.E.; Luján, R. Expression, Cellular and Subcellular Localisation of Kv4.2 and Kv4.3 Channels in the Rodent Hippocampus. Int. J. Mol. Sci. 2019, 20, 246. https://doi.org/10.3390/ijms20020246
Alfaro-Ruíz R, Aguado C, Martín-Belmonte A, Moreno-Martínez AE, Luján R. Expression, Cellular and Subcellular Localisation of Kv4.2 and Kv4.3 Channels in the Rodent Hippocampus. International Journal of Molecular Sciences. 2019; 20(2):246. https://doi.org/10.3390/ijms20020246
Chicago/Turabian StyleAlfaro-Ruíz, Rocío, Carolina Aguado, Alejandro Martín-Belmonte, Ana Esther Moreno-Martínez, and Rafael Luján. 2019. "Expression, Cellular and Subcellular Localisation of Kv4.2 and Kv4.3 Channels in the Rodent Hippocampus" International Journal of Molecular Sciences 20, no. 2: 246. https://doi.org/10.3390/ijms20020246
APA StyleAlfaro-Ruíz, R., Aguado, C., Martín-Belmonte, A., Moreno-Martínez, A. E., & Luján, R. (2019). Expression, Cellular and Subcellular Localisation of Kv4.2 and Kv4.3 Channels in the Rodent Hippocampus. International Journal of Molecular Sciences, 20(2), 246. https://doi.org/10.3390/ijms20020246