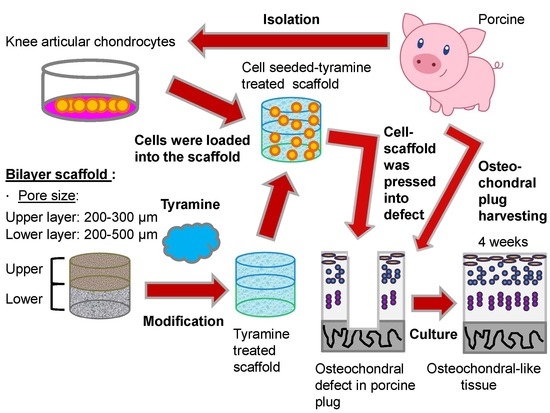
Osteochondral Tissue Regeneration Using a Tyramine-Modified Bilayered PLGA Scaffold Combined with Articular Chondrocytes in a Porcine Model
Abstract
:1. Introduction
2. Results
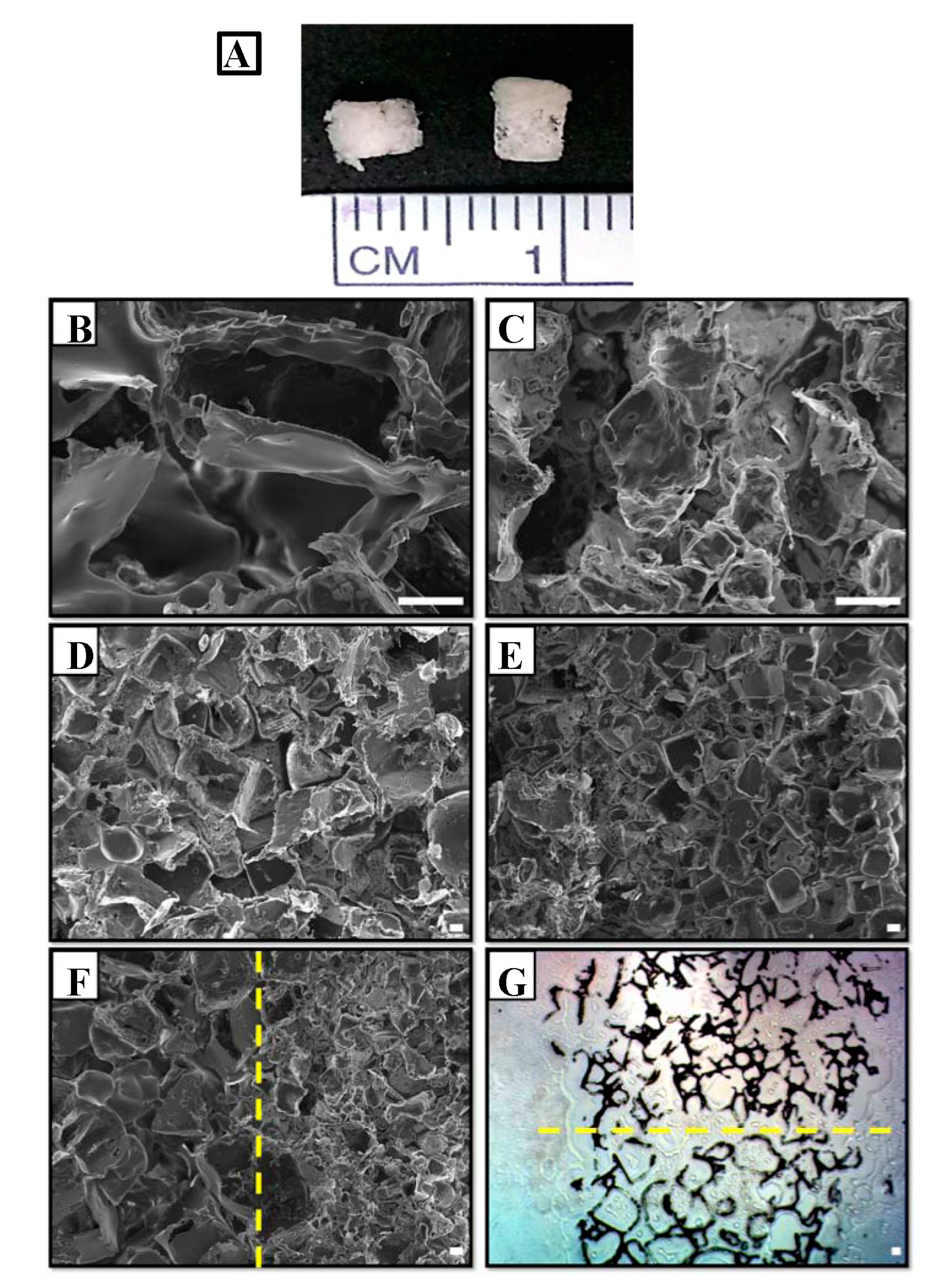
2.1. Morphology of Bilayered PLGA Scaffolds
2.2. Physical Properties of Biphasic PLGA Scaffolds
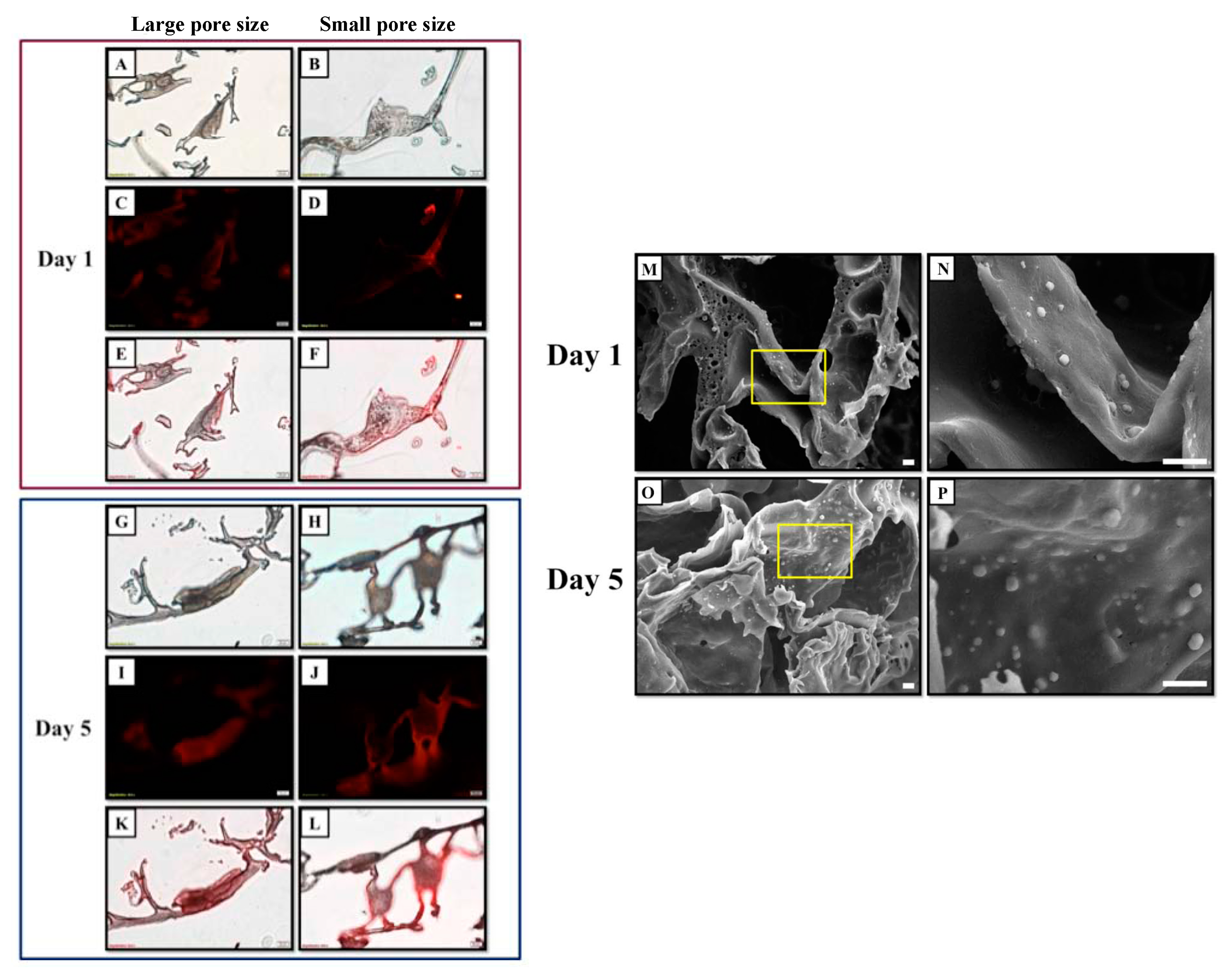
2.3. Effect of Pore Size of Bilayered PLGA Scaffolds on Chondrocyte Morphology
2.4. Characteristics of Bilayered PLGA Scaffolds with Surface Modification
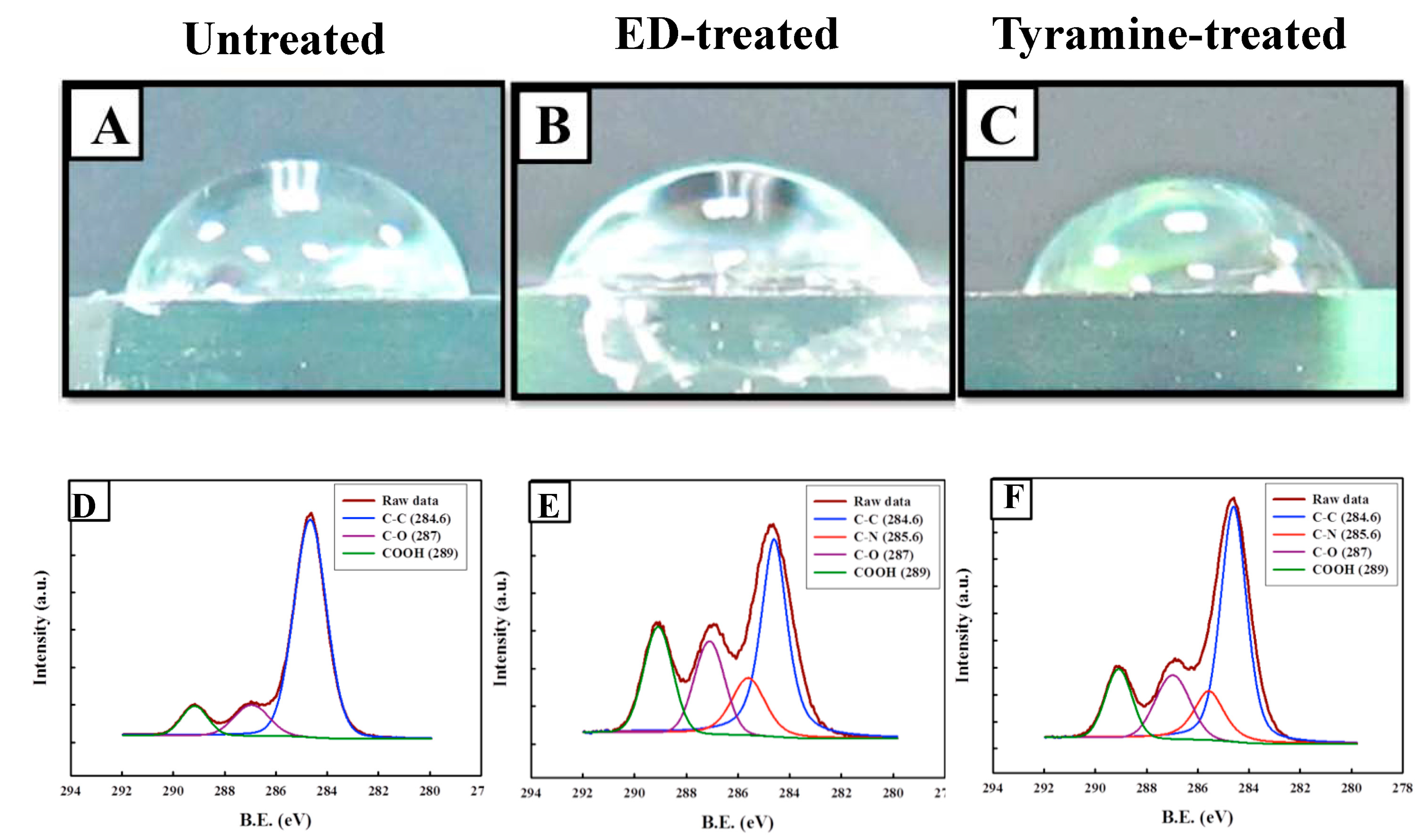
2.4.1. Water Contact Angle of Surface-Modified Bilayered PLGA Scaffolds
2.4.2. Chemical Composition of Surface-Modified Bilayered PLGA Scaffolds
2.4.3. Biocompatibility of Surface-Modified Bilayered PLGA Scaffolds
2.4.4. Adhesive Strength of the Scaffold–Cartilage Interface
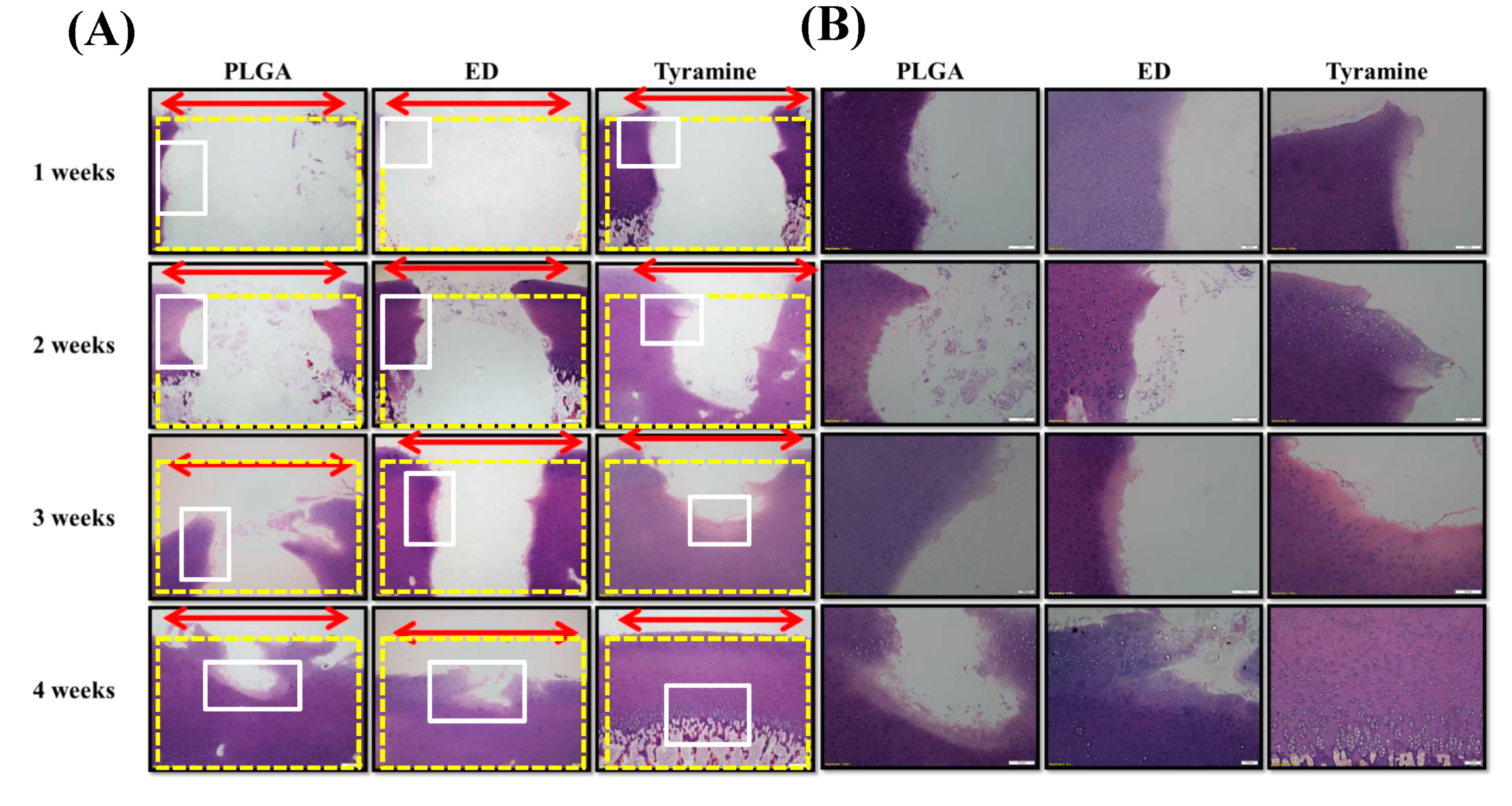
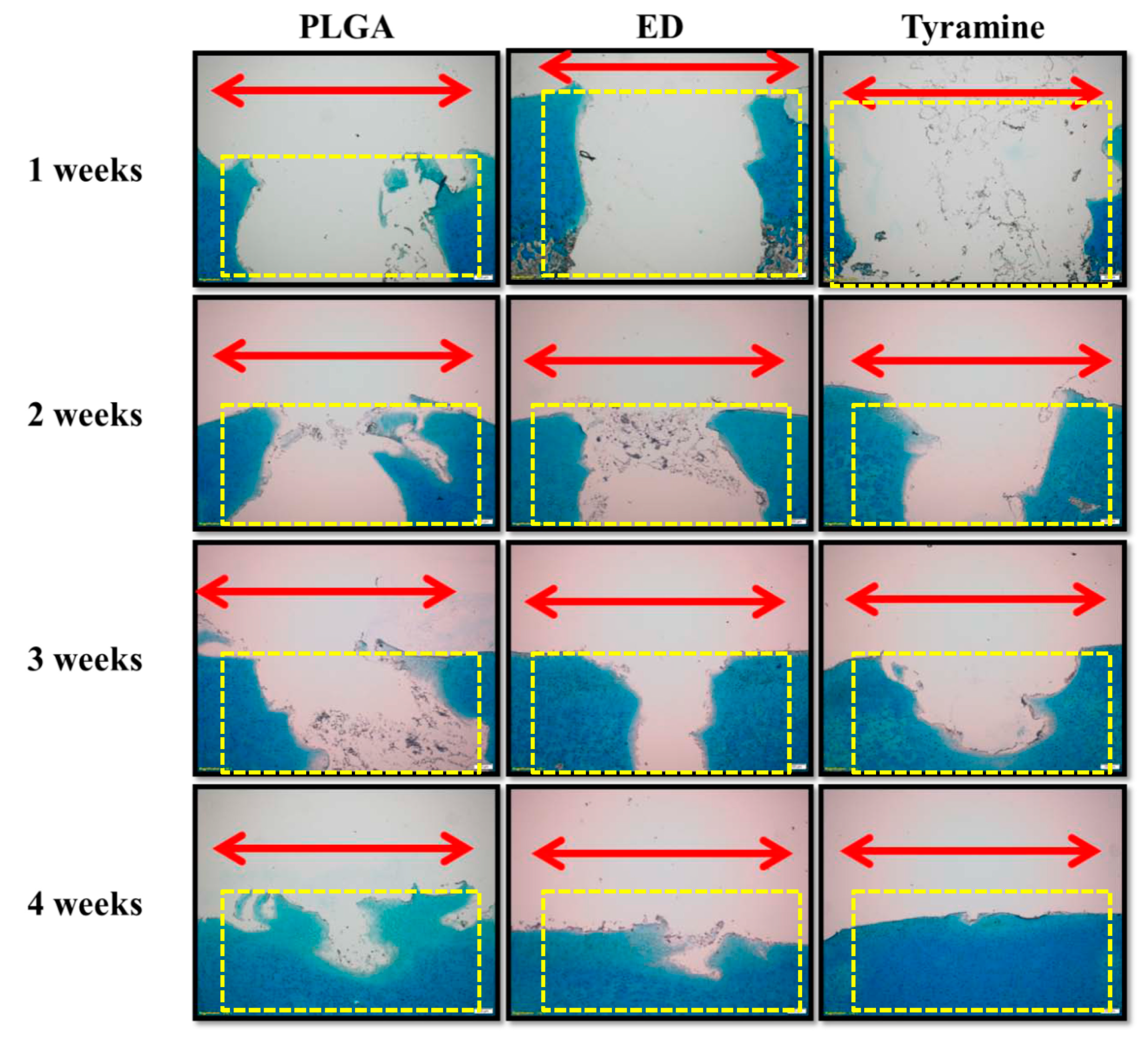
2.4.5. Histological and Immunohistochemical Analyses of Scaffold–Cartilage Constructs in Osteochondral Tissue
3. Discussion
4. Materials and Methods
4.1. Fabrication of Bilayered PLGA Scaffold
4.2. Physical Properties of Bilayered Scaffold
4.3. Chondrocyte Isolation
4.4. Chondrocyte Appearance in Bilayered PLGA Scaffold
4.5. Surface Modification of Bilayered PLGA Scaffold
4.6. Measurement of Water Contact Angle of Surface-Modified Bilayered Scaffold
4.7. XPS Analysis of Surface-Modified Bilayered Scaffold
4.8. Viability and Cell Adherence in Surface-Modified Bilayered Scaffold
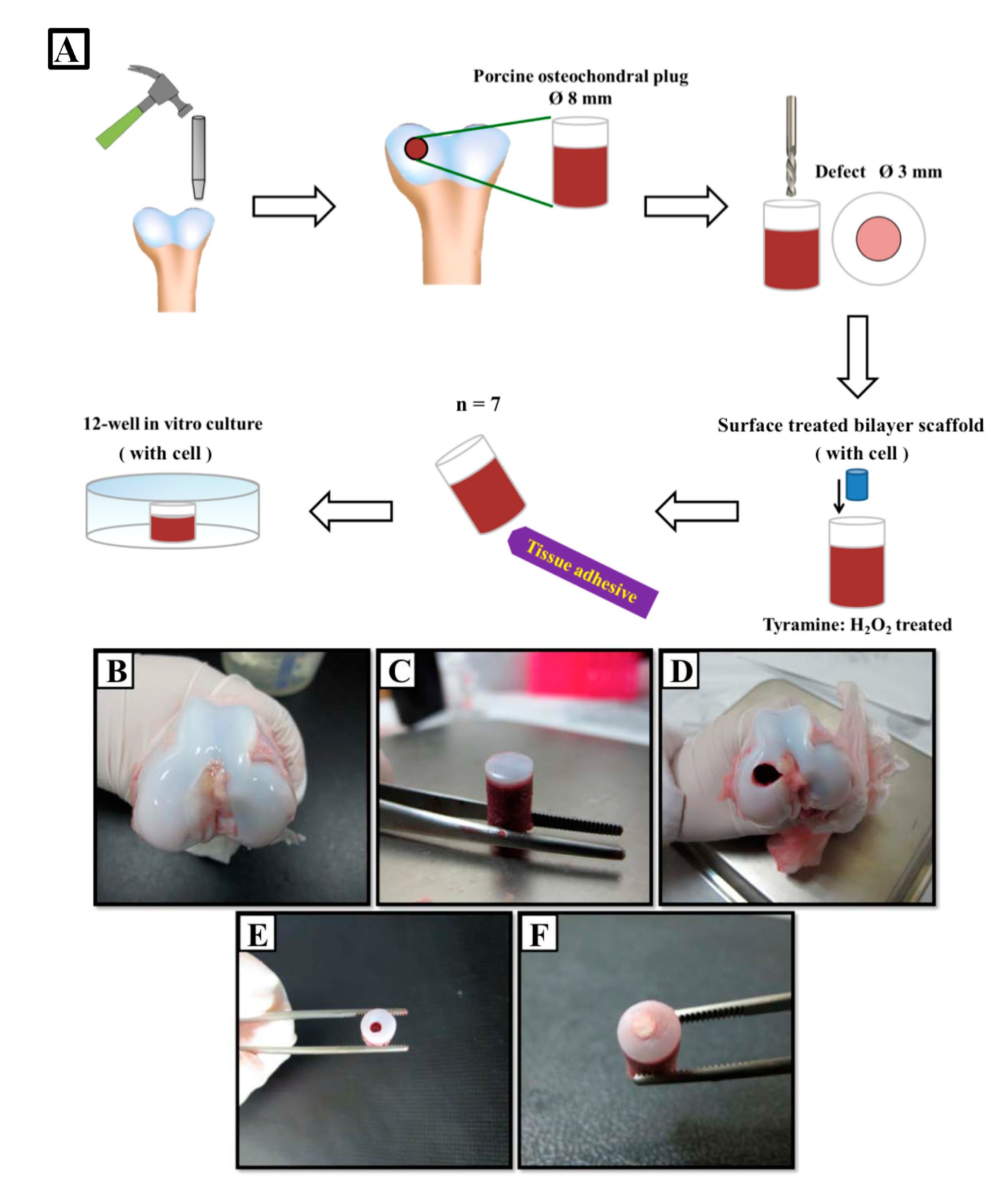
4.9. Preparation of Osteochondral Host Tissue Plug and Scaffold–Cartilage Constructs
4.10. Biomechanical Analysis of Scaffold–Cartilage Construct
4.11. Histological and Immunohistochemical Analyses
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| Col II | Collagen type II |
| Col X | Collagen type X |
| ECM | Extracellular matrix |
| ED | Ethylenediamine |
| GAG | Glycosaminoglycan |
| OCD | Osteochondritis dissecans |
| PBS | Phosphate-buffered saline |
| PLGA | Poly(lactic-co-glycolic acid) |
| SEM | Scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
References
- Kocher, M.S.; Tucker, R.; Ganley, T.J.; Flynn, J.M. Management of osteochondritis dissecans of the knee: Current concepts review. Am. J. Sports Med. 2006, 34, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.G.; Jacobs, J.C., Jr.; Carey, J.L.; Anderson, A.F.; Oxford, J.T. Osteochondritis dissecans knee histology studies have variable findings and theories of etiology. Clin. Orthop. Relat. Res. 2013, 471, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Versier, G.; Dubrana, F.; French Arthroscopy, S. Treatment of knee cartilage defect in 2010. Orthop. Traumatol. Surg. Res. 2011, 97, S140–S153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobic, V. Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction: A preliminary clinical study. Knee Surg. Sports Traumatol. Arthrosc. 1996, 3, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Kish, G.; Karpati, Z.; Udvarhelyi, I.; Szigeti, I.; Bely, M. Mosaicplasty for the treatment of articular cartilage defects: Application in clinical practice. Orthopedics 1998, 21, 751–756. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Keyhani, S.; Lee, E.H.; Hui, J.H. Evidence-based status of microfracture technique: A systematic review of level I and II studies. Arthroscopy: J. Arthrosc. Relat. Surg. 2013, 29, 1579–1588. [Google Scholar] [CrossRef]
- Solheim, E.; Hegna, J.; Oyen, J.; Austgulen, O.K.; Harlem, T.; Strand, T. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: Results at 5 to 9 years. Knee 2010, 17, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Verdonk, P.; Condello, V.; Delcogliano, M.; Dhollander, A.; Filardo, G.; Pignotti, E.; Marcacci, M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: Systematic clinical data review and study quality analysis. Am. J. Sports Med. 2009, 37 (Suppl. 1), 156S–166S. [Google Scholar] [CrossRef]
- Mase, V.J., Jr.; Hsu, J.R.; Wolf, S.E.; Wenke, J.C.; Baer, D.G.; Owens, J.; Badylak, S.F.; Walters, T.J. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 2010, 33, 511. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Li, P.; Diao, H.; Zhu, S.; Dong, L.; Wang, R.; Guo, T.; Zhao, J.; Zhang, J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 2011, 32, 4793–4805. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Du, Y.; Li, W.; Dai, X.; Zhao, T.; Yan, W. Cartilage repair using mesenchymal stem cell (MSC) sheet and MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tang, A.; Ateshian, G.A.; Guo, X.E.; Hung, C.T.; Lu, H.H. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann. Biomed. Eng. 2010, 38, 2183–2196. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Park, K.M.; Joung, Y.K.; Park, K.D.; Do, S.H. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J. Control. Release 2012, 159, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, R.A. Cell sources for the regeneration of articular cartilage: The past, the horizon and the future. Int. J. Exp. Pathol. 2012, 93, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.M.; Chiang, M.Y.; Lan, C.W.; Niu, G.C.; Chang, S.J. Evaluation of nanoarchitectured collagen type II molecules on cartilage engineering. J. Biomed. Mater. Res. Part A 2013, 101, 368–377. [Google Scholar] [CrossRef]
