Evolution of Disease Defense Genes and Their Regulators in Plants
Abstract
1. Introduction
2. Three Layers of Defense Mechanisms to Biotic Stresses in Plants
2.1. The First Layer of Defense: Defense Genes in PTI
2.2. The Second Layer of Defense: The Defense Genes in ETI
2.3. The Third Layer of Defense: Cross-Kingdom/Organism RNA Interference
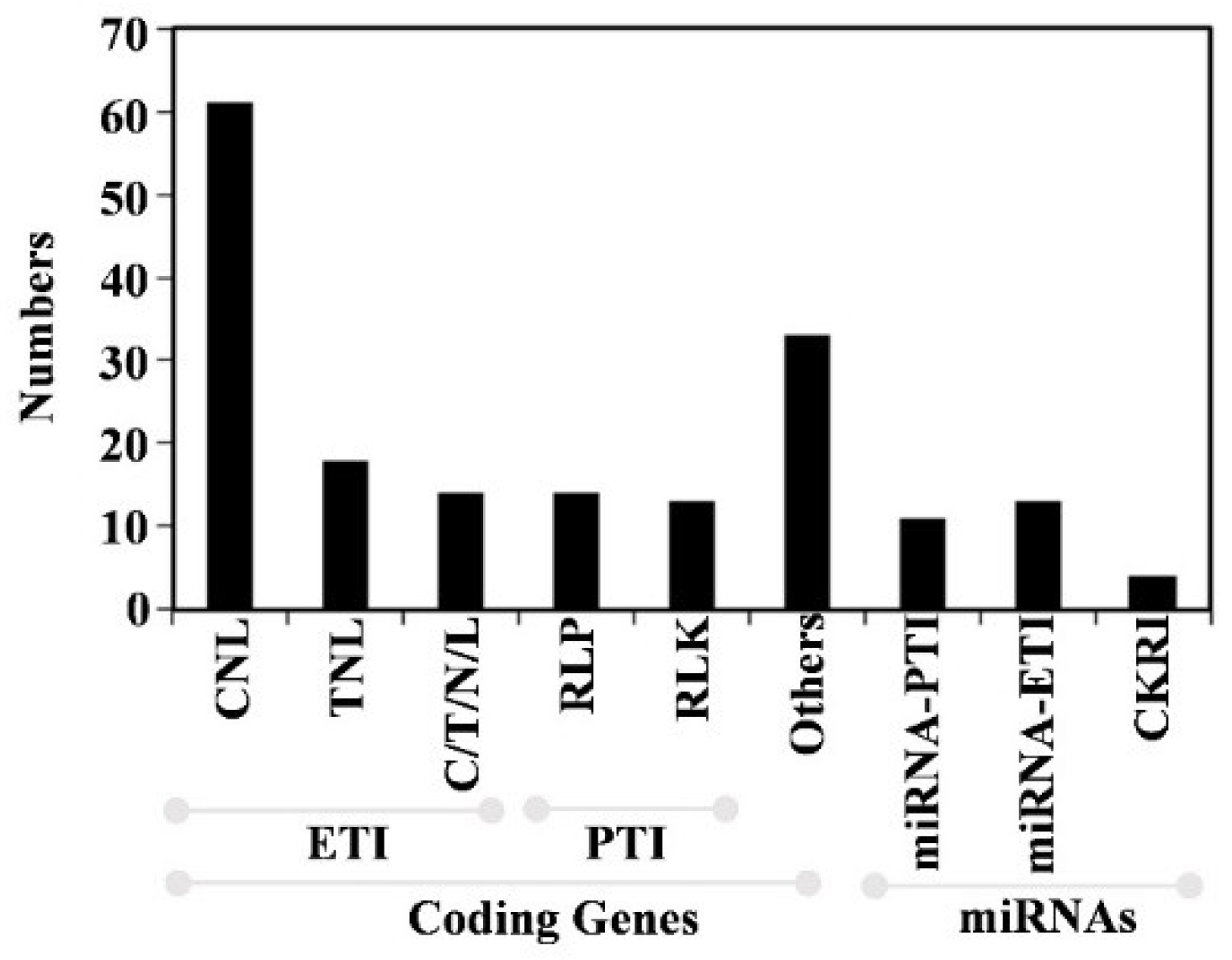
3. The Regulation of Disease Resistance Genes by Small RNAs
3.1. The First Layer of Defense Regulation: miRNAs Involved in the PTI Pathway
3.2. The Second Layer of Defense Regulation: The Defense Signal Small RNAs in ETI
4. The Evolution of Defense Genes
4.1. The Evolution of Defense Gene in PTI
4.2. The Evolution of Defense Gene in ETI
5. The Evolution of microRNAs in the Defense Pathway
5.1. The Evolution of miRNAs in PTI
5.2. The Evolution of miRNAs in ETI
6. The Strategies of Defense Pathogens in plants
6.1. The First Strategy: Utilize the Disease Resistance Genes by a Molecular Switch
6.2. The Second Strategy: Host-Induced Gene Silencing (HIGS)
6.3. The Third Strategy: Spray-Induced Gene Silencing (SIGS)
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Monaghan, J.; Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Eitas, T.K.; Dangl, J.L. NB-LRR proteins: Pairs, pieces, perception, partners, and pathways. Curr. Opin. Plant Biol. 2010, 13, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, H.D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018. [Google Scholar] [CrossRef]
- Shi, H.; Shen, Q.; Qi, Y.; Yan, H.; Nie, H.; Chen, Y.; Zhao, T.; Katagiri, F.; Tang, D. BR-SIGNALING KINASE1 physically associates with FLAGELLIN SENSING2 and regulates plant innate immunity in Arabidopsis. Plant Cell 2013, 25, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.H.; Panzeri, D.; Kadota, Y.; Huang, Y.C.; Huang, P.Y.; Tao, C.N.; Roux, M.; Chien, H.C.; Chin, T.C.; Chu, P.W.; et al. The Arabidopsis Malectin-Like/LRR-RLK IOS1 Is Critical for BAK1-Dependent and BAK1-Independent Pattern-Triggered Immunity. Plant Cell 2016, 28, 1701–1721. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Bourdais, G.; Pan, H.; Robatzek, S.; Tang, D. Arabidopsis glycosylphosphatidylinositol-anchored protein LLG1 associates with and modulates FLS2 to regulate innate immunity. Proc. Natl. Acad. Sci. USA 2017, 114, 5749–5754. [Google Scholar] [CrossRef] [PubMed]
- Erwig, J.; Ghareeb, H.; Kopischke, M.; Hacke, R.; Matei, A.; Petutschnig, E.; Lipka, V. Chitin-induced and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) phosphorylation-dependent endocytosis of Arabidopsis thaliana LYSIN MOTIF-CONTAINING RECEPTOR-LIKE KINASE5 (LYK5). New Phytol. 2017, 215, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003, 15, 809–834. [Google Scholar] [CrossRef]
- Xiao, S.; Ellwood, S.; Calis, O.; Patrick, E.; Li, T.; Coleman, M.; Turner, J.G. Broad-spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8. Science 2001, 291, 118–120. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Xue, J.Y.; Wu, P.; Zhang, Y.M.; Wu, Y.; Hang, Y.Y.; Wang, B.; Chen, J.Q. Large-Scale Analyses of Angiosperm Nucleotide-Binding Site-Leucine-Rich Repeat Genes Reveal Three Anciently Diverged Classes with Distinct Evolutionary Patterns. Plant Physiol. 2016, 170, 2095–2109. [Google Scholar] [CrossRef]
- Mukhtar, M.S.; Carvunis, A.R.; Dreze, M.; Epple, P.; Steinbrenner, J.; Moore, J.; Tasan, M.; Galli, M.; Hao, T.; Nishimura, M.T.; et al. Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science 2011, 333, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.C.; Dickerman, A.W.; Michelmore, R.W.; Sivaramakrishnan, S.; Sobral, B.W.; Young, N.D. Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant J. Cell Mol. Biol. 1999, 20, 317–332. [Google Scholar] [CrossRef]
- Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 2009, 136, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Alptekin, B.; Langridge, P.; Budak, H. Abiotic stress miRNomes in the Triticeae. Funct. Integr. Genom. 2017, 17, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Kantar, M. Harnessing NGS and Big Data Optimally: Comparison of miRNA Prediction from Assembled versus Non-assembled Sequencing Data—The Case of the Grass Aegilops tauschii Complex Genome. Omics J. Integr. Biol. 2015, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Kantar, M.; Bulut, R.; Akpinar, B.A. Stress responsive miRNAs and isomiRs in cereals. Plant Sci. Int. J. Exp. Plant Biol. 2015, 235, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Zhang, J.; Wu, L.; Qi, Y.; Zhou, J.M. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol. 2010, 152, 2222–2231. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, R.; Kuang, H.; Meyers, B.C. The Diversification of Plant NBS-LRR Defense Genes Directs the Evolution of MicroRNAs That Target Them. Mol. Biol. Evol. 2016, 33, 2692–2705. [Google Scholar] [CrossRef]
- Osuna-Cruz, C.M.; Paytuvi-Gallart, A.; Di Donato, A.; Sundesha, V.; Andolfo, G.; Aiese Cigliano, R.; Sanseverino, W.; Ercolano, M.R. PRGdb 3.0: A comprehensive platform for prediction and analysis of plant disease resistance genes. Nucleic Acids Res. 2018, 46, D1197–D1201. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.L.; Zhao, J.H.; Wang, S.; Jin, Y.; Chen, Z.Q.; Fang, Y.Y.; Hua, C.L.; Ding, S.W.; Guo, H.S. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef]
- Liu, Y.; Mittal, R.; Solis, N.V.; Prasadarao, N.V.; Filler, S.G. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog. 2011, 7, e1002305. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiao, X.; Kong, X.; Hamera, S.; Wu, Y.; Chen, X.; Fang, R.; Yan, Y. A Signaling Cascade from miR444 to RDR1 in Rice Antiviral RNA Silencing Pathway. Plant Physiol. 2016, 170, 2365–2377. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Yuan, Q.; Wang, S.; Peng, J.; Lu, Y.; Zheng, H.; Lin, L.; Chen, H.; Gong, Y.; Chen, J.; et al. Altered accumulation of osa-miR171b contributes to rice stripe virus infection by regulating disease symptoms. J. Exp. Bot. 2017, 68, 4357–4367. [Google Scholar] [CrossRef] [PubMed]
