Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis
Abstract
1. Introduction
2. Polyphenol Effects on Cardiovascular Disease (CVD)
2.1. Impact of Polyphenols on Cardiovascular Disease in Humans
2.2. Mechanisms of Cardioprotection
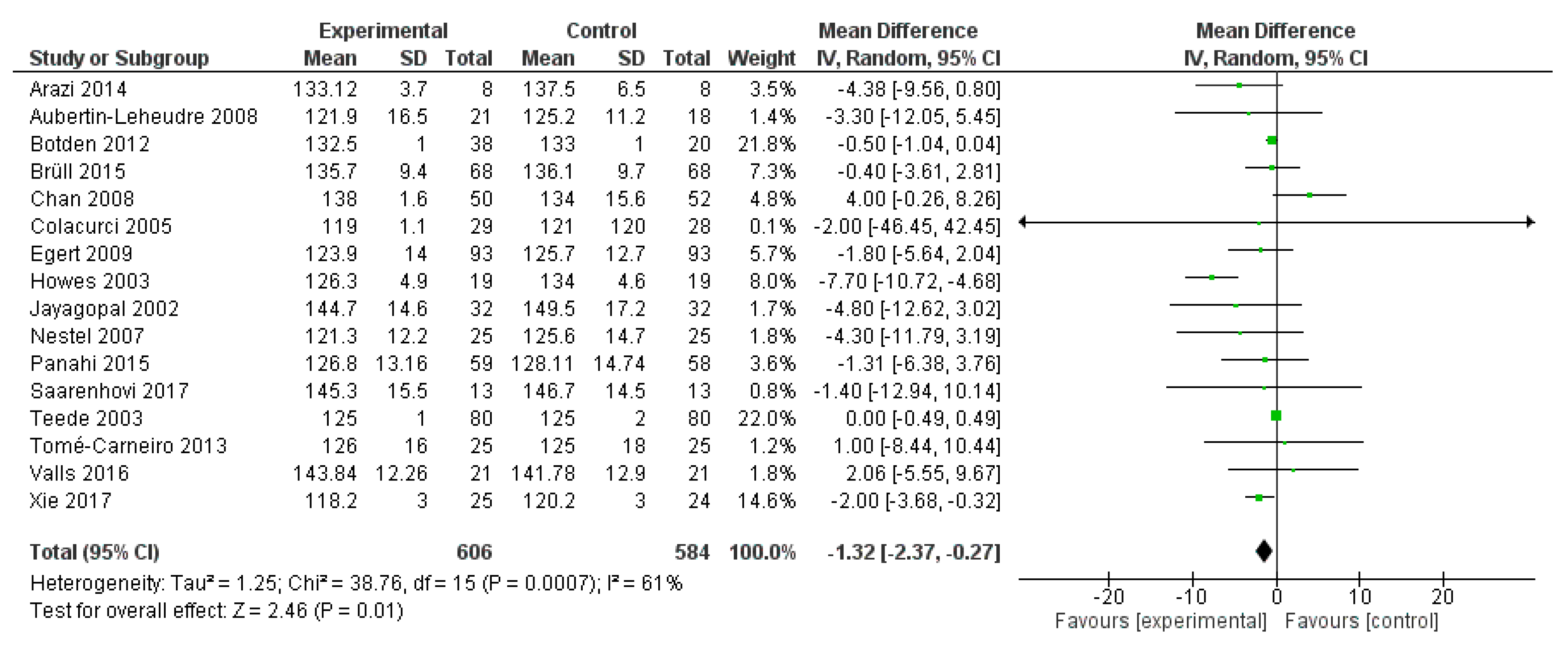
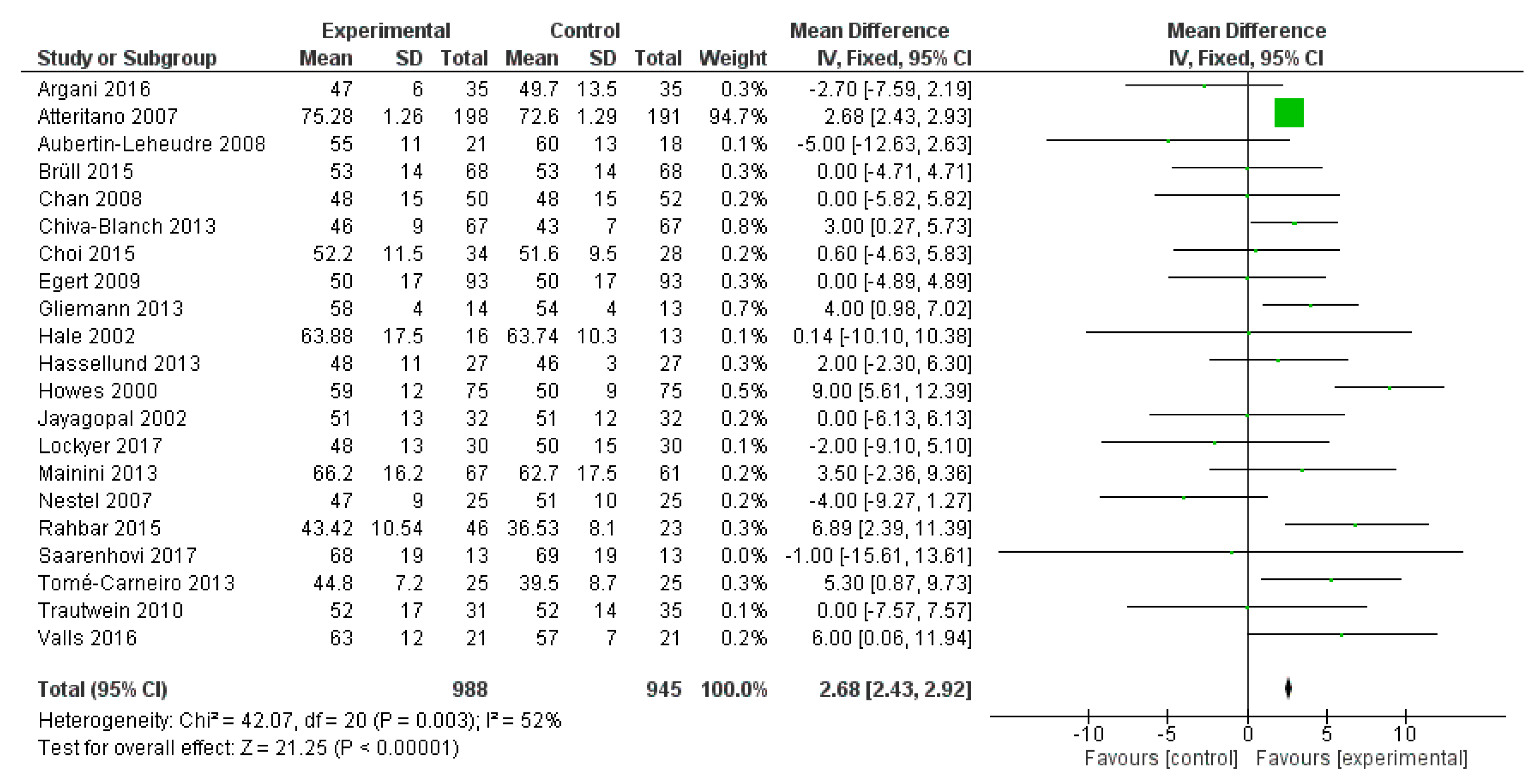
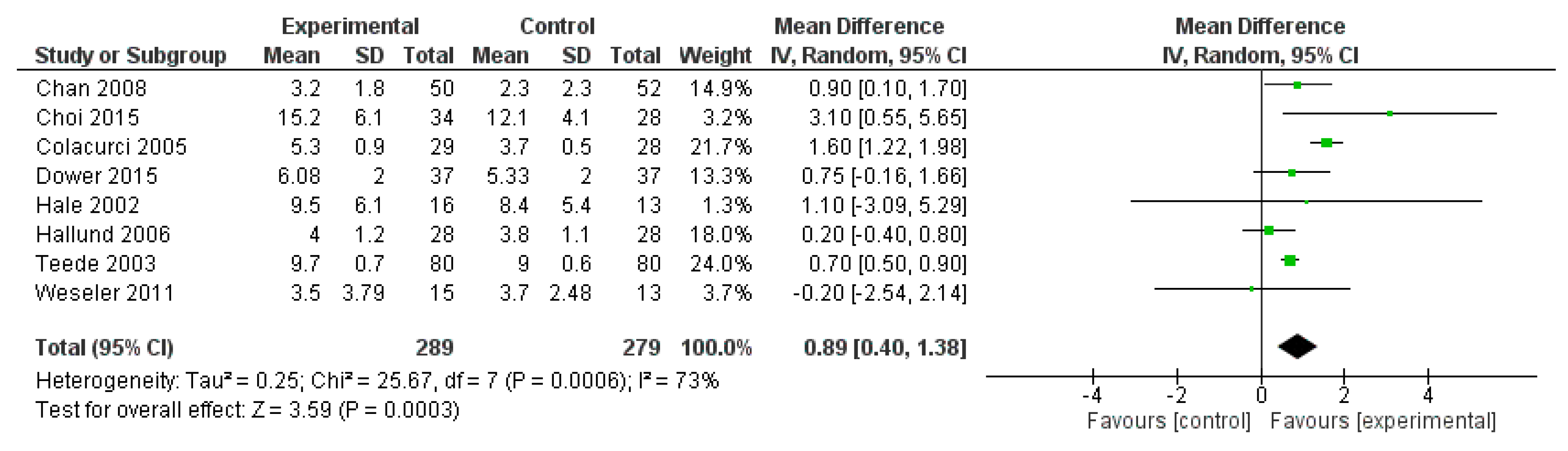
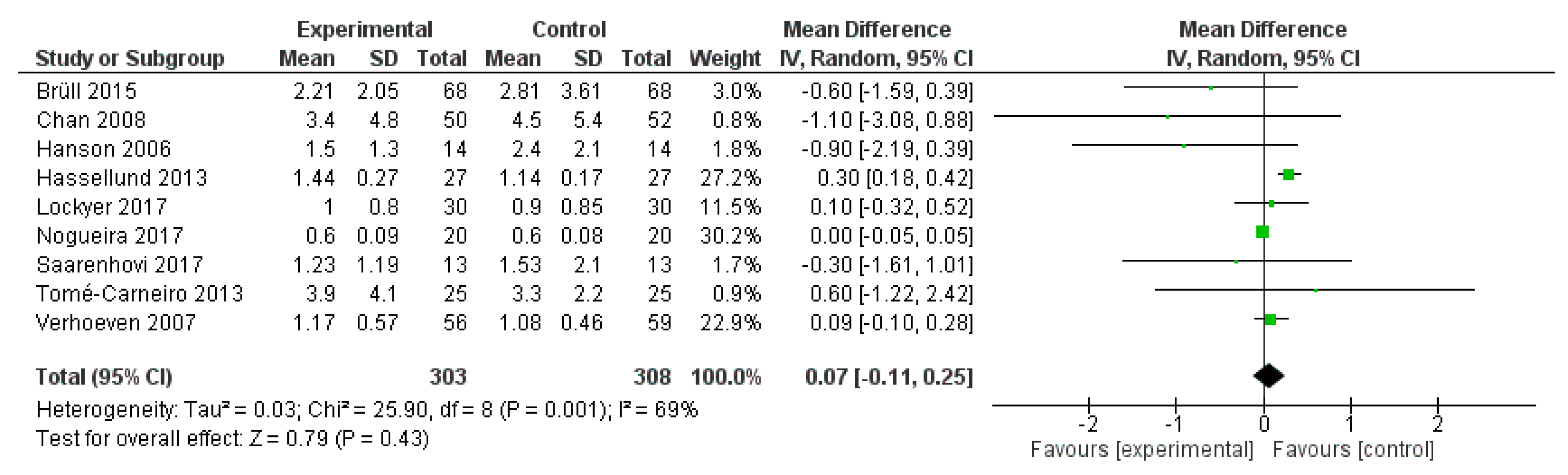
2.3. Meta-Analysis
2.3.1. Methods
2.3.2. Results
2.3.3. Discussion
3. Polyphenol Effects on Neurocognitive Functions
3.1. Impact of Polyphenols on Cognitive Functions in Humans
3.2. Mechanisms of Neuroprotection
3.3. Meta-Analysis
3.3.1. Methods
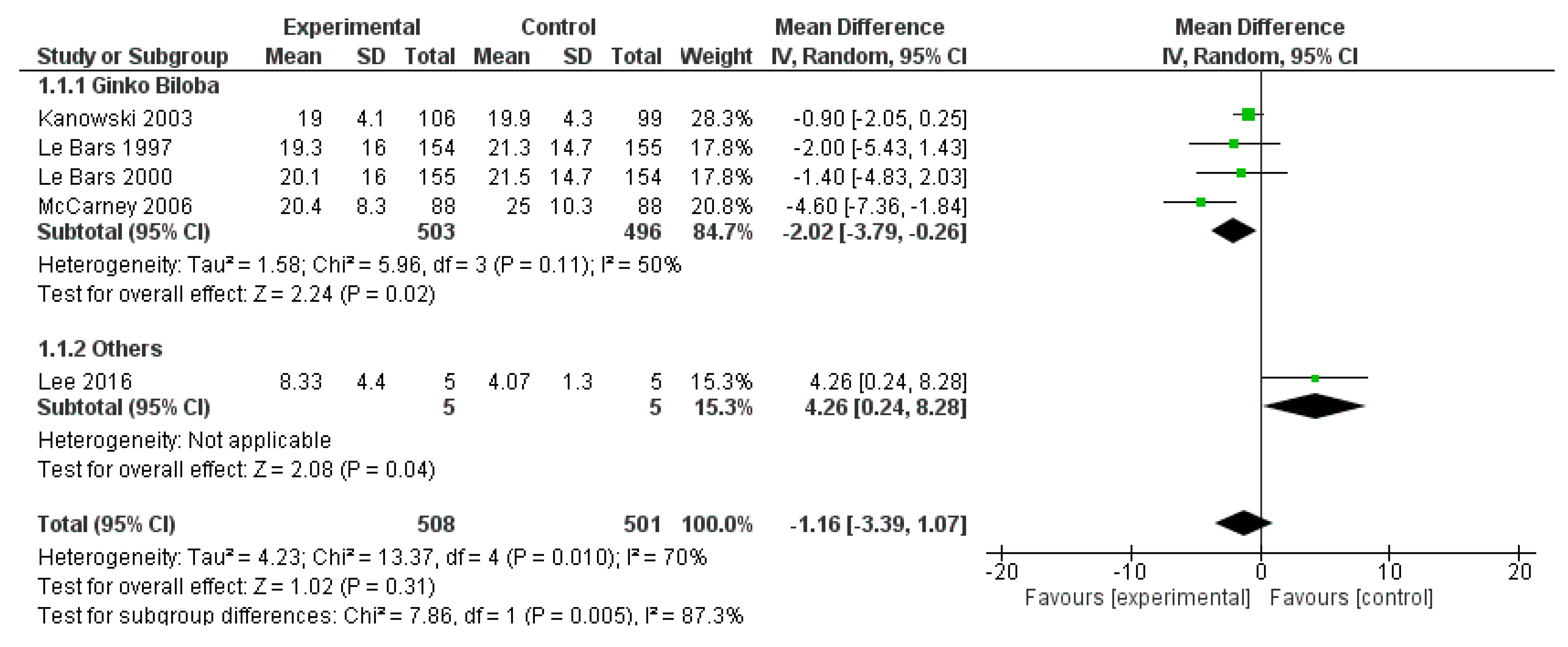
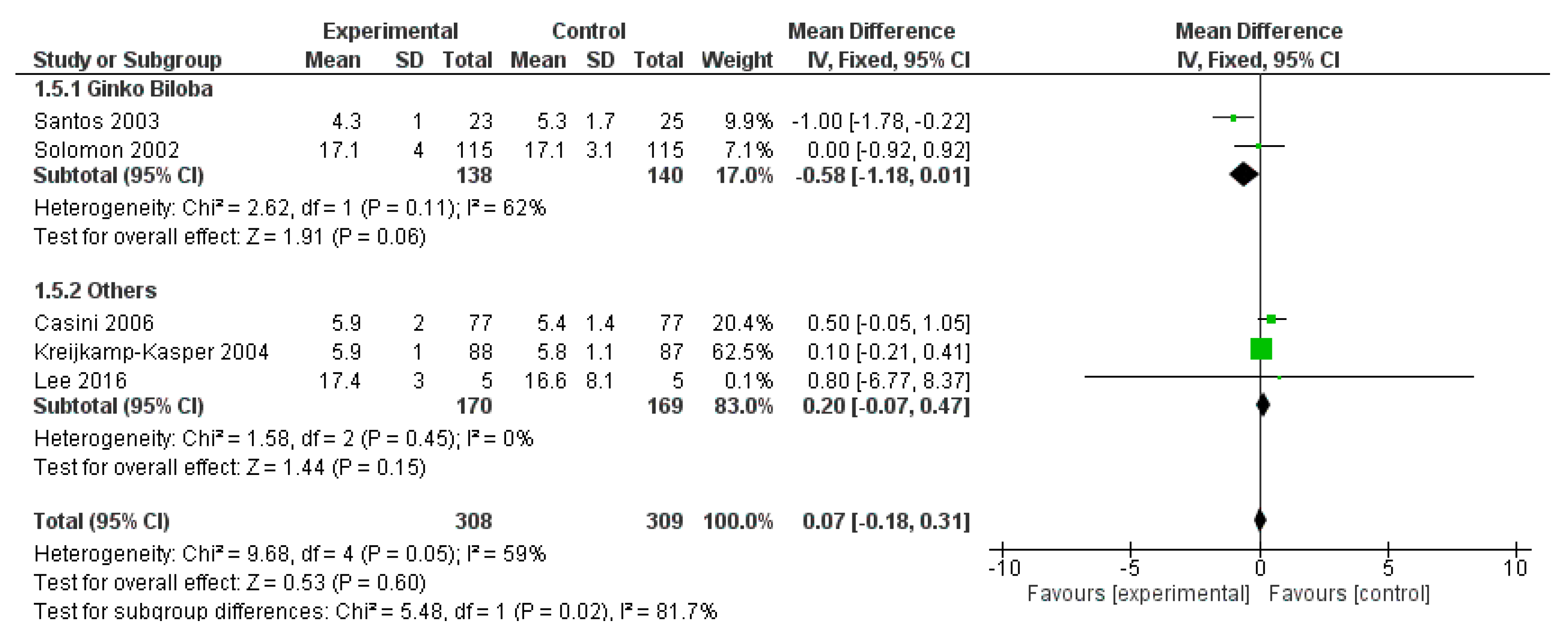
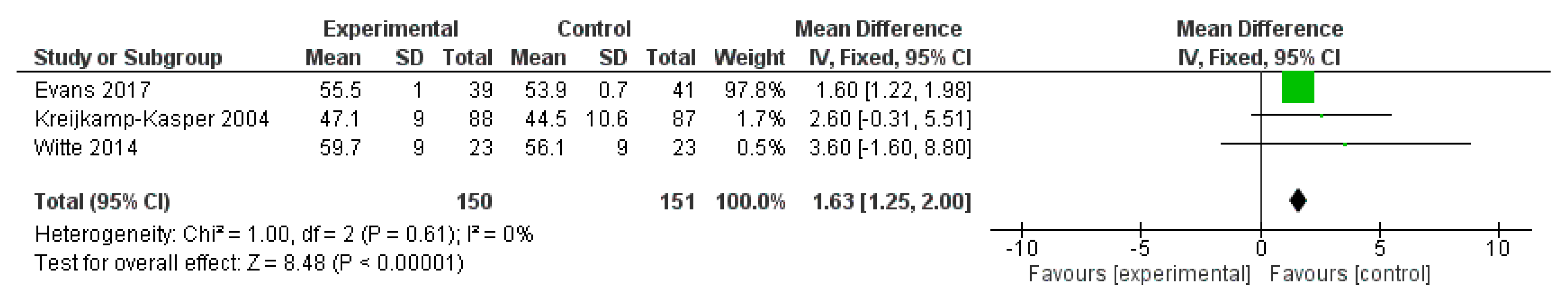
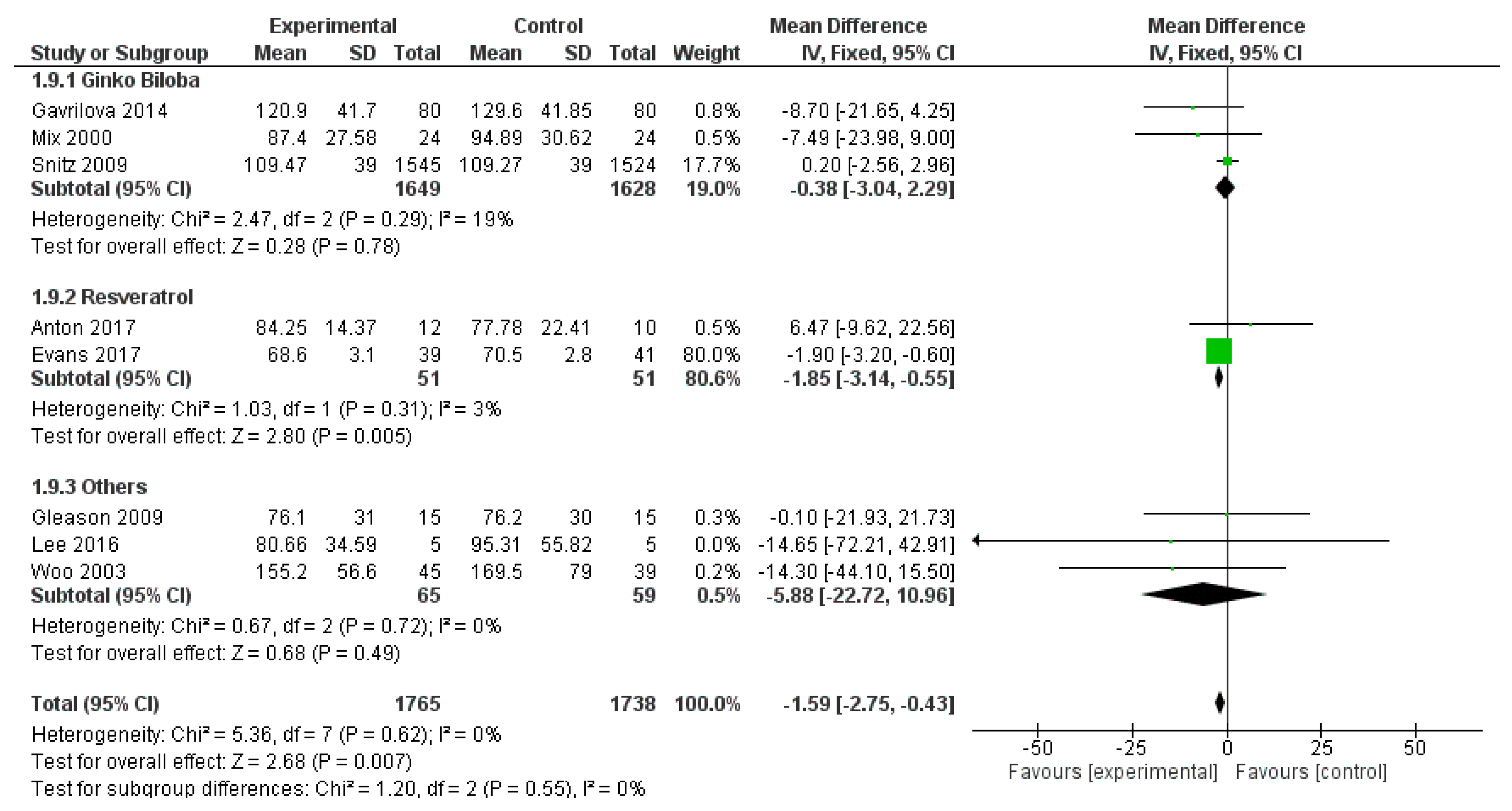
3.3.2. Results
3.3.3. Discussion
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular disease |
| LDL-C | Low density lipoprotein cholesterol |
| HDL-C | High density lipoprotein cholesterol |
| FMD | Flow mediated dilation |
| NO | Nitric oxide |
| TNF-α | Tumor necrosis factor alpha |
| hs-CRP | High-sensitive C-reactive protein |
| HOMA-AD | Homeostasis model assessment-adiponectin |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| AD | Alzheimer’s disease |
| MCI | Mild cognitive impairment |
| WCST | Wisconsin card classification test |
| RAVLT | Reys auditory verbal learning task |
| TMT | Trail making test |
| ADAS-Cog | Alzheimer’s Disease assessment scale-cognitive subscale |
| MMSE | Mini-mental state examination |
| WAIS | Wechsler adult intelligence scale |
References
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A. Polyphenols and Cardiovascular Disease: A Critical Summary of the Evidence. Mini Rev. Med. Chem. 2011, 11, 1186–1190. [Google Scholar]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1–40.
- GBD 2017 MortalityCollaborators. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1684–1735. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Mahabir, S.; Pathak, Y. Nutraceuticals and Health Review of Human Evidence; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1466517220. [Google Scholar]
- Liperoti, R.; Vetrano, D.L.; Bernabei, R.; Onder, G. Herbal Medications in Cardiovascular Medicine. J. Am. Coll. Cardiol. 2017, 69, 1188–1199. [Google Scholar] [CrossRef]
- Mena, P.; Del Rio, D. Gold Standards for Realistic (Poly)phenol Research. J. Agric. Food Chem. 2018, 66, 8221–8223. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Norata, G.D. Vascular inflammation and low-density lipoproteins: Is cholesterol the link? A lesson from the clinical trials. Br. J. Pharmacol. 2017, 174, 3973–3985. [Google Scholar] [CrossRef]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 1—Serological Markers. Heart Lung Circ. 2018. [Google Scholar] [CrossRef]
