SLC35A5 Protein—A Golgi Complex Member with Putative Nucleotide Sugar Transport Activity
Abstract
1. Introduction
2. Results
2.1. Inactivation of the SLC35A5 Gene
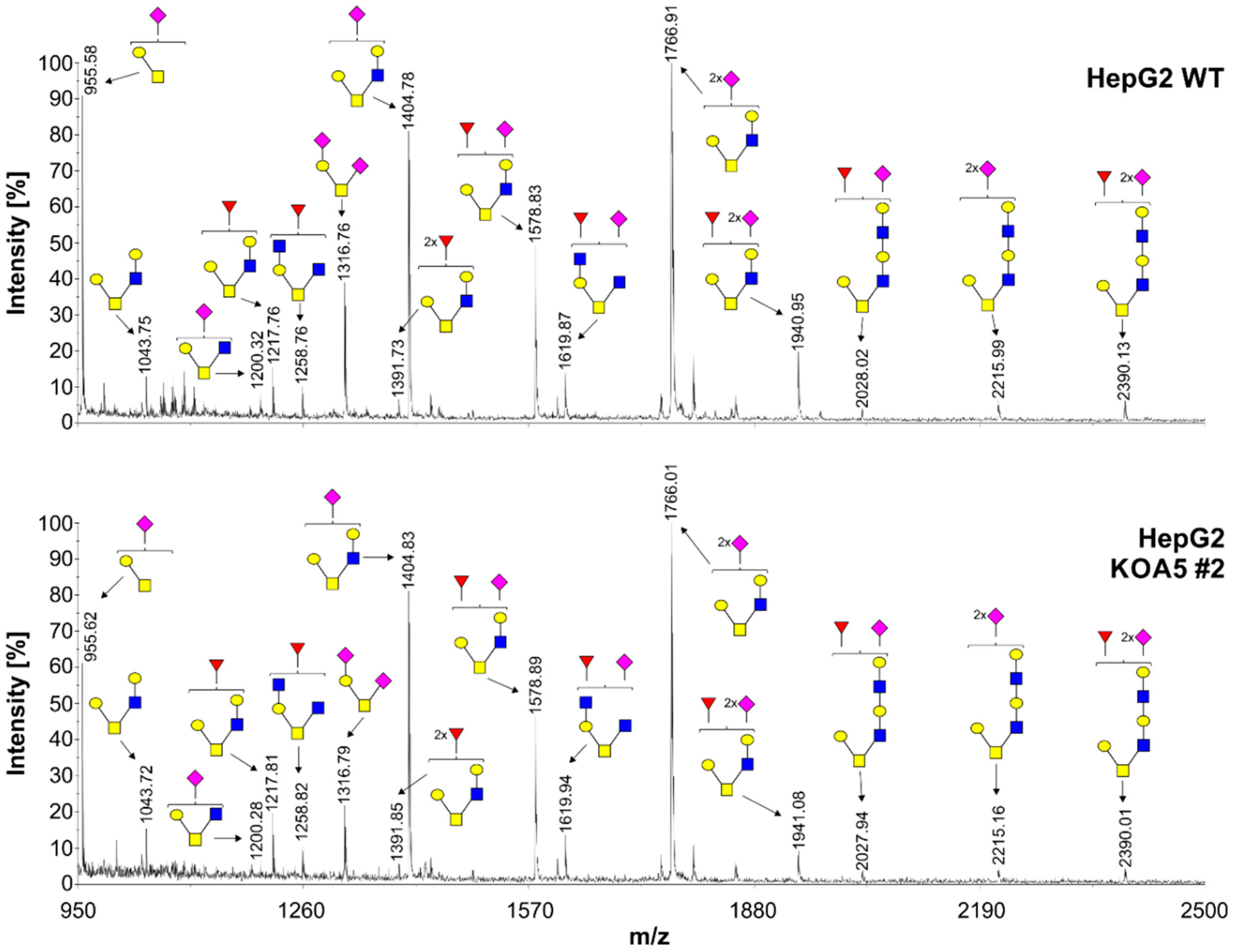
2.2. Glycosylation Analysis
2.3. UDP-Sugar Transport Assay
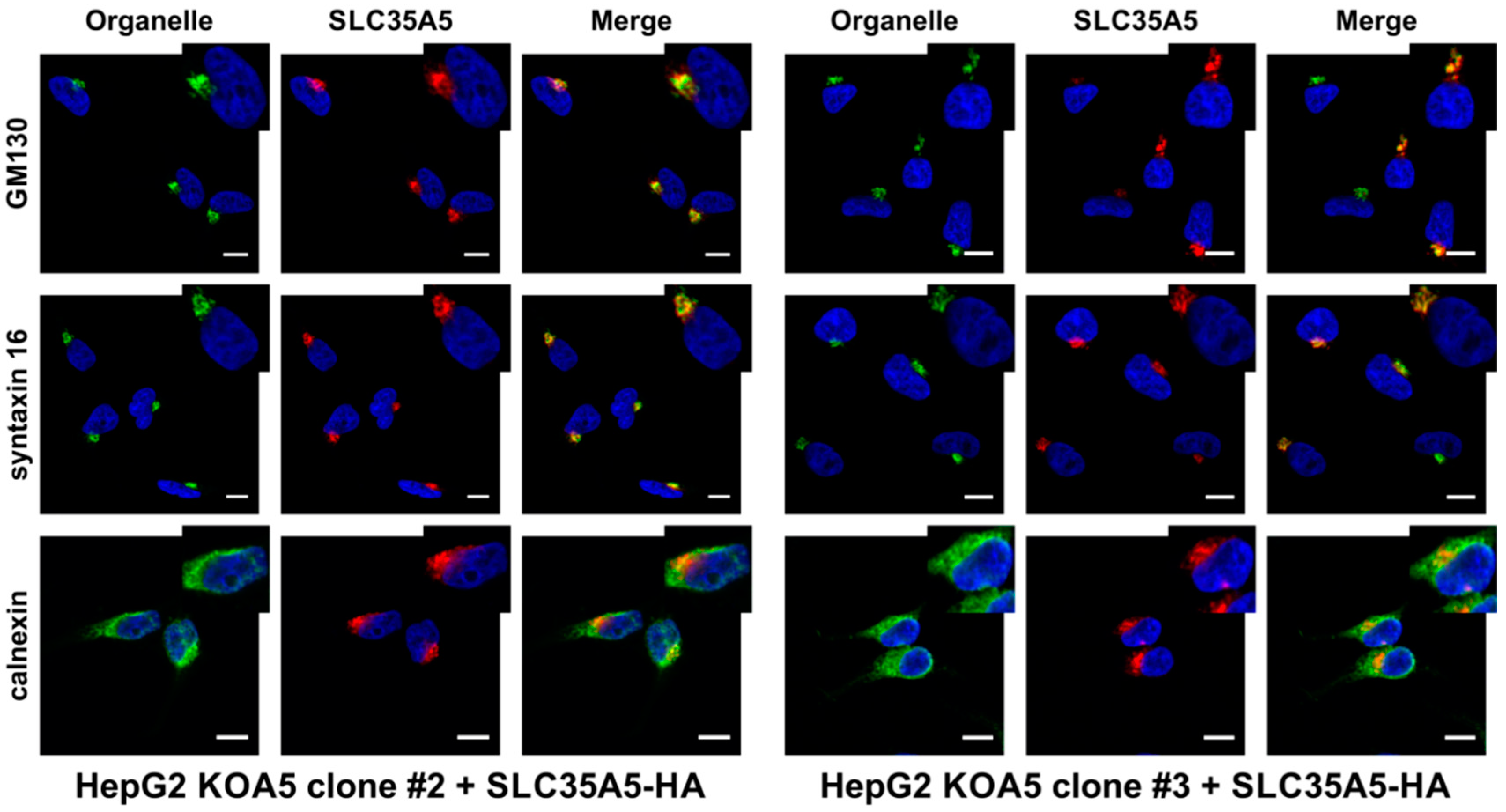
2.4. SLC35A5 Subcellular Localization
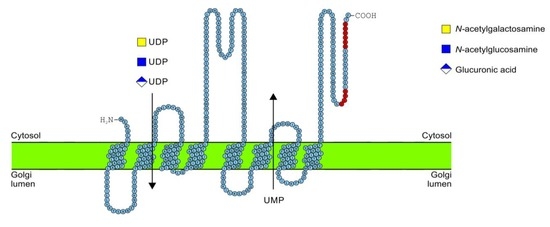
2.5. C-Terminus Topology
2.6. Fluorescence Lifetime Imaging Microscopy (FLIM)/Förster Resonance Energy Transfer (FRET) Interaction Analysis
3. Discussion
4. Materials and Methods
4.1. Construction of Plasmids
4.2. Cell Maintenance and Transfection
4.3. Evaluation of the SLC35A5 Knock-Out Efficiency
4.4. Analysis of Glycoproteins with Lectins
4.5. Analysis of Fluorescently Labeled N-Glycans
4.6. Analysis of Fluorescently Labeled O-Glycans
4.7. Isolation and Separation of Glycolipids
4.8. Subcellular Fractionation and Nucleotide Sugar Transport Assay
4.9. Western Blotting
4.10. Immunofluorescence
4.11. SLC35A5 C-Terminus Localization
4.12. Confocal and Fluorescence Lifetime Imaging Microscopy (FLIM)
4.13. Bioinformatics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ER | endoplasmic reticulum |
| NST | nucleotide sugar transporter |
| SLC | solute carrier |
| FLIM | fluorescence lifetime imaging microscopy |
| FRET | Förster resonance energy transfer |
| eGFP | enhanced green fluorescent protein |
| mRFP | monomeric red fluorescent protein |
| Mgat1 | mannosyl (α-1,3-)-glycoprotein β-1,2-N-acetylglucosaminyltransferase |
References
- Fredriksson, R.; Nordström, K.J.V.; Stephansson, O.; Hägglund, M.G.A.; Schiöth, H.B. The solute carrier (SLC) complement of the human genome: Phylogenetic classification reveals four major families. FEBS Lett. 2008, 582, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Song, Z. Roles of the nucleotide sugar transporters (SLC35 family) in health and disease. Mol. Asp. Med. 2013, 34, 590–600. [Google Scholar] [CrossRef]
- Fusella, A.; Micaroni, M.; Di Giandomenico, D.; Mironov, A.A.; Beznoussenko, G.V. Segregation of the Qb-SNAREs GS27 and GS28 into Golgi vesicles regulates intra-Golgi transport. Traffic 2013, 14, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Tie, H.C.; Ludwig, A.; Sandin, S.; Lu, L. The spatial separation of processing and transport functions to the interior and periphery of the Golgi stack. eLife 2018, 7, e41301. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Wang, Y.; Yao, Y.; Zhang, Z.; Harper, D.C.; Heijnen, H.F.G.; Sitaram, A.; Li, W.; Raposo, G.; Weiss, M.J.; et al. SLC35D3 delivery from megakaryocyte early endosomes is required for platelet dense granule biogenesis and is differentially defective in Hermansky-Pudlak syndrome models. Blood 2012, 120, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Sosicka, P.; Maszczak-Seneczko, D.; Bazan, B.; Shauchuk, Y.; Kaczmarek, B.; Olczak, M. An insight into the orphan nucleotide sugar transporter SLC35A4. Biochim. Biophys. Acta 2017, 1864, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hao, C.-J.; Li, C.-G.; Zang, D.-J.; Zhao, J.; Li, X.-N.; Wei, A.-H.; Wei, Z.-B.; Yang, L.; He, X.; et al. Mutation of SLC35D3 causes metabolic syndrome by impairing dopamine signaling in striatal D1 neurons. PLoS Genet. 2014, 10, e1004124. [Google Scholar] [CrossRef]
- Eckhardt, M.; Gotza, B.; Gerardy-Schahn, R. Membrane topology of the mammalian CMP-sialic acid transporter. J. Biol. Chem. 1999, 274, 8779–8787. [Google Scholar] [CrossRef]
- Brandan, E.; Fleischer, B. Orientation and role of nucleosidediphosphatase and 5′-nucleotidase in Golgi vesicles from rat liver. Biochemistry 1982, 21, 4640–4645. [Google Scholar] [CrossRef]
- Capasso, J.M.; Hirschberg, C.B. Mechanisms of glycosylation and sulfation in the Golgi apparatus: Evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc. Natl. Acad. Sci. USA 1984, 81, 7051–7055. [Google Scholar] [CrossRef]
- Kuhn, N.J.; White, A. Evidence for specific transport of uridine diphosphate galactose across the Golgi membrane of rat mammary gland. Biochem. J. 1976, 154, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Waldman, B.C.; Rudnick, G. UDP-GlcNAc transport across the Golgi membrane: Electroneutral exchange for dianionic UMP. Biochemistry 1990, 29, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Avalos, M.D.; Uccelletti, D.; Abeijon, C.; Hirschberg, C.B. The UDPase activity of the Kluyveromyces lactis Golgi GDPase has a role in uridine nucleotide sugar transport into Golgi vesicles. Glycobiology 2001, 11, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Briles, E.B.; Li, E.; Kornfeld, S. Isolation of wheat germ agglutinin-resistant clones of Chinese hamster ovary cells deficient in membrane sialic acid and galactose. J. Biol. Chem. 1977, 252, 1107–1116. [Google Scholar] [PubMed]
- Stanley, P.; Siminovitch, L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977, 3, 391–405. [Google Scholar] [CrossRef]
- Deutscher, S.L.; Nuwayhid, N.; Stanley, P.; Briles, E.I.; Hirschberg, C.B. Translocation across Golgi vesicle membranes: A CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 1984, 39, 295–299. [Google Scholar] [CrossRef]