- Hanemian, M.; Barlet, X.; Sorin, C.; Yadeta, K.A.; Keller, H.; Favery, B.; Simon, R.; Thomma, B.P.; Hartmann, C.; Crespi, M.; et al. Arabidopsis CLAVATA1 and CLAVATA2 receptors contribute to Ralstonia solanacearum pathogenicity through a miR169-dependent pathway. New Phytol. 2016, 211, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, S.L.; Li, J.L.; Hu, X.H.; Wang, H.; Cao, X.L.; Xu, Y.J.; Zhao, Z.X.; Xiao, Z.Y.; Yang, N.; et al. Osa-miR169 Negatively Regulates Rice Immunity against the Blast Fungus Magnaporthe oryzae. Front. Plant Sci. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Zhang, Y.; Wu, C.; Wang, S.; Hao, L.; Wang, S.; Li, T. Md-miR156ab and Md-miR395 Target WRKY Transcription Factors to Influence Apple Resistance to Leaf Spot Disease. Front. Plant Sci. 2017, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Soto-Suarez, M.; Baldrich, P.; Weigel, D.; Rubio-Somoza, I.; San Segundo, B. The Arabidopsis miR396 mediates pathogen-associated molecular pattern-triggered immune responses against fungal pathogens. Sci. Rep. 2017, 7, 44898. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Wu, K.; Yang, L.; Li, Y.; Yang, Z.; Shi, S.; Liu, X.; Zhao, S.; Yang, Z.; et al. Suppression of Jasmonic Acid-Mediated Defense by Viral-Inducible MicroRNA319 Facilitates Virus Infection in Rice. Mol. Plant 2016, 9, 1302–1314. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Y.; Shan, D.; Wang, Z.; Song, X.; Wang, Z.; Wang, J.; He, L.; Wu, L.; Zhang, Z.; et al. Magnaporthe oryzae Induces the Expression of a MicroRNA to Suppress the Immune Response in Rice. Plant Physiol. 2018, 177, 352–368. [Google Scholar] [CrossRef]
- Salvador-Guirao, R.; Baldrich, P.; Weigel, D.; Rubio-Somoza, I.; San Segundo, B. The microRNA miR773 is involved in the Arabidopsis immune response to fungal pathogens. Mol. Plant Microbe Interact. 2017. [Google Scholar] [CrossRef]
- Wang, C.; He, X.; Wang, X.; Zhang, S.; Guo, X. ghr-miR5272a-mediated regulation of GhMKK6 gene transcription contributes to the immune response in cotton. J. Exp. Bot. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Piya, S.; Qi, M.; Balasubramaniam, M.; Rice, J.H.; Baum, T.J. Arabidopsis miR827 mediates post-transcriptional gene silencing of its ubiquitin E3 ligase target gene in the syncytium of the cyst nematode Heterodera schachtii to enhance susceptibility. Plant J. Cell Mol. Biol. 2016, 88, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, H.; Gao, S.; Wang, W.C.; Katiyar-Agarwal, S.; Huang, H.D.; Raikhel, N.; Jin, H. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393(*)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol. Cell 2011, 42, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Lii, Y.E.; Chellappan, P.; Lei, L.; Peralta, K.; Jiang, C.; Guo, J.; Coaker, G.; Jin, H. miRNA863-3p sequentially targets negative immune regulator ARLPKs and positive regulator SERRATE upon bacterial infection. Nat. Commun. 2016, 7, 11324. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Traw, M.B.; Chen, J.Q.; Kreitman, M.; Bergelson, J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 2003, 423, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Jeong, D.H.; De Paoli, E.; Park, S.; Rosen, B.D.; Li, Y.; Gonzalez, A.J.; Yan, Z.; Kitto, S.L.; Grusak, M.A.; et al. MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev. 2011, 25, 2540–2553. [Google Scholar] [CrossRef]
- Fei, Q.; Xia, R.; Meyers, B.C. Phased, secondary, small interfering RNAs in posttranscriptional regulatory networks. Plant Cell 2013, 25, 2400–2415. [Google Scholar] [CrossRef]
- He, X.F.; Fang, Y.Y.; Feng, L.; Guo, H.S. Characterization of conserved and novel microRNAs and their targets, including a TuMV-induced TIR-NBS-LRR class R gene-derived novel miRNA in Brassica. FEBS Lett. 2008, 582, 2445–2452. [Google Scholar] [CrossRef]
- Li, F.; Pignatta, D.; Bendix, C.; Brunkard, J.O.; Cohn, M.M.; Tung, J.; Sun, H.; Kumar, P.; Baker, B. MicroRNA regulation of plant innate immune receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1790–1795. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, J.; Tung, J.; Liu, D.; Zhou, Y.; He, S.; Du, Y.; Baker, B.; Li, F. A role for small RNA in regulating innate immunity during plant growth. PLoS Pathog. 2018, 14, e1006756. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Park, G.; Atamian, H.S.; Han, C.S.; Stajich, J.E.; Kaloshian, I.; Borkovich, K.A. MicroRNAs suppress NB domain genes in tomato that confer resistance to Fusarium oxysporum. PLoS Pathog. 2014, 10, e1004464. [Google Scholar] [CrossRef] [PubMed]
- Boccara, M.; Sarazin, A.; Thiebeauld, O.; Jay, F.; Voinnet, O.; Navarro, L.; Colot, V. The Arabidopsis miR472-RDR6 silencing pathway modulates PAMP- and effector-triggered immunity through the post-transcriptional control of disease resistance genes. PLoS Pathog. 2014, 10, e1003883. [Google Scholar] [CrossRef] [PubMed]
- Arikit, S.; Xia, R.; Kakrana, A.; Huang, K.; Zhai, J.; Yan, Z.; Valdes-Lopez, O.; Prince, S.; Musket, T.A.; Nguyen, H.T.; et al. An atlas of soybean small RNAs identifies phased siRNAs from hundreds of coding genes. Plant Cell 2014, 26, 4584–4601. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Xu, J.; Arikit, S.; Meyers, B.C. Extensive Families of miRNAs and PHAS Loci in Norway Spruce Demonstrate the Origins of Complex phasiRNA Networks in Seed Plants. Mol. Biol. Evol. 2015, 32, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Cai, T.; Zhang, R.; Li, A.; Huo, N.; Li, S.; Gu, Y.Q.; Vogel, J.; Jia, J.; Qi, Y.; et al. Novel microRNAs uncovered by deep sequencing of small RNA transcriptomes in bread wheat (Triticum aestivum L.) and Brachypodium distachyon (L.) Beauv. Funct. Integr. Genom. 2009, 9, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cheng, X.; Liu, D.; Xu, W.; Wise, R.; Shen, Q.H. The miR9863 family regulates distinct Mla alleles in barley to attenuate NLR receptor-triggered disease resistance and cell-death signaling. PLoS Genet. 2014, 10, e1004755. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cannon, C.H.; Cobb, G.P.; Anderson, T.A. Conservation and divergence of plant microRNA genes. Plant J. Cell Mol. Biol. 2006, 46, 243–259. [Google Scholar] [CrossRef]