- Shao, X.X.; Hutmacher, D.W.; Ho, S.T.; Goh, J.C.; Lee, E.H. Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials 2006, 27, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Gotterbarm, T.; Richter, W.; Jung, M.; Berardi Vilei, S.; Mainil-Varlet, P.; Yamashita, T.; Breusch, S.J. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects. Biomaterials 2006, 27, 3387–3395. [Google Scholar] [CrossRef]
- Marquass, B.; Somerson, J.S.; Hepp, P.; Aigner, T.; Schwan, S.; Bader, A.; Josten, C.; Zscharnack, M.; Schulz, R.M. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J. Orthop. Res. 2010, 28, 1586–1599. [Google Scholar] [CrossRef]
- Getgood, A.M.; Kew, S.J.; Brooks, R.; Aberman, H.; Simon, T.; Lynn, A.K.; Rushton, N. Evaluation of early-stage osteochondral defect repair using a biphasic scaffold based on a collagen-glycosaminoglycan biopolymer in a caprine model. Knee 2012, 19, 422–430. [Google Scholar] [CrossRef]
- Schleicher, I.; Lips, K.S.; Sommer, U.; Schappat, I.; Martin, A.P.; Szalay, G.; Hartmann, S.; Schnettler, R. Biphasic scaffolds for repair of deep osteochondral defects in a sheep model. J. Surg. Res. 2013, 183, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Cui, W.; Wang, Q.; Chen, G.; Zhou, S.; Chang, Q.; Zuo, Q.; Ren, K.; Fan, W. Repair of articular cartilage defects with tissue-engineered osteochondral composites in pigs. J. Biosci. Bioeng. 2011, 111, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.J.; Jhung, Y.R.; Issariyakul, N.; Yao, C.K.; Yeh, M.L. Synergistic Stimuli by Hydrodynamic Pressure and Hydrophilic Coating on PLGA Scaffolds for Extracellular Matrix Synthesis of Engineered Cartilage. J. Biomater. Sci. Polym. Ed. 2012, 23, 2133–2151. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.J.; Lin, C.C.; Li, C.F.; Wang, D.A.; Issariyaku, N.; Yeh, M.L. The combined effects of continuous passive motion treatment and acellular PLGA implants on osteochondral regeneration in the rabbit. Biomaterials 2012, 33, 3153–3163. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.J.; Lam, C.F.; Lin, C.C.; Chen, W.L.; Li, C.F.; Lin, Y.T.; Yeh, M.L. Transplantation of autologous endothelial progenitor cells in porous PLGA scaffolds create a microenvironment for the regeneration of hyaline cartilage in rabbits. Osteoarthr. Cartel. 