- Brändli, A.W.; Hansson, G.C.; Rodriguez-Boulan, E.; Simons, K. A polarized epithelial cell mutant deficient in translocation of UDP-galactose into the Golgi complex. J. Biol. Chem. 1988, 263, 16283–16290. [Google Scholar]
- Ishida, N.; Yoshioka, S.; Iida, M.; Sudo, K.; Miura, N.; Aoki, K.; Kawakita, M. Indispensability of transmembrane domains of Golgi UDP-galactose transporter as revealed by analysis of genetic defects in UDP-galactose transporter-deficient murine had-1 mutant cell lines and construction of deletion mutants. J. Biochem. 1999, 126, 1107–1117. [Google Scholar] [CrossRef]
- Oelmann, S.; Stanley, P.; Gerardy-Schahn, R. Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J. Biol. Chem. 2001, 276, 26291–26300. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Olczak, T.; Jakimowicz, P.; Olczak, M. Overexpression of UDP-GlcNAc transporter partially corrects galactosylation defect caused by UDP-Gal transporter mutation. FEBS Lett. 2011, 585, 3090–3094. [Google Scholar] [CrossRef] [PubMed]
- Abeijon, C.; Robbins, P.W.; Hirschberg, C.B. Molecular cloning of the Golgi apparatus uridine diphosphate-N-acetylglucosamine transporter from Kluyveromyces lactis. Proc. Natl. Acad. Sci. USA 1996, 93, 5963–5968. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Sosicka, P.; Olczak, T.; Jakimowicz, P.; Majkowski, M.; Olczak, M. UDP-N-acetylglucosamine transporter (SLC35A3) regulates biosynthesis of highly branched N-glycans and keratan sulfate. J. Biol. Chem. 2013, 288, 21850–21860. [Google Scholar] [CrossRef] [PubMed]
- Lühn, K.; Wild, M.K.; Eckhardt, M.; Gerardy-Schahn, R.; Vestweber, D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat. Genet. 2001, 28, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, S.; Ichimiya, T.; Ikehara, Y.; Takase, T.; Fujimoto, I.; Suda, T.; Nakamori, S.; Nakamura, M.; Nakayama, F.; Irimura, T.; et al. Expression and the role of 3′-phosphoadenosine 5′-phosphosulfate transporters in human colorectal carcinoma. Glycobiology 2011, 21, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, S.; Sasaki, N.; Goda, E.; Ui-Tei, K.; Saigo, K.; Narimatsu, H.; Jigami, Y.; Kannagi, R.; Irimura, T.; Nishihara, S. Molecular cloning and characterization of a novel 3′-phosphoadenosine 5′-phosphosulfate transporter, PAPST2. J. Biol. Chem. 2006, 281, 10945–10953. [Google Scholar] [CrossRef]
- Kamiyama, S.; Suda, T.; Ueda, R.; Suzuki, M.; Okubo, R.; Kikuchi, N.; Chiba, Y.; Goto, S.; Toyoda, H.; Saigo, K.; et al. Molecular cloning and identification of 3′-phosphoadenosine 5′-phosphosulfate transporter. J. Biol. Chem. 2003, 278, 25958–25963. [Google Scholar] [CrossRef]