- Madonna, R.; Selvaggio, S.; Selvaggio, G.; Coronelli, M.; Cocco, N. “State-of-Art” paper of the Italian Working Group on Atherosclerosis: Preclinical assessment of early coronary atherosclerosis. Int. J. Cardiol. 2016, 214, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 2–Genetic and Imaging Markers. Heart Lung Circ. 2018. [Google Scholar] [CrossRef] [PubMed]
- Argani, H.; Ghorbanihaghjo, A.; Vatankhahan, H.; Rashtchizadeh, N.; Raeisi, S.; Ilghami, H. The effect of red grape seed extract on serum paraoxonase activity in patients with mild to moderate hyperlipidemia. Sao Paulo Med. J. 2016, 134, 234–239. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P.; et al. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-)hypertension: A randomized double-blinded, placebo-controlled crossover trial. Eur. J. Nutr. 2017, 56, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Lee, H.; Woo, J.S.; Jang, H.H.; Hwang, S.J.; Kim, H.S.; Kim, W.S.; Kim, Y.S.; Choue, R.; Cha, Y.J.; et al. Effect of onion peel extract on endothelial function and endothelial progenitor cells in overweight and obese individuals. Nutrition 2015, 31, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the Pure Flavonoids Epicatechin and Quercetin Affects Some Biomarkers of Endothelial Dysfunction and Inflammation in (Pre) Hypertensive Adults: A Randomized Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C.H. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. Inflammation Revisited: Atherosclerosis in The Post-CANTOS Era. Eur. Cardiol. 2017, 12, 89–91. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and fl avonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Iranshahy, M.; Iranshahi, M.; Abtahi, S.R.; Karimi, G. The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: A review. Food Chem. Toxicol. 2018, 120, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Callcott, E.T.; Thompson, K.; Oli, P.; Blanchard, C.L.; Santhakumar, A.B. Coloured rice-derived polyphenols reduce lipid peroxidation and pro-inflammatory cytokines ex vivo. Food Funct. 2018, 9, 5169–5175. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tao, M.; Zhao, Y.; Hu, X.; Wang, M. 4′-Methoxyresveratrol Alleviated AGE-Induced Inflammation via RAGE-Mediated NF-κB and NLRP3 Inflammasome Pathway. Molecules 2018, 23, 1447. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Vaccaro, O.; Masulli, M.; Bonora, E.; Del Prato, S.; Giorda, C.B.; Nicolucci, A.; Squatrito, S.; Auciello, S.; Babini, A.C.; et al. Polyphenol intake and cardiovascular risk factors in a population with type 2 diabetes: The TOSCA.IT study. Clin. Nutr. 2017, 36, 1686–1692. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Hurtubise, J.; McLellan, K.; Durr, K.; Onasanya, O.; Nwabuko, D.; Ndisang, J.F. The Different Facets of Dyslipidemia and Hypertension in Atherosclerosis. Curr. Atheroscler. Rep. 2016, 18, 82. [Google Scholar] [CrossRef]