2013, 21, 1613–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraj, K.A.; van Kuppevelt, T.H.; Daamen, W.F. Construction of collagen scaffolds that mimic the three-dimensional architecture of specific tissues. Tissue Eng. 2007, 13, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Woodfield, T.B.; Malda, J.; de Wijn, J.; Peters, F.; Riesle, J.; van Blitterswijk, C.A. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 2004, 25, 4149–4161. [Google Scholar] [CrossRef]
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan scaffolds: Interconnective pore size and cartilage engineering. Acta Biomater. 2006, 2, 313–320. [Google Scholar] [CrossRef]
- Choi, S.W.; Xie, J.; Xia, Y. Chitosan-Based Inverse Opals: Three-Dimensional Scaffolds with Uniform Pore Structures for Cell Culture. Adv. Mater. 2009, 21, 2997–3001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, G.I.; Ko, J.Y.; Lee, J.H. Chondrogenesis of adipose stem cells in a porous polymer scaffold: Influence of the pore size. Cell Transplant. 2012, 21, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Nava, M.M.; Draghi, L.; Giordano, C.; Pietrabissa, R. The effect of scaffold pore size in cartilage tissue engineering. J. appl. Biomater. Funct. Mater. 2016, 14, e223–e229. [Google Scholar] [CrossRef] [PubMed]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Young, G.; Shortkroff, S.; Louie, L.K.; Sledge, C.B.; Yannas, I.V.; Spector, M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials 1997, 18, 769–776. [Google Scholar] [CrossRef]
- Stenhamre, H.; Nannmark, U.; Lindahl, A.; Gatenholm, P.; Brittberg, M. Influence of pore size on the redifferentiation potential of human articular chondrocytes in poly(urethane urea) scaffolds. J. Tissue Eng. Regener. Med. 2011, 5, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Joint Surg. 2001, 83, S105–S115. [Google Scholar] [CrossRef]
- Roosa, S.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. Part A 2010, 92, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Pan, Z.; Cao, L.; He, Y.; Wang, H.; Qu, Z.; Dong, J.; Ding, J. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J. Biomed. Mater. Res. Part A 2014, 102, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hong, Z.; Yu, T.; Chen, X.; Jing, X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(L-lactide). Biomaterials 2009, 30, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.