- Sunkar, R.; Jagadeeswaran, G. In silico identification of conserved microRNAs in large number of diverse plant species. BMC Plant Biol. 2008, 8, 37. [Google Scholar] [CrossRef]
- Sorin, C.; Declerck, M.; Christ, A.; Blein, T.; Ma, L.; Lelandais-Briere, C.; Njo, M.F.; Beeckman, T.; Crespi, M.; Hartmann, C. A miR169 isoform regulates specific NF-YA targets and root architecture in Arabidopsis. New Phytol. 2014, 202, 1197–1211. [Google Scholar] [CrossRef]
- Zhao, M.; Ding, H.; Zhu, J.K.; Zhang, F.; Li, W.X. Involvement of miR169 in the nitrogen-starvation responses in Arabidopsis. New Phytol. 2011, 190, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Oono, Y.; Zhu, J.; He, X.J.; Wu, J.M.; Iida, K.; Lu, X.Y.; Cui, X.; Jin, H.; Zhu, J.K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 2008, 20, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.E.; Ercoli, M.F.; Debernardi, J.M.; Breakfield, N.W.; Mecchia, M.A.; Sabatini, M.; Cools, T.; De Veylder, L.; Benfey, P.N.; Palatnik, J.F. MicroRNA miR396 Regulates the Switch between Stem Cells and Transit-Amplifying Cells in Arabidopsis Roots. Plant Cell 2015, 27, 3354–3366. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, K.; Liu, Y.; Chen, Y.; Chen, P.; Shi, Z.; Luo, J.; Jiang, D.; Fan, F.; Zhu, Y.; et al. Blocking miR396 increases rice yield by shaping inflorescence architecture. Nat. Plants 2015, 2, 15196. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H. The miR156/SPL Module, a Regulatory Hub and Versatile Toolbox, Gears up Crops for Enhanced Agronomic Traits. Mol. Plant 2015, 8, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Yuan, S.; Li, Z.; Li, D.; Hu, Q.; Luo, H. Heterologous expression of a rice miR395 gene in Nicotiana tabacum impairs sulfate homeostasis. Sci. Rep. 2016, 6, 28791. [Google Scholar] [CrossRef]
- Liang, G.; Yang, F.; Yu, D. MicroRNA395 mediates regulation of sulfate accumulation and allocation in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2010, 62, 1046–1057. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wang, M.; Wang, Z.M.; Fang, R.X.; Wang, X.J.; Jia, Y.T. Dynamic and Coordinated Expression Changes of Rice Small RNAs in Response to Xanthomonas oryzae pv. oryzae. J. Genet. Genom. 2015, 42, 625–637. [Google Scholar] [CrossRef]
- Li, Q.; Deng, C.; Xia, Y.; Kong, L.; Zhang, H.; Zhang, S.; Wang, J. Identification of novel miRNAs and miRNA expression profiling in embryogenic tissues of Picea balfouriana treated by 6-benzylaminopurine. PLoS ONE 2017, 12, e0176112. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, H.; Arikit, S.; Huang, K.; Nan, G.L.; Walbot, V.; Meyers, B.C. Spatiotemporally dynamic, cell-type-dependent premeiotic and meiotic phasiRNAs in maize anthers. Proc. Natl. Acad. Sci. USA 2015, 112, 3146–3151. [Google Scholar] [CrossRef]
- Johnson, C.; Kasprzewska, A.; Tennessen, K.; Fernandes, J.; Nan, G.L.; Walbot, V.; Sundaresan, V.; Vance, V.; Bowman, L.H. Clusters and superclusters of phased small RNAs in the developing inflorescence of rice. Genome Res. 2009, 19, 1429–1440. [Google Scholar] [CrossRef]
- Song, X.; Li, P.; Zhai, J.; Zhou, M.; Ma, L.; Liu, B.; Jeong, D.H.; Nakano, M.; Cao, S.; Liu, C.; et al. Roles of DCL4 and DCL3b in rice phased small RNA biogenesis. Plant J. Cell Mol. Biol. 2012, 69, 462–474. [Google Scholar] [CrossRef]
- Tang, D.; Wang, G.; Zhou, J.M. Receptor Kinases in Plant-Pathogen Interactions: More Than Pattern Recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef]
- Kourelis, J.; van der Hoorn, R.A.L. Defended to the Nines: 25 Years of Resistance Gene Cloning Identifies Nine Mechanisms for R Protein Function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, L.; Boller, T. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 2000, 5, 1003–1011. [Google Scholar] [CrossRef]
- Trda, L.; Fernandez, O.; Boutrot, F.; Heloir, M.C.; Kelloniemi, J.; Daire, X.; Adrian, M.; Clement, C.; Zipfel, C.; Dorey, S.; et al. The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin-derived epitopes from the endophytic growth-promoting bacterium Burkholderia phytofirmans and plant pathogenic bacteria. New Phytol. 2014, 201, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Pitino, M.; Duan, Y.; Stover, E. Reduced Susceptibility to Xanthomonas citri in Transgenic Citrus Expressing the FLS2 Receptor From Nicotiana benthamiana. Mol. Plant Microbe Interact. 2016, 29, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, Y.; Takai, R.; Furukawa, T.; Hirai, H.; Morimoto, T.; Katayama, T.; Murakami, T.; Che, F.S. CD2-1, the C-Terminal Region of Flagellin, Modulates the Induction of Immune Responses in Rice. Mol. Plant Microbe Interact. 2015, 28, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Robatzek, S.; Bittel, P.; Chinchilla, D.; Kochner, P.; Felix, G.; Shiu, S.H.; Boller, T. Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities. Plant Mol. Biol. 2007, 64, 539–547. [Google Scholar] [CrossRef]
