Functionalized ?-Cyclodextrin Immobilized on Ag-Embedded Silica Nanoparticles as a Drug Carrier
Abstract
:1. Introduction
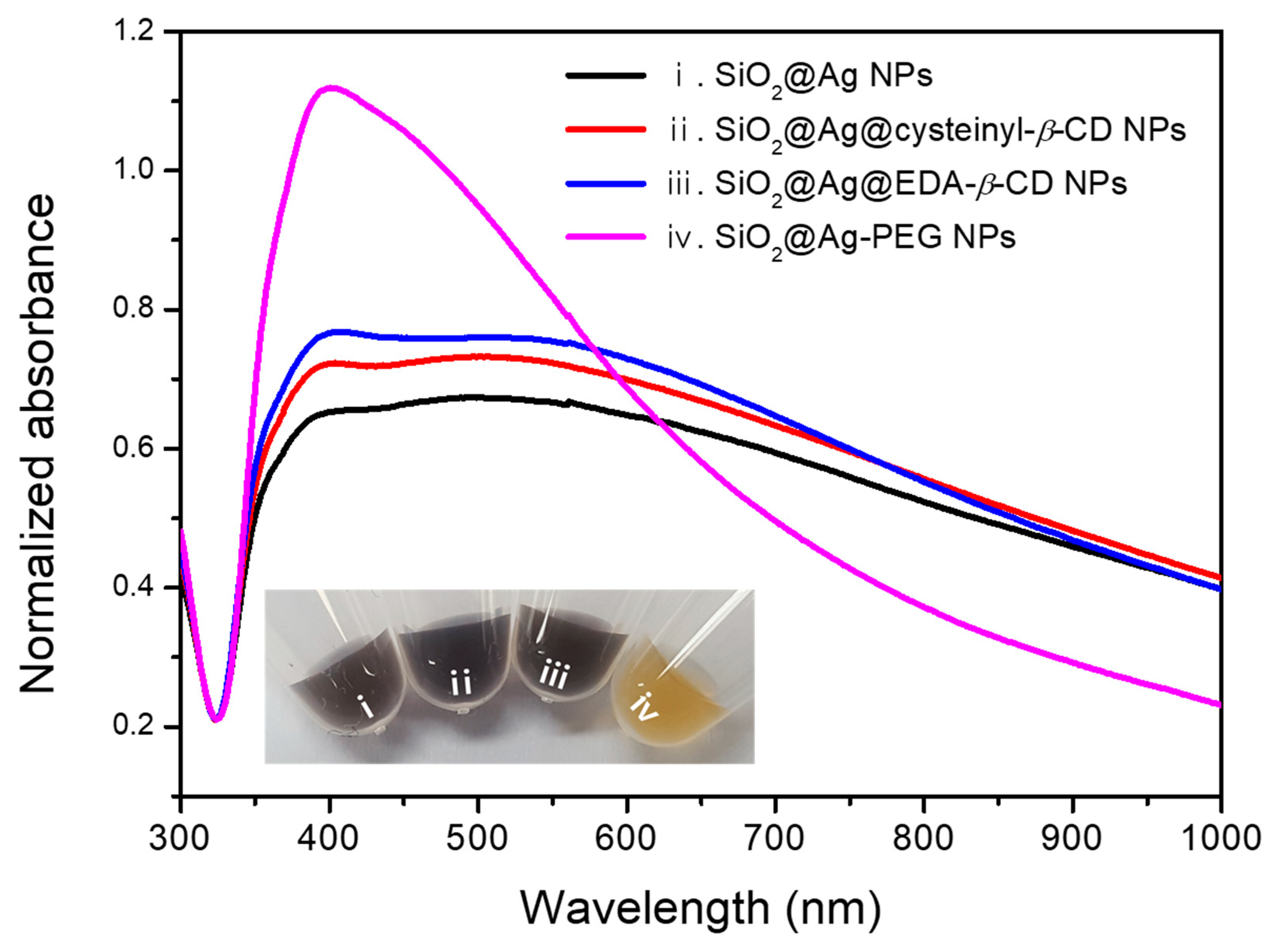
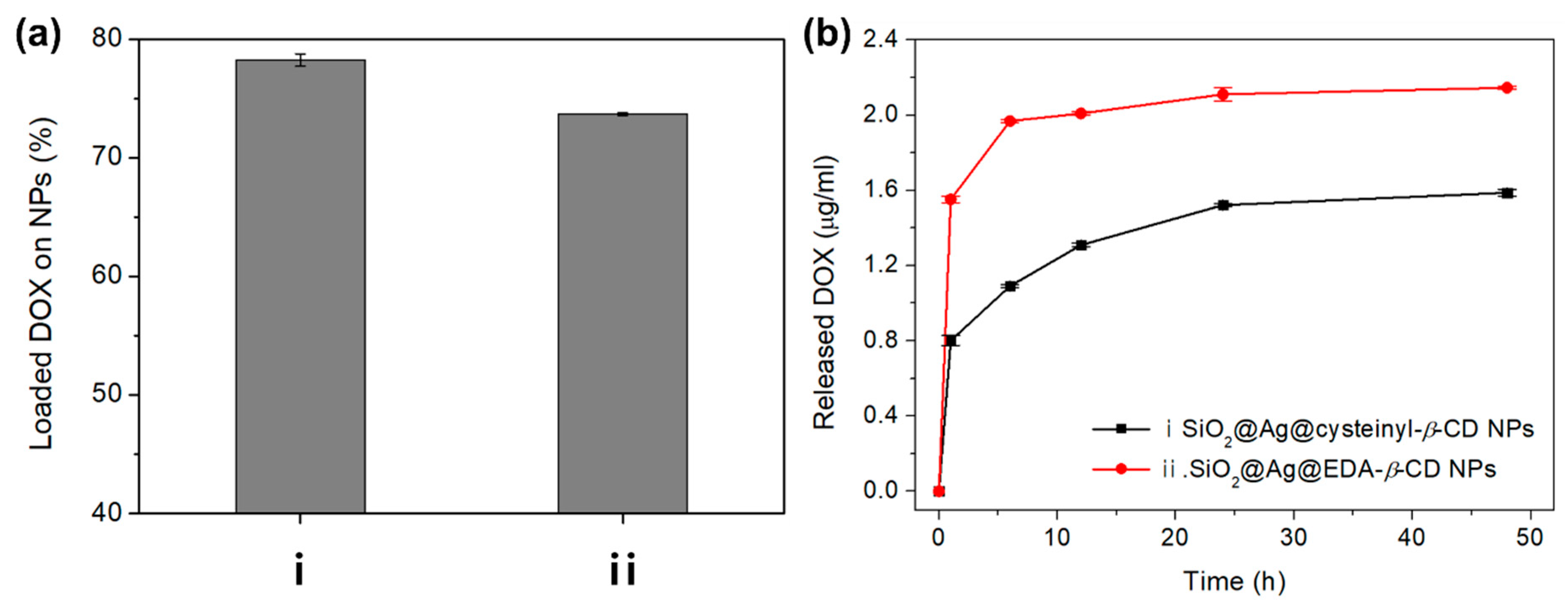
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of SiO2@Ag NPs
3.3. Preparation of SiO2@Ag@cysteinyl-β-CD NPs, SiO2@Ag@EDA-β-CD NPs, and SiO2@Ag-PEG NPs
3.4. Loading of DOX on SiO2@Ag NPs with Ligands (β-CD Derivatives and PEG)
3.5. DOX Release
3.6. Cell Culture and Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of nanoparticle-based carriers for targeted drug delivery. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug. Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gatón, L.; Espuelas, S.; Larrañeta, E.; Reviakine, I.; Yate, L.A.; Irache, J.M. Pegylated poly (anhydride) nanoparticles for oral delivery of docetaxel. Eur. J. Pharm. Sci. 2018, 118, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Van De Manakker, F.; Vermonden, T.; Van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-based polymeric materials: Synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Givord, C.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Elaboration of a new antibacterial bio-nano-material for food-packaging by synergistic action of cyclodextrin and microfibrillated cellulose. Innov. Food Sci. Emerg. 2014, 26, 330–340. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. B-cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloid Surf. B-Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Huarte, J.; Espuelas, S.; Lai, Y.; He, B.; Tang, J.; Irache, J.M. Oral delivery of camptothecin using cyclodextrin/poly (anhydride) nanoparticles. Int. J. Pharm. 2016, 506, 116–128. [Google Scholar] [CrossRef]
- Calleja, P.; Espuelas, S.; Corrales, L.; Pio, R.; Irache, J.M. Pharmacokinetics and antitumor efficacy of paclitaxel–cyclodextrin complexes loaded in mucus-penetrating nanoparticles for oral administration. Nanomedicine 2014, 9, 2109–2121. [Google Scholar] [CrossRef] [Green Version]
- Bekers, O.; Kettenes, J.J.V.D.B.; Van Helden, S.P.; Seijkens, D.; Beijnen, J.H.; Bulti, A.; Underberg, W.J. Inclusion complex formation of anthracycline antibiotics with cyclodextrins; a proton nuclear magnetic resonance and molecular modelling study. J. Inclus. Phenom. Mol. 1991, 11, 185–193. [Google Scholar] [CrossRef]