-S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(L-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Liu, X.; He, T.; Shen, J. Immobilization of biomacromolecules onto aminolyzed poly(L-lactic acid) toward acceleration of endothelium regeneration. Tissue Eng. 2004, 10, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.C.; Tutt, T.J.; Galloway, M.; Davidson, Y.Y.; McWhorter, C.S.; Soper, S.A.; McCarley, R.L. Surface modification of poly(methyl methacrylate) used in the fabrication of microanalytical devices. Anal. Chem. 2000, 72, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Dominick, W.D.; Berhane, B.T.; Mecomber, J.S.; Limbach, P.A. Covalent immobilization of proteases and nucleases to poly(methylmethacrylate). Anal. Bioanal. Chem. 2003, 376, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable surface modification of poly(lactic-co-glycolic acid) (PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Fixe, F.; Dufva, M.; Telleman, P.; Christensen, C.B. Functionalization of poly(methyl methacrylate) (PMMA) as a substrate for DNA microarrays. Nucleic Acids Res. 2004, 32, e9. [Google Scholar] [CrossRef] [PubMed]
- Fixe, F.; Dufva, M.; Telleman, P.; Christensen, C.B. One-step immobilization of aminated and thiolated DNA onto poly(methylmethacrylate) (PMMA) substrates. Lab Chip 2004, 4, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; He, T.; Shen, J. Endothelium regeneration on luminal surface of polyurethane vascular scaffold modified with diamine and covalently grafted with gelatin. Biomaterials 2004, 25, 423–430. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, Y.; Shen, J. Endothelial cell functions in vitro cultured on poly(L-lactic acid) membranes modified with different methods. J. Biomed. Mater. Res. Part A 2004, 69, 436–443. [Google Scholar] [CrossRef]
- Brown, L.; Koerner, T.; Horton, J.H.; Oleschuk, R.D. Fabrication and characterization of poly(methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66–73. [Google Scholar] [CrossRef]
- Jin, R.; Hiemstra, C.; Zhong, Z.Y.; Feijen, J. Enzyme-mediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials 2007, 28, 2791–2800. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; Zhong, Z.Y.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically Crosslinked Dextran-Tyramine Hydrogels as Injectable Scaffolds for Cartilage Tissue Engineering. Tissue Eng. Part A 2010, 16, 2429–2440. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Chondrogenesis in injectable enzymatically crosslinked heparin/dextran hydrogels. J. Control Release 2011, 152, 186–195. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Bijl, S.; Pully, V.V.; Otto, C.; Jin, R.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Self-attaching and cell-attracting in-situ forming dextran-tyramine conjugates hydrogels for arthroscopic cartilage repair. Biomaterials 2012, 33, 3164–3174. [Google Scholar] [CrossRef]