- Zanotti, I.; Dall’Asta, M.; Mena, P.; Mele, L.; Bruni, R.; Ray, S.; Del Rio, D. Atheroprotective effects of (poly)phenols: A focus on cell cholesterol metabolism. Food Funct. 2015, 6, 13–31. [Google Scholar] [CrossRef]
- Sae-Tan, S.; Grove, K.A.; Lambert, J.D. Weight control and prevention of metabolic syndrome by green tea. Pharmacol. Res. 2011, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Kolouchova, G.; Dolezelova, E.; Cermanova, J.; Hyspler, R.; Kadova, Z.; Micuda, S. Epigallocatechin gallate enhances biliary cholesterol secretion in healthy rats and lowers plasma and liver cholesterol in ethinylestradiol-treated rats. Eur. J. Pharmacol. 2012, 691, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nishizawa, M.; Inoue, N.; Hosoya, T.; Yoshida, M.; Ukawa, Y.; Sagesaka, Y.M.; Doi, T.; Nakayama, T.; Kumazawa, S.; et al. Epigallocatechin gallate decreases the micellar solubility of cholesterol via specific interaction with phosphatidylcholine. J. Agric. Food Chem. 2014, 62, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Xiao, C.; Morgantini, C.; Szeto, L.; Lewis, G.F. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Azorin-Ortuno, M.; Yanez-Gascon, M.J.; Gonzalez-Sarrias, A.; Larrosa, M.; Vallejo, F.; Pallares, F.J.; Lucas, R.; Morales, J.C.; Tomas-Barberan, F.A.; Garcia-Conesa, M.-T.; et al. Effects of long-term consumption of low doses of resveratrol on diet-induced mild hypercholesterolemia in pigs: A transcriptomic approach to disease prevention. J. Nutr. Biochem. 2012, 23, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, A.; Natsume, M.; Osakabe, N.; Kawahata, K.; Koga, J. Cacao polyphenols influence the regulation of apolipoprotein in HepG2 and Caco2 cells. J. Agric. Food Chem. 2011, 59, 1470–1476. [Google Scholar] [CrossRef]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; et al. Sustained Benefits in Vascular Function Through Flavanol-Containing Cocoa in Medicated Diabetic Patients A. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef]
- Leikert, J.F.; Räthel, T.R.; Wohlfart, P.; Cheynier, V.; Vollmar, A.M.; Dirsch, V.M. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 2002, 106, 1614–1617. [Google Scholar] [CrossRef]