- Ashikov, A.; Routier, F.; Fuhlrott, J.; Helmus, Y.; Wild, M.; Gerardy-Schahn, R.; Bakker, H. The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J. Biol. Chem. 2005, 280, 27230–27235. [Google Scholar] [CrossRef]
- Muraoka, M.; Kawakita, M.; Ishida, N. Molecular characterization of human UDP-glucuronic acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide sugar transporter with dual substrate specificity. FEBS Lett. 2001, 495, 87–93. [Google Scholar] [CrossRef]
- Ishida, N.; Kuba, T.; Aoki, K.; Miyatake, S.; Kawakita, M.; Sanai, Y. Identification and characterization of human Golgi nucleotide sugar transporter SLC35D2, a novel member of the SLC35 nucleotide sugar transporter family. Genomics 2005, 85, 106–116. [Google Scholar] [CrossRef]
- Lu, L.; Hou, X.; Shi, S.; Körner, C.; Stanley, P. Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells. J. Biol. Chem. 2010, 285, 36245–36254. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Zhou, M.; Li, X.; Chen, W.; Wang, Y.; Gu, S.; Cao, Y.; Chu, C.; Liu, X.; et al. Solute carrier family 35 member F2 is indispensable for papillary thyroid carcinoma progression through activation of transforming growth factor-β type I receptor/apoptosis signal-regulating kinase 1/mitogen-activated protein kinase signaling axis. Cancer Sci. 2018, 109, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, N.; Furukawa, J.; Araki, K.; Fujioka, T.; Takegawa, Y.; Piao, J.; Nishioka, T.; Tamura, T.; Nikaido, T.; Ito, M.; et al. Total cellular glycomics allows characterizing cells and streamlining the discovery process for cellular biomarkers. Proc. Natl. Acad. Sci. USA 2013, 110, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Hadley, B.; Maggioni, A.; Ashikov, A.; Day, C.J.; Haselhorst, T.; Tiralongo, J. Structure and function of nucleotide sugar transporters: Current progress. Comput. Struct. Biotechnol. J. 2014, 10, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Wainwright, L.J. Molecular cloning of eukaryotic glycoprotein and glycolipid glycosyltransferases: A survey. Glycobiology 1995, 5, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Hashimoto, H.; Yoda, K. Molecular characterization of Vig4/Vrg4 GDP-mannose transporter of the yeast Saccharomyces cerevisiae. FEBS Lett. 1999, 458, 309–312. [Google Scholar] [CrossRef]
- Hong, K.; Ma, D.; Beverley, S.M.; Turco, S.J. The Leishmania GDP-mannose transporter is an autonomous, multi-specific, hexameric complex of LPG2 subunits. Biochemistry 2000, 39, 2013–2022. [Google Scholar] [CrossRef]
- Maszczak-Seneczko, D.; Sosicka, P.; Majkowski, M.; Olczak, T.; Olczak, M. UDP-N-acetylglucosamine transporter and UDP-galactose transporter form heterologous complexes in the Golgi membrane. FEBS Lett. 2012, 586, 4082–4087. [Google Scholar] [CrossRef]