- Scholes, P.D.; Coombes, A.G.; Illum, L.; Davis, S.S.; Watts, J.F.; Ustariz, C.; Vert, M.; Davies, M.C. Detection and determination of surface levels of poloxamer and PVA surfactant on biodegradable nanospheres using SSIMS and XPS. J. of Control. Release 1999, 59, 261–278. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Reis, R.L. Bilayered chitosan-based scaffolds for osteochondral tissue engineering: Influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. Acta Biomater. 2009, 5, 644–660. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.M.; Rodrigues, M.T.; Silva, S.S.; Malafaya, P.B.; Gomes, M.E.; Viegas, C.A.; Dias, I.R.; Azevedo, J.T.; Mano, J.F.; Reis, R.L. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 2006, 27, 6123–6137. [Google Scholar] [CrossRef] [Green Version]
- Mesallati, T.; Buckley, C.T.; Nagel, T.; Kelly, D.J. Scaffold architecture determines chondrocyte response to externally applied dynamic compression. Biomech. Model. Mechanobiol. 2013, 12, 889–899. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef]
- Yannas, I.V. Tissue regeneration by use of collagen-glycosaminoglycan copolymers. Clin. Mater. 1992, 9, 179–187. [Google Scholar] [CrossRef]
- Emans, P.J.; Jansen, E.J.; van Iersel, D.; Welting, T.J.; Woodfield, T.B.; Bulstra, S.K.; Riesle, J.; van Rhijn, L.W.; Kuijer, R. Tissue-engineered constructs: The effect of scaffold architecture in osteochondral repair. J. Tissue Eng. Regener. Med. 2013, 7, 751–756. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef]
- Hegert, C.; Kramer, J.; Hargus, G.; Muller, J.; Guan, K.; Wobus, A.M.; Muller, P.K.; Rohwedel, J. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J. Cell Sci. 2002, 115, 4617–4628. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fan, W.; Ma, Z.; Wu, C.; Fang, W.; Liu, G.; Xiao, Y. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 2010, 6, 3021–3028. [Google Scholar] [CrossRef] [Green Version]
- Ashman, A.; Moss, M.L. Implantation of porous polymethylmethacrylate resin for tooth and bone replacement. J. prosthet. Dent. 1977, 37, 657–665. [Google Scholar] [CrossRef]
- Gotz, H.E.; Muller, M.; Emmel, A.; Holzwarth, U.; Erben, R.G.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, C. Topographical control of cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef]