- Fisher, N.D.L.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003, 21, 2281–2286. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schomig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Shao, P.; Lee, R.P.; Huang, J.; Ly, A.; Hsu, M.; Lu, Q.Y.; Thames, G.; Heber, D.; et al. Health benefit of vegetable/fruit juice-based diet: Role of microbiome. Sci. Rep. 2017, 7, 2167. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-zacas, D.; Porrini, M.; Alimentari, T.; Celoria, V. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Muñoz-gonzález, I.; Cueva, C.; Jiménez-girón, A.; Sánchez-patán, F.; Santos-buelga, C.; Moreno-arribas, M.V.; Bartolomé, B. A Survey of Modulation of Gut Microbiota by Dietary Polyphenols, A Survey of Modulation of Gut Microbiota by Dietary Polyphenols. BioMed Res. Int. 2015, 2015, e850902. [Google Scholar]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Cheng, H.; Jenner, A.M.; Seng, C.; Kun, Y. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Med. Food 2000, 3, 121–125. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Atteritano, M.; Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Mazzaferro, S.; D’Anna, R.; Cannata, M.L.; Gaudio, A.; et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: A two-year randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2007, 92, 3068–3075. [Google Scholar] [CrossRef]
- Kawashiri, M.; Tada, H.; Nomura, A.; Yamagishi, M. Mendelian randomization: Its impact on cardiovascular disease. J. Cardiol. 2018, 72, 307–313. [Google Scholar] [CrossRef]
- Tall, A.R. Plasma high density lipoproteins: Therapeutic targeting and links to atherogenic inflammation. Atherosclerosis 2018, 276, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Rossi, S.; Biasini, B.; Zavaroni, I.; Bedogni, G.; Musci, M.; Pruneti, C.; Passeri, G.; Ventura, M.; Di Nuzzo, S.; et al. Claimed effects, outcome variables and methods of measurement for health claims proposed under European Community Regulation 1924/2006 in the framework of protection against oxidative damage and cardiovascular health. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 473–503. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Heal. 2017, 5, 335. [Google Scholar]
- Chung, C.G.; Lee, H.; Lee, S.B. Mechanisms of protein toxicity in neurodegenerative diseases. Cell. Mol. Life Sci. 2018, 75, 3159–3180. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Niranjan, R. Recent advances in the mechanisms of neuroinflammation and their roles in neurodegeneration. Neurochem. Int. 2018, 120, 13–20. [Google Scholar] [CrossRef]
- Cristani, M.; Speciale, A.; Saija, A.; Gangemi, S.; Minciullo, P.L.; Cimino, F. Circulating advanced oxidation protein products as oxidative stress biomarkers and progression mediators in pathological conditions related to inflammation and immune dysregulation. Curr. Med. Chem. 2016, 23, 3862–3882. [Google Scholar] [CrossRef]