- Zipfel, C.; Kunze, G.; Chinchilla, D.; Caniard, A.; Jones, J.D.; Boller, T.; Felix, G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 2006, 125, 749–760. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Huffaker, A.; Bryan, A.C.; Tax, F.E.; Ryan, C.A. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 2010, 22, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Albert, I.; Bohm, H.; Albert, M.; Feiler, C.E.; Imkampe, J.; Wallmeroth, N.; Brancato, C.; Raaymakers, T.M.; Oome, S.; Zhang, H.; et al. An RLP23-SOBIR1-BAK1 complex mediates NLP-triggered immunity. Nat. Plants 2015, 1, 15140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fraiture, M.; Kolb, D.; Loffelhardt, B.; Desaki, Y.; Boutrot, F.F.; Tor, M.; Zipfel, C.; Gust, A.A.; Brunner, F. Arabidopsis receptor-like protein30 and receptor-like kinase suppressor of BIR1-1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell 2013, 25, 4227–4241. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, L.; Macho, A.P.; Han, Z.; Hu, Z.; Zipfel, C.; Zhou, J.M.; Chai, J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 2013, 342, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Z.; Song, C.; Hu, Y.; Han, Z.; She, J.; Fan, F.; Wang, J.; Jin, C.; Chang, J.; et al. Chitin-induced dimerization activates a plant immune receptor. Science 2012, 336, 1160–1164. [Google Scholar] [CrossRef]
- Hayafune, M.; Berisio, R.; Marchetti, R.; Silipo, A.; Kayama, M.; Desaki, Y.; Arima, S.; Squeglia, F.; Ruggiero, A.; Tokuyasu, K.; et al. Chitin-induced activation of immune signaling by the rice receptor CEBiP relies on a unique sandwich-type dimerization. Proc. Natl. Acad. Sci. USA 2014, 111, E404–E413. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Bi, G.; Zhou, Z.; Wang, W.; Li, L.; Rao, S.; Wu, Y.; Zhang, X.; Menke, F.L.H.; Chen, S.; Zhou, J.M. Receptor-like Cytoplasmic Kinases Directly Link Diverse Pattern Recognition Receptors to the Activation of Mitogen-activated Protein Kinase Cascades in Arabidopsis. Plant Cell 2018. [Google Scholar] [CrossRef]
- Li, W.; Zhong, S.; Li, G.; Li, Q.; Mao, B.; Deng, Y.; Zhang, H.; Zeng, L.; Song, F.; He, Z. Rice RING protein OsBBI1 with E3 ligase activity confers broad-spectrum resistance against Magnaporthe oryzae by modifying the cell wall defence. Cell Res. 2011, 21, 835–848. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, H.; Deng, Y.; Xiao, J.; Li, X.; Wang, S. The WRKY45-2 WRKY13 WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol. 2015, 167, 1087–1099. [Google Scholar] [CrossRef]
- Qiu, D.; Xiao, J.; Ding, X.; Xiong, M.; Cai, M.; Cao, Y.; Li, X.; Xu, C.; Wang, S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant Microbe Interact. 2007, 20, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Boden, E.; Arias, J. Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell 2003, 15, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yuan, M.; Ai, C.; Liu, L.; Zhuang, E.; Karapetyan, S.; Wang, S.; Dong, X. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature 2017, 545, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Huang, G.; Allen, R.; Davis, E.L.; Baum, T.J.; Hussey, R.S. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA 2006, 103, 14302–14306. [Google Scholar] [CrossRef]
- Pliego, C.; Nowara, D.; Bonciani, G.; Gheorghe, D.M.; Xu, R.; Surana, P.; Whigham, E.; Nettleton, D.; Bogdanove, A.J.; Wise, R.P.; et al. Host-induced gene silencing in barley powdery mildew reveals a class of ribonuclease-like effectors. Mol. Plant Microbe Interact. 2013, 26, 633–642. [Google Scholar] [CrossRef]
- Panwar, V.; McCallum, B.; Bakkeren, G. Host-induced gene silencing of wheat leaf rust fungus Puccinia triticina pathogenicity genes mediated by the Barley stripe mosaic virus. Plant Mol. Biol. 2013, 81, 595–608. [Google Scholar] [CrossRef]
- Panwar, V.; McCallum, B.; Bakkeren, G. Endogenous silencing of Puccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J. Cell Mol. Biol. 2013, 73, 521–532. [Google Scholar] [CrossRef]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.H. Host-induced gene silencing of cytochrome P450 lanosterol C14alpha-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef]
- Jahan, S.N.; Asman, A.K.; Corcoran, P.; Fogelqvist, J.; Vetukuri, R.R.; Dixelius, C. Plant-mediated gene silencing restricts growth of the potato late blight pathogen Phytophthora infestans. J. Exp. Bot. 2015, 66, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Vega-Arreguin, J.C.; Jalloh, A.; Bos, J.I.; Moffett, P. Recognition of an Avr3a homologue plays a major role in mediating nonhost resistance to Phytophthora capsici in Nicotiana species. Mol. Plant Microbe Interact. 2014, 27, 770–780. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.; Huang, H.D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef] [PubMed]
- Timmons, L.; Court, D.L.; Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001, 263, 103–112. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-Based Control of Fusarium graminearum Infections Through Spraying of Long dsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Murat, F.; Pont, C.; Langin, T.; Salse, J. Paleo-evolutionary plasticity of plant disease resistance genes. BMC Genom. 2014, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- El Kasmi, F.; Chung, E.H.; Anderson, R.G.; Li, J.; Wan, L.; Eitas, T.K.; Gao, Z.; Dangl, J.L. Signaling from the plasma-membrane localized plant immune receptor RPM1 requires self-association of the full-length protein. Proc. Natl. Acad. Sci. USA 2017, 114, E7385–E7394. [Google Scholar] [CrossRef]
- Ilag, L.L.; Yadav, R.C.; Huang, N.; Ronald, P.C.; Ausubel, F.M. Isolation and characterization of disease resistance gene homologues from rice cultivar IR64. Gene 2000, 255, 245–255. [Google Scholar] [CrossRef]