- Freed, L.E.; Marquis, J.C.; Nohria, A.; Emmanual, J.; Mikos, A.G.; Langer, R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J. Biomed. Mater. Res. 1993, 27, 11–23. [Google Scholar] [CrossRef]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable polymer scaffolds for tissue engineering. Nat. Biotechnol. 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Freed, L.E.; Hollander, A.P.; Martin, I.; Barry, J.R.; Langer, R.; Vunjak-Novakovic, G. Chondrogenesis in a cell-polymer-bioreactor system. Exp. Cell Res. 1998, 240, 58–65. [Google Scholar] [CrossRef]
- Li, W.J.; Jiang, Y.J.; Tuan, R.S. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006, 12, 1775–1785. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, C.; Wluka, A.E.; Davis, S.; Ebeling, P.R.; Jones, G.; Cicuttini, F.M. Factors affecting progression of knee cartilage defects in normal subjects over 2 years. Rheumatology 2006, 45, 79–84. [Google Scholar] [CrossRef]
- Guettler, J.H.; Demetropoulos, C.K.; Yang, K.H.; Jurist, K.A. Osteochondral defects in the human knee: Influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am. J. Sports Med. 2004, 32, 1451–1458. [Google Scholar] [CrossRef]
- Homminga, G.N.; Buma, P.; Koot, H.W.; van der Kraan, P.M.; van den Berg, W.B. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop. Scand. 1993, 64, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Hoemann, C.D.; Sun, J.; Legare, A.; McKee, M.D.; Buschmann, M.D. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr. Cartel. 2005, 13, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Bos, P.K.; DeGroot, J.; Budde, M.; Verhaar, J.A.; van Osch, G.J. Specific enzymatic treatment of bovine and human articular cartilage: Implications for integrative cartilage repair. Arthritis Rheum. 2002, 46, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Lauffenburger, D.A.; Morales, T.I. Motile chondrocytes from newborn calf: Migration properties and synthesis of collagen II. Osteoarthr. Cartel. 2003, 11, 603–612. [Google Scholar] [CrossRef]
- Theodoropoulos, J.S.; De Croos, J.N.; Park, S.S.; Pilliar, R.; Kandel, R.A. Integration of tissue-engineered cartilage with host cartilage: An in vitro model. Clin. Orthop. Relat. Res. 2011, 469, 2785–2795. [Google Scholar] [CrossRef]
- Ng, K.W.; Wanivenhaus, F.; Chen, T.; Hsu, H.C.; Allon, A.A.; Abrams, V.D.; Torzilli, P.A.; Warren, R.F.; Maher, S.A. A novel macroporous polyvinyl alcohol scaffold promotes chondrocyte migration and interface formation in an in vitro cartilage defect model. Tissue Eng. Part A 2012, 18, 1273–1281. [Google Scholar] [CrossRef]