- Daniele, S.; Giacomelli, C.; Martini, C. Brain ageing and neurodegenerative disease: The role of cellular waste management. Biochem. Pharmacol. 2018, 158, 207–216. [Google Scholar] [CrossRef]
- Gan, L.; Cookson, M.R.; Petrucelli, L.; La Spada, A.R. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 2018, 21, 1300–1309. [Google Scholar] [CrossRef]
- Katsuno, M.; Sahashi, K.; Iguchi, Y.; Hashizume, A. Preclinical progression of neurodegenerative diseases. Nagoya J. Med. Sci. 2018, 80, 289–298. [Google Scholar]
- Gaugler, J.; James, B.; Johnson, T.; Scholz, K.; Weuve, J. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016, 12, 459–509. [Google Scholar]
- Bature, F.; Guinn, B.A.; Pang, D.; Pappas, Y. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: A systematic scoping review of literature from 1937 to 2016. BMJ Open 2017, 7, e015746. [Google Scholar] [CrossRef] [PubMed]
- Janoutová, J.; Šerý, O.; Hosák, L.; Janout, V. Is mild cognitive impairment a precursor of alzheimer’s disease? Short review. Cent. Eur. J. Public Health 2015, 23, 365–367. [Google Scholar] [PubMed]
- Hadjichrysanthou, C.; Mcrae-mckee, K.; Evans, S.; Wolf, F. De Potential Factors Associated with Cognitive Improvement of Individuals Diagnosed with Mild Cognitive Impairment or Dementia in Longitudinal Studies. J. Alzheimer’s Dis. 2018, 66, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Alegret, M.; Valero, S.; Vinyes-Junqué, G.; Hernández, I.; Mauleón, A.; Rosende-Roca, M.; Ruiz, A.; López, O.; Tárraga, L.; et al. A Longitudinal Follow-Up of 550 Mild Cognitive Impairment Patients: Evidence for Large Conversion to Dementia Rates and Detection of Major Risk Factors Involved. J. Alzheimer’s Dis. 2013, 34, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Botanicals and phytochemicals active on cognitive decline: The clinical evidence. Pharmacol. Res. 2018, 130, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Montandon, M.L.; Rodriguez, C.; Herrmann, F.; Giannakopoulos, P. Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients 2018, 10, 1391. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A review of the cognitive effects observed in humans following acute supplementation with flavonoids, and their associated mechanisms of action. Nutrients 2015, 7, 10290–10306. [Google Scholar] [CrossRef]