- Qi, D.; DeYoung, B.J.; Innes, R.W. Structure-function analysis of the coiled-coil and leucine-rich repeat domains of the RPS5 disease resistance protein. Plant Physiol. 2012, 158, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Shirano, Y.; Kachroo, P.; Shah, J.; Klessig, D.F. A gain-of-function mutation in an Arabidopsis Toll Interleukin1 receptor-nucleotide binding site-leucine-rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell 2002, 14, 3149–3162. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, M.; Shirasu, K.; Noutoshi, Y.; Kubo, Y.; Shiraishi, T.; Iwabuchi, M.; Narusaka, Y. RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. Plant J. Cell Mol. Biol. 2009, 60, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Bittner-Eddy, P.D.; Crute, I.R.; Holub, E.B.; Beynon, J.L. RPP13 is a simple locus in Arabidopsis thaliana for alleles that specify downy mildew resistance to different avirulence determinants in Peronospora parasitica. Plant J. 2000, 21, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Botella, M.A.; Parker, J.E.; Frost, L.N.; Bittner-Eddy, P.D.; Beynon, J.L.; Daniels, M.J.; Holub, E.B.; Jones, J.D. Three genes of the Arabidopsis RPP1 complex resistance locus recognize distinct Peronospora parasitica avirulence determinants. Plant Cell 1998, 10, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Van der Biezen, E.A.; Freddie, C.T.; Kahn, K.; Parker, J.E.; Jones, J.D. Arabidopsis RPP4 is a member of the RPP5 multigene family of TIR-NB-LRR genes and confers downy mildew resistance through multiple signalling components. Plant J. Cell Mol. Biol. 2002, 29, 439–451. [Google Scholar] [CrossRef]
- Parker, J.E.; Coleman, M.J.; Szabo, V.; Frost, L.N.; Schmidt, R.; van der Biezen, E.A.; Moores, T.; Dean, C.; Daniels, M.J.; Jones, J.D. The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell 1997, 9, 879–894. [Google Scholar] [CrossRef]
- Noel, L.; Moores, T.L.; van Der Biezen, E.A.; Parniske, M.; Daniels, M.J.; Parker, J.E.; Jones, J.D. Pronounced intraspecific haplotype divergence at the RPP5 complex disease resistance locus of Arabidopsis. Plant Cell 1999, 11, 2099–2112. [Google Scholar] [CrossRef]
- Bryan, G.T.; Wu, K.S.; Farrall, L.; Jia, Y.; Hershey, H.P.; McAdams, S.A.; Faulk, K.N.; Donaldson, G.K.; Tarchini, R.; Valent, B. tA single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 2000, 12, 2033–2046. [Google Scholar] [CrossRef]
- Cesari, S.; Thilliez, G.; Ribot, C.; Chalvon, V.; Michel, C.; Jauneau, A.; Rivas, S.; Alaux, L.; Kanzaki, H.; Okuyama, Y.; et al. The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell 2013, 25, 1463–1481. [Google Scholar] [CrossRef]
- Liu, X.; Lin, F.; Wang, L.; Pan, Q. The in silico map-based cloning of Pi36, a rice coiled-coil nucleotide-binding site leucine-rich repeat gene that confers race-specific resistance to the blast fungus. Genetics 2007, 176, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Liu, G.; Zhou, B.; Bellizzi, M.; Zeng, L.; Dai, L.; Han, B.; Wang, G.L. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 2006, 172, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Qu, S.; Liu, G.; Dolan, M.; Sakai, H.; Lu, G.; Bellizzi, M.; Wang, G.L. The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol. Plant Microbe Interact. 2006, 19, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Tao, Y.; Chen, X.; Zou, Y.; Lei, C.; Wang, J.; Li, X.; Zhao, X.; Zhang, M.; Lu, Z.; et al. Identification of a new rice blast resistance gene, Pid3, by genomewide comparison of paired nucleotide-binding site--leucine-rich repeat genes and their pseudogene alleles between the two sequenced rice genomes. Genetics 2009, 182, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Song, M.Y.; Seo, Y.S.; Kim, H.K.; Ko, S.; Cao, P.J.; Suh, J.P.; Yi, G.; Roh, J.H.; Lee, S.; et al. Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes. Genetics 2009, 181, 1627–1638. [Google Scholar] [CrossRef]
- Kawano, Y.; Akamatsu, A.; Hayashi, K.; Housen, Y.; Okuda, J.; Yao, A.; Nakashima, A.; Takahashi, H.; Yoshida, H.; Wong, H.L.; et al. Activation of a Rac GTPase by the NLR family disease resistance protein Pit plays a critical role in rice innate immunity. Cell Host Microbe 2010, 7, 362–375. [Google Scholar] [CrossRef]
- Yuan, B.; Zhai, C.; Wang, W.; Zeng, X.; Xu, X.; Hu, H.; Lin, F.; Wang, L.; Pan, Q. The Pik-p resistance to Magnaporthe oryzae in rice is mediated by a pair of closely linked CC-NBS-LRR genes. Theor. Appl. Genet. 2011, 122, 1017–1028. [Google Scholar] [CrossRef]
- Okuyama, Y.; Kanzaki, H.; Abe, A.; Yoshida, K.; Tamiru, M.; Saitoh, H.; Fujibe, T.; Matsumura, H.; Shenton, M.; Galam, D.C.; et al. A multifaceted genomics approach allows the isolation of the rice Pia-blast resistance gene consisting of two adjacent NBS-LRR protein genes. Plant J. Cell Mol. Biol. 2011, 66, 467–479. [Google Scholar] [CrossRef]
- Lin, F.; Chen, S.; Que, Z.; Wang, L.; Liu, X.; Pan, Q. The blast resistance gene Pi37 encodes a nucleotide binding site leucine-rich repeat protein and is a member of a resistance gene cluster on rice chromosome 1. Genetics 2007, 177, 1871–1880. [Google Scholar] [CrossRef]
- Sakamoto, K.; Tada, Y.; Yokozeki, Y.; Akagi, H.; Hayashi, N.; Fujimura, T.; Ichikawa, N. Chemical induction of disease resistance in rice is correlated with the expression of a gene encoding a nucleotide binding site and leucine-rich repeats. Plant Mol. Biol. 1999, 40, 847–855. [Google Scholar] [CrossRef]
- Bai, S.; Liu, J.; Chang, C.; Zhang, L.; Maekawa, T.; Wang, Q.; Xiao, W.; Liu, Y.; Chai, J.; Takken, F.L.; et al. Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 2012, 8, e1002752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Kurth, J.; Wei, F.; Elliott, C.; Vale, G.; Yahiaoui, N.; Keller, B.; Somerville, S.; Wise, R.; Schulze-Lefert, P. Cell-autonomous expression of barley Mla1 confers race-specific resistance to the powdery mildew fungus via a Rar1-independent signaling pathway. Plant Cell 2001, 13, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hein, I.; Campbell, E.I.; Woodhead, M.; Hedley, P.E.; Young, V.; Morris, W.L.; Ramsay, L.; Stockhaus, J.; Lyon, G.D.; Newton, A.C.; et al. Characterisation of early transcriptional changes involving multiple signalling pathways in the Mla13 barley interaction with powdery mildew (Blumeria graminis f. sp. hordei). Planta 2004, 218, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Lawrence, G.J.; Ellis, J.G. Six amino acid changes confined to the leucine-rich repeat beta-strand/beta-turn motif determine the difference between the P and P2 rust resistance specificities in flax. Plant Cell 2001, 13, 163–178. [Google Scholar] [CrossRef]