- van de Breevaart Bravenboer, J.; In der Maur, C.D.; Bos, P.K.; Feenstra, L.; Verhaar, J.A.; Weinans, H.; van Osch, G.J. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res. Ther. 2004, 6, R469–R476. [Google Scholar] [CrossRef]
- Chan, Y.; Walmsley, R.P. Learning and understanding the Kruskal-Wallis one-way analysis-of-variance-by-ranks test for differences among three or more independent groups. Phys. Ther. 1997, 77, 1755–1762. [Google Scholar] [CrossRef]
- Horn, M. The Wilcoxon, Mann and Whitney test: Conditions under which and hypotheses for which it is applicable. Z. Versuchstierk. 1990, 33, 109–114. [Google Scholar]


| Pore Type | Compressive Modulus (MPa) | Porosity (%) |
|---|---|---|
| Large pores | 0.517 ± 0.01 | 88.807 ± 1.29 |
| Small pores | 0.516 ± 0.01 | 89.532 ± 1.38 |
| Mixed sized pores | 0.517 ± 0.01 | 88.997 ± 1.28 |
| Contact Angle Analysis | Untreated PLGA | Ethylenediamine-Treated PLGA | Tyramine-Treated PLGA |
|---|---|---|---|
| Contact angle (°) | 68.08 ± 1.8° | ** 57.10 ± 2.14° | **,## 52.58 ± 1.43° |
| Surface Treatment | Atom | Atomic Percentage (%) |
|---|---|---|
| Untreated | C | 76.53 |
| O | 22.60 | |
| N | 0.87 | |
| Ethylenediamine-treated | C | 69.26 |
| O | 29.50 | |
| N | 1.24 | |
| Tyramine-treated | C | 71.54 |
| O | 26.89 | |
| N | 1.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-H.; Wang, H.-C.; Cheng, W.-H.; Hsu, H.-C.; Yeh, M.-L. Osteochondral Tissue Regeneration Using a Tyramine-Modified Bilayered PLGA Scaffold Combined with Articular Chondrocytes in a Porcine Model. Int. J. Mol. Sci. 2019, 20, 326. https://doi.org/10.3390/ijms20020326
Lin T-H, Wang H-C, Cheng W-H, Hsu H-C, Yeh M-L. Osteochondral Tissue Regeneration Using a Tyramine-Modified Bilayered PLGA Scaffold Combined with Articular Chondrocytes in a Porcine Model. International Journal of Molecular Sciences. 2019; 20(2):326. https://doi.org/10.3390/ijms20020326
Chicago/Turabian StyleLin, Tzu-Hsiang, Hsueh-Chun Wang, Wen-Hui Cheng, Horng-Chaung Hsu, and Ming-Long Yeh. 2019. "Osteochondral Tissue Regeneration Using a Tyramine-Modified Bilayered PLGA Scaffold Combined with Articular Chondrocytes in a Porcine Model" International Journal of Molecular Sciences 20, no. 2: 326. https://doi.org/10.3390/ijms20020326
APA StyleLin, T.-H., Wang, H.-C., Cheng, W.-H., Hsu, H.-C., & Yeh, M.-L. (2019). Osteochondral Tissue Regeneration Using a Tyramine-Modified Bilayered PLGA Scaffold Combined with Articular Chondrocytes in a Porcine Model. International Journal of Molecular Sciences, 20(2), 326. https://doi.org/10.3390/ijms20020326