- Radd-Vagenas, S.; Duffy, S.L.; Naismith, S.L.; Brew, B.J.; Flood, V.M.; Fiatarone Singh, M.A. Effect of the Mediterranean diet on cognition and brain morphology and function: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2018, 107, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Roozbeh, N.; Kashef, R.; Ghazanfarpour, M.; Kargarfard, L.; Darvish, L.; Khadivzadeh, T.; Dizavandi, F.R.; Afiat, M. Overview of the Effect of Herbal Medicines and Isoflavones on the Treatment of Cognitive Function. J. Menopausal Med. 2018, 24, 113. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.; Sun, J.; Zhang, K.; Liu, J. Ginkgo Biloba for Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Top. Med. Chem. 2016, 16, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055. [Google Scholar] [PubMed]
- Flanagan, E.; Müller, M.; Hornberger, M.; Vauzour, D. Impact of Flavonoids on Cellular and Molecular Mechanisms Underlying Age-Related Cognitive Decline and Neurodegeneration. Neurol. Dis. Cogn. Funct. 2018, 7, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Espargaró, A.; Ginex, T.; del Mar Vadell, M.; Busquets, M.A.; Estelrich, J.; Muñoz-Torrero, D.; Luque, F.J.; Sabate, R. Combined in Vitro Cell-Based/in Silico Screening of Naturally Occurring Flavonoids and Phenolic Compounds as Potential Anti-Alzheimer Drugs. J. Nat. Prod. 2017, 80, 278–289. [Google Scholar]
- Amit, T.; Avramovich-Tirosh, Y.; Youdim, M.B; Mandel, S. Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J. 2008, 22, 1296–1305. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.F.; Zhang, Z.F.; Liu, Z.G.; Pei, X.R.; Wang, J.B.; Li, Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Aβ 1-42 oligomers and upregulating synaptic plasticity–related proteins in the hippocampus. Neuroscience 2009, 163, 741–749. [Google Scholar] [CrossRef]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The Effect of Flavanol-rich Cocoa on the fMRI Response to a Cognitive Task in Healthy Young People. J. Cardiovasc. Pharmacol. 2006, 47, 215–220. [Google Scholar] [CrossRef]
- Sorond, F.A.; Hurwitz, S.; Salat, D.H.; Greve, D.N.; Fisher, N.D. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology 2013, 81, 904–909. [Google Scholar] [CrossRef]
- Chun, O.K.; Chung, S.-J.; Claycombe, K.J.; Song, W.O. Serum C-Reactive Protein Concentrations Are Inversely Associated with Dietary Flavonoid Intake in U.S. Adults. J. Nutr. 2008, 138, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Kjølsrud-Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins Inhibit Nuclear Factor-κB Activation in Monocytes and Reduce Plasma Concentrations of Pro-Inflammatory Mediators in Healthy Adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Freese, R.; Vaarala, O.; Turpeinen, A.M.; Mutanen, M. No difference in platelet activation of inflammation markers after diets rich of poor in vegetables, berries and apple in healthy subjects. Eur. J. Nutr. 2004, 43, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Duffy, S.J.; Hamburg, N.M.; Gokce, N.; Warden, B.A.; Wiseman, S.; Keaney, J.F.; Frei, B.; Vita, J.A. Effects