- Swiech, O.; Mieczkowska, A.; Chmurski, K.; Bilewicz, R. Intermolecular interactions between doxorubicin and β-cyclodextrin 4-methoxyphenol conjugates. J. Phys. Chem. B 2012, 116, 1765–1771. [Google Scholar] [CrossRef]
- Anand, R.; Manoli, F.; Manet, I.; Daoud-Mahammed, S.; Agostoni, V.; Gref, R.; Monti, S. β-cyclodextrin polymer nanoparticles as carriers for doxorubicin and artemisinin: A spectroscopic and photophysical study. Photochem. Photobiol. Sci. 2012, 11, 1285–1292. [Google Scholar] [CrossRef]
- Yousef, T.; Hassan, N. Supramolecular encapsulation of doxorubicin with β-cyclodextrin dendrimer: In vitro evaluation of controlled release and cytotoxicity. J. Incl. Phenom. Macrocycl. Chem. 2017, 87, 105–115. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, S.; Park, Y.; Yim, J.; Jun, B.H.; Kyeong, S.; Yang, J.K.; Kim, G.; Hong, S.; Lee, L.P. Near-infrared sers nanoprobes with plasmonic au/ag hollow-shell assemblies for in vivo multiplex detection. Adv. Funct. Mater. 2013, 23, 3719–3727. [Google Scholar] [CrossRef]
- Noh, M.S.; Lee, S.; Kang, H.; Yang, J.-K.; Lee, H.; Hwang, D.; Lee, J.W.; Jeong, S.; Jang, Y.; Jun, B.-H. Target-specific near-ir induced drug release and photothermal therapy with accumulated au/ag hollow nanoshells on pulmonary cancer cell membranes. Biomaterials 2015, 45, 81–92. [Google Scholar] [CrossRef]
- Jun, B.H.; Kim, G.; Jeong, S.; Noh, M.S.; Pham, X.H.; Kang, H.; Cho, M.H.; Kim, J.H.; Lee, Y.S.; Jeong, D.H. Silica core-based surface-enhanced raman scattering (sers) tag: Advances in multifunctional sers nanoprobes for bioimaging and targeting of biomarkers#. Bull. Korean Chem. Soc. 2015, 36, 963–978. [Google Scholar]
- Chang, H.; Kang, H.; Ko, E.; Jun, B.-H.; Lee, H.-Y.; Lee, Y.-S.; Jeong, D.H. Psa detection with femtomolar sensitivity and a broad dynamic range using sers nanoprobes and an area-scanning method. ACS Sens. 2016, 1, 645–649. [Google Scholar] [CrossRef]