- Olczak, M.; Guillen, E. Characterization of a mutation and an alternative splicing of UDP-galactose transporter in MDCK-RCAr cell line. Biochim. Biophys. Acta 2006, 1763, 82–92. [Google Scholar] [CrossRef]
- Parker, J.L.; Newstead, S. Structural basis of nucleotide sugar transport across the Golgi membrane. Nature 2017, 551, 521–524. [Google Scholar] [CrossRef]
- Puglielli, L.; Hirschberg, C.B. Reconstitution, identification, and purification of the rat liver golgi membrane GDP-fucose transporter. J. Biol. Chem. 1999, 274, 35596–35600. [Google Scholar] [CrossRef]
- Puglielli, L.; Mandon, E.C.; Rancour, D.M.; Menon, A.K.; Hirschberg, C.B. Identification and purification of the rat liver Golgi membrane UDP-N-acetylgalactosamine transporter. J. Biol. Chem. 1999, 274, 4474–4479. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Sosicka, P.; Kaczmarek, B.; Majkowski, M.; Luzarowski, M.; Olczak, T.; Olczak, M. UDP-galactose (SLC35A2) and UDP-N-acetylglucosamine (SLC35A3) Transporters Form Glycosylation-related Complexes with Mannoside Acetylglucosaminyltransferases (Mgats). J. Biol. Chem. 2015, 290, 15475–15486. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Olczak, T.; Olczak, M. Subcellular localization of UDP-GlcNAc, UDP-Gal and SLC35B4 transporters. Acta Biochim. Pol. 2011, 58, 413–419. [Google Scholar] [PubMed]
- Bossuyt, X.; Blanckaert, N. Carrier-mediated transport of intact UDP-glucuronic acid into the lumen of endoplasmic-reticulum-derived vesicles from rat liver. Biochem. J. 1994, 302 Pt 1, 261–269. [Google Scholar] [CrossRef]
- Häcker, U.; Nybakken, K.; Perrimon, N. Heparan sulphate proteoglycans: The sweet side of development. Nat. Rev. Mol. Cell Biol. 2005, 6, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Baasanjav, S.; Al-Gazali, L.; Hashiguchi, T.; Mizumoto, S.; Fischer, B.; Horn, D.; Seelow, D.; Ali, B.R.; Aziz, S.A.A.; Langer, R.; et al. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am. J. Hum. Genet. 2011, 89, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Sleeman, J.E.; Coughtrie, M.W.H.; Burchell, B. Molecular and functional characterization of microsomal UDP-glucuronic acid uptake by members of the nucleotide sugar transporter (NST) family. Biochem. J. 2006, 400, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kabuss, R.; Ashikov, A.; Oelmann, S.; Gerardy-Schahn, R.; Bakker, H. Endoplasmic reticulum retention of the large splice variant of the UDP-galactose transporter is caused by a dilysine motif. Glycobiology 2005, 15, 905–911. [Google Scholar] [CrossRef]