of black tea consumption on plasma catechins and markers of oxidative stress and inflammation in patients with coronary artery disease. Free Radic. Biol. Med. 2005, 38, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Schmatz, R.; Mazzanti, C.M.; Spanevello, R.; Stefanello, N.; Gutierres, J.; Correa, M.; da Rosa, M.M.; Rubin, M.A.; Chitolina Schetinger, M.R.; Morsch, V.M. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2009, 610, 42–48. [Google Scholar] [CrossRef]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D’Arcy, B.R.; Williams, B.A.; et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef]
- Heaton, R.K.; Chelune, G.J.; Talley, J.L.; Kay, G.G.; Curtiss, G. Wisconsin Card Sorting Test Manual: Revised and Expanded; Psychological Assessment Resources: Odessa, FL, USA, 1993. [Google Scholar]
- Auditory, S.M.; Test, V.L. Auditory and Verbal Learning Test: A Handbook; Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Sanchez-Cubillo, I.; Perianez, J.A.; Adrover-Roig, D.; Rodriguez-Sanchez, J.M.; Rios-Lago, M.; Tirapu, J.; Barcelo, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef]
- Rosen, W.G.; Mohs, R.C.; Davis, K.L. A new rating scale for Alzheimer’s disease. Am. J. Psychiatry 1984, 141, 1356–1364. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-III Administration and Scoring Manual; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Hashiguchi, M.; Ohta, Y.; Shimizu, M.; Maruyama, J.; Mochizuki, M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J. Pharm. Health Care Sci. 2015, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.F.; Galduroz, J.C.; Barbieri, A.; Castiglioni, M.L.; Ytaya, L.Y.; Bueno, O.F. Cognitive performance, SPECT, and blood viscosity in elderly non-demented people using Ginkgo biloba. Pharmacopsychiatry 2003, 36, 127–133. [Google Scholar] [PubMed]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Kelly, J.T.; Marshall, S.; Cutajar, J.; Annois, B.; Pipingas, A.; Tierney, A.; Itsiopoulos, C. Effect of resveratrol supplementation on cognitive performance and mood in adults: A systematic literature review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-F.; Chen, J.-J.; Zhou, X.-Y.; Ren, Y.-F.; Huang, W.; Zhou, J.-J.; Xie, P. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause 2015, 22, 198–206. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Nikfar, S.; Abdollahi, M. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients. Pharmacol. Res. 2018, 128, 338–344. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Evans, H.M.; Howe, P.R.C.; Wong, R.H.X. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to functions of the nervous system, including psychological functions 1. EFSA J. 2012, 10, 1–13. [Google Scholar]