- Noh, M.S.; Jun, B.-H.; Kim, S.; Kang, H.; Woo, M.-A.; Minai-Tehrani, A.; Kim, J.-E.; Kim, J.; Park, J.; Lim, H.-T. Magnetic surface-enhanced raman spectroscopic (m-sers) dots for the identification of bronchioalveolar stem cells in normal and lung cancer mice. Biomaterials 2009, 30, 3915–3925. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, J.-S.; Choi, H.; Lee, S.-M.; Jun, B.-H.; Yu, K.-N.; Kuk, E.; Kim, Y.-K.; Jeong, D.H.; Cho, M.-H. Nanoparticle probes with surface enhanced raman spectroscopic tags for cellular cancer targeting. Anal. Chem. 2006, 78, 6967–6973. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.H.; Noh, M.S.; Kim, J.; Kim, G.; Kang, H.; Kim, M.S.; Seo, Y.T.; Baek, J.; Kim, J.H.; Park, J. Multifunctional silver-embedded magnetic nanoparticles as sers nanoprobes and their applications. Small 2010, 6, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.G.; Kim, H.-M.; Kang, Y.-L.; Lee, M.; Kang, H.; Kim, J.; Pham, X.-H.; Kim, T.H.; Hahm, E.; Lee, Y.-S. Thin silica shell coated ag assembled nanostructures for expanding generality of sers analytes. PLoS ONE 2017, 12, e0178651. [Google Scholar] [CrossRef] [PubMed]
- Ueno, R.; Kim, B. Reliable transfer technique of gold micro heater through different affinities of thiol (sh) and amine (nh2) groups. Microelectron. Eng. 2017, 171, 6–10. [Google Scholar] [CrossRef]
- Hahm, E.; Jeong, D.; Cha, M.G.; Choi, J.M.; Pham, X.-H.; Kim, H.-M.; Kim, H.; Lee, Y.-S.; Jeong, D.H.; Jung, S. β-cd dimer-immobilized ag assembly embedded silica nanoparticles for sensitive detection of polycyclic aromatic hydrocarbons. Sci. Rep. 2016, 6, 26082. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Hahm, E.; Park, K.; Jeong, D.; Rho, W.-Y.; Kim, J.; Jeong, D.H.; Lee, Y.-S.; Jhang, S.H.; Chung, H.J. Sers-based flavonoid detection using ethylenediamine-β-cyclodextrin as a capturing ligand. Nanomaterials 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Yiluo, H.; Park, S.; Lee, J.Y.; Cho, E.; Jung, S. Characterization and enhanced antioxidant activity of the cysteinyl β-cyclodextrin-baicalein inclusion complex. Molecules 2016, 21, 703. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Liu, J.; Xu, Q.; Xiao, H.; Wang, X.; Xu, H.; Zhou, J. Thiol modified fe3o4@ sio2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem. Eng. J. 2013, 226, 30–38. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Qian, Y.; Hu, X.; Liu, S. Multifunctional ph-disintegrable micellar nanoparticles of asymmetrically functionalized β-cyclodextrin-based star copolymer covalently conjugated with doxorubicin and dota-gd moieties. Biomaterials 2012, 33, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S.; Al-Jamal, W.T.; Bossche, J.V.; Bui, T.T.; Drake, A.F.; Mason, A.J.; Kostarelos, K. Lipid–peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano 2012, 6, 9335–9346. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, A.; Mahmoodian, R.; Abdullah, B.J.J.; Abdullah, H.; Hamdi, M.; Abu Kasim, N.H. Low-melting-point polymeric nanoshells for thermal-triggered drug release under hyperthermia condition. Int. J. Hyperthermia 2015, 31, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Viale, M.; Giglio, V.; Monticone, M.; Maric, I.; Lentini, G.; Rocco, M.; Vecchio, G. New doxorubicin nanocarriers based on cyclodextrins. Investig. New Drugs 2017, 35, 539–544. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, E.J.; Baek, Y.M.; Hahm, E.; Lee, S.H.; Pham, X.-H.; Noh, M.S.; Kim, D.-E.; Jun, B.-H. Functionalized ?-Cyclodextrin Immobilized on Ag-Embedded Silica Nanoparticles as a Drug Carrier. Int. J. Mol. Sci. 2019, 20, 315. https://doi.org/10.3390/ijms20020315
Kang EJ, Baek YM, Hahm E, Lee SH, Pham X-H, Noh MS, Kim D-E, Jun B-H. Functionalized ?-Cyclodextrin Immobilized on Ag-Embedded Silica Nanoparticles as a Drug Carrier. International Journal of Molecular Sciences. 2019; 20(2):315. https://doi.org/10.3390/ijms20020315
Chicago/Turabian StyleKang, Eun Ji, Yu Mi Baek, Eunil Hahm, Sang Hun Lee, Xuan-Hung Pham, Mi Suk Noh, Dong-Eun Kim, and Bong-Hyun Jun. 2019. "Functionalized ?-Cyclodextrin Immobilized on Ag-Embedded Silica Nanoparticles as a Drug Carrier" International Journal of Molecular Sciences 20, no. 2: 315. https://doi.org/10.3390/ijms20020315
APA StyleKang, E. J., Baek, Y. M., Hahm, E., Lee, S. H., Pham, X.-H., Noh, M. S., Kim, D.-E., & Jun, B.-H. (2019). Functionalized ?-Cyclodextrin Immobilized on Ag-Embedded Silica Nanoparticles as a Drug Carrier. International Journal of Molecular Sciences, 20(2), 315. https://doi.org/10.3390/ijms20020315