- Esther, C.R.; Sesma, J.I.; Dohlman, H.G.; Ault, A.D.; Clas, M.L.; Lazarowski, E.R.; Boucher, R.C. Similarities between UDP-glucose and adenine nucleotide release in yeast: Involvement of the secretory pathway. Biochemistry 2008, 47, 9269–9278. [Google Scholar] [CrossRef]
- Dörre, K.; Olczak, M.; Wada, Y.; Sosicka, P.; Grüneberg, M.; Reunert, J.; Kurlemann, G.; Fiedler, B.; Biskup, S.; Hörtnagel, K.; et al. A new case of UDP-galactose transporter deficiency (SLC35A2-CDG): Molecular basis, clinical phenotype, and therapeutic approach. J. Inherit. Metab. Dis. 2015, 38, 931–940. [Google Scholar] [CrossRef]
- Bazan, B.; Wiktor, M.; Maszczak-Seneczko, D.; Olczak, T.; Kaczmarek, B.; Olczak, M. Lysine at position 329 within a C-terminal dilysine motif is crucial for the ER localization of human SLC35B4. PLoS ONE 2018, 13, e0207521. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Mühlenhoff, M.; Bethe, A.; Gerardy-Schahn, R. Expression cloning of the Golgi CMP-sialic acid transporter. Proc. Natl. Acad. Sci. USA 1996, 93, 7572–7576. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Márquez, J.; Reguillo, B.; Paniagua, R. Cloning of the cDNA and mRNA expression of CLRP, a complex leucine repeat protein of the Golgi apparatus expressed by specific neurons of the rat brain. J. Neurobiol. 2002, 52, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Segawa, H.; Kawakita, M.; Ishida, N. Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur. J. Biochem. 2002, 269, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Hofmann, F.; Selzer, J.; Munro, S.; Jeckel, D.; Aktories, K. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J. Biol. Chem. 1998, 273, 19566–19572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-M.; Hsu, C.-H.; Wang, C.-H.; Liu, W.; Chang, W.-H.; Lin, C.-S. Role of the DxxDxD motif in the assembly and stability of betanodavirus particles. Arch. Virol. 2008, 153, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Hanton, S.L.; Renna, L.; Bortolotti, L.E.; Chatre, L.; Stefano, G.; Brandizzi, F. Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. Plant Cell 2005, 17, 3081–3093. [Google Scholar] [CrossRef]
- Sevier, C.S.; Weisz, O.A.; Davis, M.; Machamer, C.E. Efficient export of the vesicular stomatitis virus G protein from the endoplasmic reticulum requires a signal in the cytoplasmic tail that includes both tyrosine-based and di-acidic motifs. Mol. Biol. Cell 2000, 11, 13–22. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, C.; Wu, Q.J.; Balch, W.E.; Wu, G. Di-acidic motifs in the membrane-distal C termini modulate the transport of angiotensin II receptors from the endoplasmic reticulum to the cell surface. J. Biol. Chem. 2011, 286, 20525–20535. [Google Scholar] [CrossRef]