- Lawrence, G.; Finnegan, J.; Ellis, J. Instability of the L6 gene for rust resistance in flax is correlated with the presence of a linked Ac element. Plant J. Cell Mol. Biol. 1993, 4, 659–669. [Google Scholar] [CrossRef]
- Lawrence, G.J.; Anderson, P.A.; Dodds, P.N.; Ellis, J.G. Relationships between rust resistance genes at the M locus in flax. Mol. Plant Pathol. 2010, 11, 19–32. [Google Scholar] [CrossRef]
- Ellis, J.G.; Lawrence, G.J.; Luck, J.E.; Dodds, P.N. Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 1999, 11, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Ravensdale, M.; Bernoux, M.; Ve, T.; Kobe, B.; Thrall, P.H.; Ellis, J.G.; Dodds, P.N. Intramolecular interaction influences binding of the Flax L5 and L6 resistance proteins to their AvrL567 ligands. PLoS Pathog. 2012, 8, e1003004. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G.; Lawrence, G.J.; Dodds, P.N. Further analysis of gene-for-gene disease resistance specificity in flax. Mol. Plant Pathol. 2007, 8, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Farah, N.; Rolston, L.; Ericsson, D.J.; Catanzariti, A.M.; Bernoux, M.; Ve, T.; Bendak, K.; Chen, C.; Mackay, J.P.; et al. Crystal structure of the Melampsora lini effector AvrP reveals insights into a possible nuclear function and recognition by the flax disease resistance protein P. Mol. Plant Pathol. 2018, 19, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Yamanouchi, U.; Katayose, Y.; Toki, S.; Wang, Z.X.; Kono, I.; Kurata, N.; Yano, M.; Iwata, N.; Sasaki, T. Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc. Natl. Acad. Sci. USA 1998, 95, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Bendahmane, A.; Querci, M.; Kanyuka, K.; Baulcombe, D.C. Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: Application to the Rx2 locus in potato. Plant J. Cell Mol. Biol. 2000, 21, 73–81. [Google Scholar] [CrossRef]
- Mago, R.; Zhang, P.; Vautrin, S.; Simkova, H.; Bansal, U.; Luo, M.C.; Rouse, M.; Karaoglu, H.; Periyannan, S.; Kolmer, J.; et al. The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus. Nat. Plants 2015, 1, 15186. [Google Scholar] [CrossRef] [PubMed]
- Saintenac, C.; Zhang, W.; Salcedo, A.; Rouse, M.N.; Trick, H.N.; Akhunov, E.; Dubcovsky, J. Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science 2013, 341, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Kema, G.H.J.; Mirzadi Gohari, A.; Aouini, L.; Gibriel, H.A.Y.; Ware, S.B.; van den Bosch, F.; Manning-Smith, R.; Alonso-Chavez, V.; Helps, J.; Ben M’Barek, S.; et al. Stress and sexual reproduction affect the dynamics of the wheat pathogen effector AvrStb6 and strobilurin resistance. Nat. Genet. 2018, 50, 375–380. [Google Scholar] [CrossRef]
- Tian, D.; Wang, J.; Zeng, X.; Gu, K.; Qiu, C.; Yang, X.; Zhou, Z.; Goh, M.; Luo, Y.; Murata-Hori, M.; et al. The rice TAL effector-dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell 2014, 26, 497–515. [Google Scholar] [CrossRef]
- Wang, J.; Tian, D.; Gu, K.; Yang, X.; Wang, L.; Zeng, X.; Yin, Z. Induction of Xa10-like Genes in Rice Cultivar Nipponbare Confers Disease Resistance to Rice Bacterial Blight. Mol. Plant Microbe Interact. 2017, 30, 466–477. [Google Scholar] [CrossRef]
- Brunner, S.; Stirnweis, D.; Diaz Quijano, C.; Buesing, G.; Herren, G.; Parlange, F.; Barret, P.; Tassy, C.; Sautter, C.; Winzeler, M.; et al. Transgenic Pm3 multilines of wheat show increased powdery mildew resistance in the field. Plant Biotechnol. J. 2012, 10, 398–409. [Google Scholar] [CrossRef]
- Molnar, A.; Melnyk, C.W.; Bassett, A.; Hardcastle, T.J.; Dunn, R.; Baulcombe, D.C. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 2010, 328, 872–875. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef]
- Shahid, S.; Kim, G.; Johnson, N.R.; Wafula, E.; Wang, F.; Coruh, C.; Bernal-Galeano, V.; Phifer, T.; dePamphilis, C.W.; Westwood, J.H.; et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Thomas, N.; Jin, H. Cross-kingdom RNA trafficking and environmental RNAi for powerful innovative pre- and post-harvest plant protection. Curr. Opin. Plant Biol. 2017, 38, 133–141. [Google Scholar] [CrossRef]
- Shiu, S.H.; Karlowski, W.M.; Pan, R.; Tzeng, Y.H.; Mayer, K.F.; Li, W.H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004, 16, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Dievart, A.; Droc, G.; Dufayard, J.F.; Chantret, N. Evolutionary Dynamics of the Leucine-Rich Repeat Receptor-Like Kinase (LRR-RLK) Subfamily in Angiosperms. Plant Physiol. 2016, 170, 1595–1610. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.L.; Du, L.; Huang, Y.; Gao, S.M.; Yu, M. Origin and diversification of leucine-rich repeat receptor-like protein kinase (LRR-RLK) genes in plants. BMC Evol. Biol. 2017, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Akita, M.; Valkonen, J.P. A novel gene family in moss (Physcomitrella patens) shows sequence homology and a phylogenetic relationship with the TIR-NBS class of plant disease resistance genes. J. Mol. Evol. 2002, 55, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Bertin, J.; Nir, W.J.; Fischer, C.M.; Tayber, O.V.; Errada, P.R.; Grant, J.R.; Keilty, J.J.; Gosselin, M.L.; Robison, K.E.; Wong, G.H.; et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-κB. J. Biol. Chem. 1999, 274, 12955–12958. [Google Scholar] [CrossRef]
- Meyers, B.C.; Morgante, M.; Michelmore, R.W. TIR-X and TIR-NBS proteins: Two new families related to disease resistance TIR-NBS-LRR proteins encoded in Arabidopsis and other plant genomes. Plant J. Cell Mol. Biol. 2002, 32, 77–92. [Google Scholar] [CrossRef]
- Liu, J.J.; Ekramoddoullah, A.K. Isolation, genetic variation and expression of TIR-NBS-LRR resistance gene analogs from western white pine (Pinus monticola Dougl. ex. D. Don.). Mol. Genet. Genom. 2003, 270, 432–441. [Google Scholar] [CrossRef]
- Girardin, S.E.; Sansonetti, P.J.; Philpott, D.J. Intracellular vs extracellular recognition of pathogens--common concepts in mammals and flies. Trends Microbiol. 2002, 10, 193–199. [Google Scholar] [CrossRef]