- Votsmeier, C.; Gallwitz, D. An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export. EMBO J. 2001, 20, 6742–6750. [Google Scholar] [CrossRef]
- Behnia, R.; Panic, B.; Whyte, J.R.C.; Munro, S. Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p. Nat. Cell Biol. 2004, 6, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; Gougeon, P.Y.; Prosser, D.C.; Da-Silva, L.F.; Ngsee, J.K. PRA isoforms are targeted to distinct membrane compartments. J. Biol. Chem. 2001, 276, 6225–6233. [Google Scholar] [CrossRef] [PubMed]
- Sosicka, P.; Jakimowicz, P.; Olczak, T.; Olczak, M. Short N-terminal region of UDP-galactose transporter (SLC35A2) is crucial for galactosylation of N-glycans. Biochem. Biophys. Res. Commun. 2014, 454, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kudelka, M.R.; Antonopoulos, A.; Wang, Y.; Duong, D.M.; Song, X.; Seyfried, N.T.; Dell, A.; Haslam, S.M.; Cummings, R.D.; Ju, T. Cellular O-Glycome Reporter/Amplification to explore O-glycans of living cells. Nat. Methods 2016, 13, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Puglielli, L.; Tonetti, M.; Berninsone, P.; Hirschberg, C.B.; De Flora, A.; Etzioni, A. Impairment of the Golgi GDP-l-fucose transport and unresponsiveness to fucose replacement therapy in LAD II patients. Pediatr. Res. 2001, 49, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosicka, P.; Bazan, B.; Maszczak-Seneczko, D.; Shauchuk, Y.; Olczak, T.; Olczak, M. SLC35A5 Protein—A Golgi Complex Member with Putative Nucleotide Sugar Transport Activity. Int. J. Mol. Sci. 2019, 20, 276. https://doi.org/10.3390/ijms20020276
Sosicka P, Bazan B, Maszczak-Seneczko D, Shauchuk Y, Olczak T, Olczak M. SLC35A5 Protein—A Golgi Complex Member with Putative Nucleotide Sugar Transport Activity. International Journal of Molecular Sciences. 2019; 20(2):276. https://doi.org/10.3390/ijms20020276
Chicago/Turabian StyleSosicka, Paulina, Bożena Bazan, Dorota Maszczak-Seneczko, Yauhen Shauchuk, Teresa Olczak, and Mariusz Olczak. 2019. "SLC35A5 Protein—A Golgi Complex Member with Putative Nucleotide Sugar Transport Activity" International Journal of Molecular Sciences 20, no. 2: 276. https://doi.org/10.3390/ijms20020276
APA StyleSosicka, P., Bazan, B., Maszczak-Seneczko, D., Shauchuk, Y., Olczak, T., & Olczak, M. (2019). SLC35A5 Protein—A Golgi Complex Member with Putative Nucleotide Sugar Transport Activity. International Journal of Molecular Sciences, 20(2), 276. https://doi.org/10.3390/ijms20020276