- Bai, J.; Pennill, L.A.; Ning, J.; Lee, S.W.; Ramalingam, J.; Webb, C.A.; Zhao, B.; Sun, Q.; Nelson, J.C.; Leach, J.E.; et al. Diversity in nucleotide binding site-leucine-rich repeat genes in cereals. Genome Res. 2002, 12, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, X.; Yue, J.X.; Tian, D.; Chen, J.Q. Recent duplications dominate NBS-encoding gene expansion in two woody species. Mol. Genet. Genom. 2008, 280, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.W.; Paidi, M.; Ming, R.; Alam, M.; Nishijima, W.T.; Zhu, Y.J. Genome-wide analysis of Carica papaya reveals a small NBS resistance gene family. Mol. Genet. Genom. 2009, 281, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, Y.; Hu, Q.; Chen, J.; Li, K.; Lu, C.; Liu, H.; Wang, W.; Kuang, H. Dynamic nucleotide-binding site and leucine-rich repeat-encoding genes in the grass family. Plant Physiol. 2012, 159, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Fitz, J.; Schneeberger, K.; Ossowski, S.; Cao, J.; Weigel, D. Genome-wide comparison of nucleotide-binding site-leucine-rich repeat-encoding genes in Arabidopsis. Plant Physiol. 2011, 157, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Araki, H.; Chen, L.; Chen, J.Q.; Tian, D. Unique evolutionary mechanism in R-genes under the presence/absence polymorphism in Arabidopsis thaliana. Genetics 2006, 172, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, Z.; Zhang, X.; Jiang, K.; Jin, X.; Hang, Y.; Chen, J.Q.; Tian, D. Genome-wide investigation on the genetic variations of rice disease resistance genes. Plant Mol. Biol. 2006, 62, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wu, H.; Zhang, R.; Li, S.; He, W.; Wong, F.L.; Li, G.; Zhao, S.; Lam, H.M. Molecular phylogeny and dynamic evolution of disease resistance genes in the legume family. BMC Genom. 2016, 17, 402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qi, T.; Yang, Q.; He, F.; Tan, C.; Ma, W.; Voegele, R.T.; Kang, Z.; Guo, J. Host-Induced Gene Silencing of the MAPKK Gene PsFUZ7 Confers Stable Resistance to Wheat Stripe Rust. Plant Physiol. 2017, 175, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Zhu, X.; Tan, C.; Liu, P.; Guo, J.; Kang, Z.; Guo, J. Host-induced gene silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnol. J. 2018, 16, 797–807. [Google Scholar] [CrossRef]

| Species | Nb Chr. | Size (Mbp) | Nb Gene | R-Genes | |||
|---|---|---|---|---|---|---|---|
| Nb R-Genes | (%) 1 | Nb MiRNA Targets | (%) 2 | ||||
| Monocots | |||||||
| Oryza sativa (rice) | 12 | 372 | 41,046 | 1196 | 2.91 | 144 | 12.04 |
| Sorghum bicolor | 10 | 659 | 34,008 | 625 | 1.84 | 109 | 17.44 |
| Zea mays (maize) | 10 | 2365 | 32,540 | 673 | 2.07 | 0 | 0 |
| Brachypodium distachyon | 5 | 271 | 25,504 | 537 | 2.11 | 149 | 27.75 |
| Eudicots | |||||||
| Arabidopsis thaliana | 5 | 119 | 33,198 | 503 | 1.52 | 81 | 16.1 |
| Populus trichocarpa | 19 | 294 | 30,260 | 446 | 1.47 | 382 | 85.65 |
| Carica papaya | 9 | 234 | 19,205 | 228 | 1.19 | 0 | 0 |
| Glycine max | 20 | 949 | 46,164 | 1171 | 2.54 | 290 | 24.77 |
| Malusxdomestica (apple) | 17 | 742 | 58,979 | 2052 | 3.48 | 256 | 12.48 |
| Plant Species | Disease | Pathogens | Avirus Genes | Types of Pathogens | Genes | Types | GenBank Locus |
|---|---|---|---|---|---|---|---|
| Arabidopsis thaliana | White rust of crucifers | Albugo candida | Oomycetes | RAC1 | TNL | AY522496 | |
| Cucumber Mosaic Virus | Cucumber mosaic virus | Virus | RCY1 | CNL | AB087829 | ||
| Bacterial Blight | Pseudomonas syringae/Xanthomonas oryzae | avrRpm1; avrRpt2; avrPphB; N; avrRps4 | Bacterium | RPM1; Rps2; RPS5; SSI4; Rps4 | CNL; CNL; CNL; TNL; TNL | NM_111584; NM_118742; NM_101094; AY179750; NM_123893 | |
| Downy mildew of cucurbits | Pseudoperonospora cubensis | Oomycetes | RPP13/RPP8; RPP1/RPP4; RPP5 | CNL/CNL; TNL/TNL; TNL | NM_114520/NM_123713; NM_114316/NM_117790; NM_117798 | ||
| Bacterial wilt of potato | Ralstonia solanacearum | Bacterium | RRS1 | TNL | NM_001085246 | ||
| Turnip crinkle virus | Turnip crinkle virus | Virus | HRT | CNL | NM_128190 | ||
| Aegilops tauschii | Cereal cyst nematode | Heterodera avenae | Nematode | Cre1 | CNL | AY124651 | |
| Capsicum chacoense | Bacterial spot of tomato | Xanthomonas campestris | AvrBs2 | Bacterium | Bs2 | CNL | AF202179 |
| Capsicum chinense | Pepper mild mottle virus | Pepper mild mottle virus | Virus | L3 | CNL | BAJ33559 | |
| Cucumis melo | Fusarium Wilt | Fusarium oxysporum | Fungal | FOM-2 | CNL | DQ287965 | |
| Melon aphid disease | Aphis gossypii | insect | VAT | CNL | AGH33848 | ||
| zucchini yellow mosaic virus | zucchini yellow mosaic virus | Virus | FOM1 | TNL | AIU36098 | ||
| Glycine max | Soybean mosaic virus | soybean mosaic virus | Virus | KR1 | TNL | AF327903 | |
| Helianthus annuus | Downy mildew of sunflower | Plasmopara halstedii | Oomycetes | Pl8 | CNL | AY490793 | |
| Hordeum vulgare | Powdery mildew (barley) | Blumeria graminis | Fungal | MLA10/MLA1/MLA13 | CNL | AY266445; GU245961; AF523683 | |
| Lactuca sativa | Downy mildew of lettuce | Bremia lactucae | Avr3 | Oomycetes | Dm3 (RGC2B) | CNL | AH007213 |
| Linum usitatissimum | Flax rust | Melampsora lini | Fungal | P2/L6/M; L,L1-L11; P,P1-4 | TNL; TNL; TNL | AF310960/U27081/U73916; AAD25974/AAK28806 | |
| Nicotiana glutinosa | Tobacco Mosaic Virus | Tobacco mosaic virus | Virus | N | TNL | U15605 | |
| Oryza sativa | Rice blast disease | Magnaporthe grisea | Avr-Pita | Fungal | Pi-ta/PIB | CNL | AY196754 |
| Bacterial Blight | Pseudomonas syringae/Xanthomonas oryzae | Bacterium | XA1 | CNL | AB002266 | ||
| Rice blast disease | Magnaporthe oryzae | Fungal | RGA5 | CNL | EU883792 | ||
| Oryza sativa Indica Group | Rice blast disease | Magnaporthe grisea | Fungal | Pi36/Pi9/Pi2 | CNL | DQ900896/DQ285630/DQ352453 | |
| Oryza sativa Japonica Group | Rice blast disease | Magnaporthe grisea | Fungal | Piz-t/Pikm1-TS/Pikm2-TS/Pid3/Pi5-1/Pi5-2/Pit/Pikp-2 | CNL | DQ352040/AB462324/AB462325/FJ773286/EU869185/EU869186/AB379816/HM035360 | |
| Rice blast disease | Magnaporthe oryzae | Fungal | Pia; Pi37; Rpr1 | CNL; CNL; CNL | AB604626; DQ923494; AC119670 | ||
| Solanum acaule | Latent mosaic of potato/Beet cyst nematode | Potato virus X/Heterodera schachtii | Virus/Nematode | Rx2 | CNL | AJ249448 | |
| Solanum bulbocastanum | Late Blight of tomato | Phytophthora infestans | Oomycete | Rpi-blb1/Rpi-blb2; RB | CNL; CNL | AY336128; DQ122125; AY426259 | |
| Solanum demissum | Late Blight of tomato | Phytophthora infestans | Oomycete | R1 | CNL | AF447489 | |
| Solanum lycopersicum | Bacterial spot of tomato | Xanthomonas campestris | Hax4/AvrBs4 | Bacterium | Bs4 | TNL | AY438027 |
| Yellow potato cyst nematode | Yellow potato cyst nematode | Nematode | Hero | CNL | AJ457052 | ||
| root-knot nematode | Meloidogyne incognita | Nematode | Mi1.2 | CNL | AF039682 | ||
| Tomato Spotted Wilt | Tomato spotted wilt virus | Virus | Sw-5 | CNL | AY007366 | ||
| Tobacco Mosaic Virus | Tobacco mosaic virus | MP | Virus | Tm-2a/Tm-2 | CNL | F536201/AF536200 | |
| Solanum pimpinellifolium | Bacterial Speck of tomato | Pseudomonas syringae | AvrPto/AvrPtoB | Bacterium | Prf | CNL | AF220602 |
| Late blight | Phytophthora infestans | Oomycete | Ph-3 | CNL | KJ563933 | ||
| Solanum tuberosum | Yellow potato cyst nematode | Globodera | Nematode | Gpa2 | CNL | AF195939 | |
| Late Blight of potato | Phytophthora infestans | Nematode | Gro1.4 | TNL | AY196151 | ||
| Latent mosaic of potato/Beet cyst nematode | Potato virus X/Heterodera schachtii | Virus | Rx | CNL | AJ011801 | ||
| Solanum tuberosum subsp andigena | Potato virus Y | Potato virus Y | Virus | RY-1 | TNL | AJ300266 | |
| Triticum aestivum | Brown wheat rust of potato | Puccinia triticina | Fungal | Lr10/Lr21/Lr1 | CNL | AY270157/FJ876280/EF439840 | |
| powdery mildew | Blumeria graminis f. sp. Tritici | Fungal | Pm3 | CNL | AY325736 | ||
| stem rust | Puccinia graminis f. sp. Tritici | Fungal | Sr33 | CNL | KF031303 | ||
| Nematode disease | Heterodera avenae | Nematode | Cre3 | CNL | AF052641 | ||
| Yellow rust | Puccinia striiformis Westend. f.sp. Tritici | Fungal | Yr10 | CNL | AF149114 | ||
| Triticum monococcum subsp. Monococcum | stem rust | Puccinia graminis f. sp. Tritici | Fungal | Sr35 | CNL | AGP75918 | |
| Zea mays | Common rust of maize | Puccinia sorghi | Fungal | Rp1-D | CNL | AF107293 |
| Plant miRNAs | Immunity Response | Targets in Plants or Pathogens | Positive (+)/Negative (−) Regulator | Pathogens | |
|---|---|---|---|---|---|
| Classification | Pathogen/Plant | ||||
| miR393 | PTI | F-box auxin receptors | Positive | Bacteria | Pseudomonas syringae/Arabidopsis |
| miR160a | PTI | auxin response factors16 | Positive | Bacteria | Pseudomonas syringae/Arabidopsis |
| miR319 | PTI | TCP21 | Negative | Virus | Rice ragged stunt virus (RRSV)/RICE |
| miR773 | PTI | METHYLTRANSFERASE 2 | Negative | Bacteria; Fungul | Pseudomonas syringae/Arabidopsis; Plectosphaerella cucumerina/Arabidopsis |
| miR169 | PTI | NFYA | Negative | Bacteria; Fungul | Magnaporthe oryzae/RICE |
| miR396 | PTI | GRF | Negative | Fungul | Plectosphaerella cucumerina/Arabidopsis |
| miR156 | PTI | MdWRKYN1 | Negative | Fungul | Alternaria alternaria/APPLE |
| miR395 | PTI | MdWRKY26 | Negative | Fungul | Alternaria alternaria/APPLE |
| miR5272 | PTI | MKK6 | Negative | Fungul | Fusarium oxysporum/COTTON |
| MIR398 | PTI | CSD2 | Negative | Bacteria | Pseudomonas syringae/Arabidopsis |
| miR164 | PTI | NAC | Negative | Fungul | Magnaporthe oryzae/RICE |
| miR393* | ETI | MEMB12 (Membrin 12) | Positive | Bacteria | Pseudomonas syringae/Arabidopsis |
| miR444 | ETI | MADS | Positive | Virus | Rice stripe virus (RSV)/RICE |
| miR171 | ETI | OsSCL6-Iia/b/c | Positive | Virus | Rice stripe virus (RSV)/RICE |
| miR863-3p | ETI | ARLPK1&ARLPK2 | Positive | Bacteria | Pseudomonas syringae/Arabidopsis |
| miR863-3p | ETI | SERRATE | Negative | Bacteria | Pseudomonas syringae/Arabidopsis |
| MIR9863 | ETI | NBS-LRR | Negative | Fungul | Blumeria graminis/Barley |
| MIR482 | ETI | NBS-LRR | Negative | Fungul | Fusarium oxysporum/Tomato |
| MIR5300 | ETI | NBS-LRR | Negative | Fungul | Fusarium oxysporum/Tomato |
| miR1510 | ETI | NBS-LRR | Negative | Fungul | Phytophthora sojae/Soybean |
| miR6019 | ETI | NBS-LRR | Negative | Virus | Tobacco mosaic virus/Tobacco |
| miR6020 | ETI | NBS-LRR | Negative | Virus | Tobacco mosaic virus/Tobacco |
| miR1885 | ETI | NBS-LRR | Negative | Virus | Turnip mosaic virus/Brassica |
| miR472 | ETI | NBS-LRR | Negative | Bacteria | Pseudomonas syringae/Arabidopsis |
| miR166 | CKRI | Clp-1 1 | Positive | Fungul | Verticillium dahliae/Cotton |
| miR159 | CKRI | HiC-15 1 | Positive | Fungul | Verticillium dahliae/Cotton |
| TAS1c-siR483 | CKRI | Bc-Vps51&Bc-DCTN1 1 | Positive | Fungul | Botrytis cinerea /Arabidopsis |
| TAS2-siR453 | CKRI | BC1T_08464 1 | Positive | Fungul | Botrytis cinerea /Arabidopsis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zheng, F.; Wei, S.; Zhang, S.; Li, G.; Cao, P.; Zhao, S. Evolution of Disease Defense Genes and Their Regulators in Plants. Int. J. Mol. Sci. 2019, 20, 335. https://doi.org/10.3390/ijms20020335
Zhang R, Zheng F, Wei S, Zhang S, Li G, Cao P, Zhao S. Evolution of Disease Defense Genes and Their Regulators in Plants. International Journal of Molecular Sciences. 2019; 20(2):335. https://doi.org/10.3390/ijms20020335
Chicago/Turabian StyleZhang, Rongzhi, Fengya Zheng, Shugen Wei, Shujuan Zhang, Genying Li, Peijian Cao, and Shancen Zhao. 2019. "Evolution of Disease Defense Genes and Their Regulators in Plants" International Journal of Molecular Sciences 20, no. 2: 335. https://doi.org/10.3390/ijms20020335
APA StyleZhang, R., Zheng, F., Wei, S., Zhang, S., Li, G., Cao, P., & Zhao, S. (2019). Evolution of Disease Defense Genes and Their Regulators in Plants. International Journal of Molecular Sciences, 20(2), 335. https://doi.org/10.3390/ijms20020335