| Study Group | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Compound or Extract | Duration | Inclusion Criteria | n | Age ± SD | n | Age ± SD | Cardiovascular Endpoint |
| Arazi H | 2014 | Green tea extract | 3 weeks | Hypertensive | 8 | 46.12 ± 5.4 | 8 | 48.0 ± 7.1 | Blood pressure |
| Argani H | 2016 | Red grape seed extract | 8 weeks | moderate hyperlipidaemia | 35 | 47.3 ± 9.3 | 35 | 46.6 ± 8.4 | HDL-C, LDL-C, apolipoprotein A-I, paraoxonase |
| Atteritano M | 2007 | Genistein | 104 weeks | Osteopenia | 198 | 54.7 ± 0.25 | 191 | 54.2 ± 0.19 | Lipid status, ICAM-1, VCAM-1 |
| Aubertin-Leheudre M | 2008 | Isoflavone | 26 weeks | Postmenopausal, obese | 21 | 57.1 ± 5.6 | 18 | 57.7 ± 5.2 | Lipid status |
| Botden IP | 2012 | Red wine polyphenols | 4 weeks | Healthy subjects | 38 | 61 ± 8 | 20 | 61 ± 8 | Blood pressure, aortic augmentation index, pulse wave reflection index |
| Brüll V | 2015 | Quercetin | 6 weeks | Overweight | 68 | 47.4 ± 10.5 | 68 | 47.4 ± 10.5 | Blood pressure, ET-1, ICAM-1, VCAM-1, hs-CRP, HDL-C, LDL-C, triglycerides |
| Chan YH | 2008 | Isoflavone | 12 weeks | Ischaemic stroke | 50 | 66.8 ± 9.6 | 52 | 65.8 ± 10.3 | FMD, hs-CRP, blood pressure, superoxide dismutase |
| Chiva-Blanch G | 2013 | Red wine polyphenols | 4 weeks | Healthy subjects | 67 | 60 ± 8 | 67 | 60 ± 8 | HDL-C, LDL-C, apolipoprotein A-I |
| Choi EY | 2015 | Onion peel extract | 12 weeks | Overweight | 34 | 43.6 ± 9.1 | 28 | 42.5 ± 8.9 | FMD, Endothelial progenitor cells |
| Colacurci N | 2005 | Isoflavone | 26 weeks | Postmenopausal | 29 | 55.4 ± 3.7 | 28 | 54.9 ± 4 | FMD, ICAM-1, VCAM-1, E-selectin |
| Dower JI | 2015 | Epicatechin | 4 weeks | Healthy subjects | 37 | 66.4 ± 7.9 | 37 | 66.4 ± 7.9 | Blood pressure |
| Egert S | 2009 | Quercetin | 6 weeks | Overweight | 93 | 45.1 ± 10.53 | 93 | 45.1 ± 10.53 | HDL-C, LDL-C, triglycerides, TNF-α |
| Gliemann L | 2013 | Resveratrol | 8 weeks | cardiovascular risk increased | 14 | 65 ± 1 | 13 | 65 ± 1 | HDL-C, LDL-C, triglycerides, ICAM-1, VCAM-1 |
| Hale G | 2002 | Isoflavone | 2 weeks | Postmenopausal | 16 | 56.5 ± 4.5 | 13 | 58 ± 7.2 | FMD |
| Hallund J | 2006 | Isoflavone | 8 weeks | Postmenopausal | 28 | 57 ± 5 | 28 | 57 ± 5 | FMD, ET-1, blood pressure, |
| Hanson LN | 2006 | Isoflavone | 6 weeks | Postmenopausal | 14 | 60 (60–70) | 14 | 56 (49–70) | hs-CRP |
| Hassellund SS | 2013 | Anthocyanins | 4 weeks | Hypertension | 27 | 41 ± 3 | 27 | 41 ± 3 | HDL-C, LDL-C, triglycerides |
| Howes JB | 2000 | Red-clover isoflavones | 4 weeks | Type 2 diabetes mellitus | 75 | 58 ± 7.3 | 75 | 58 ± 7.3 | HDL-C, LDL-C, triglycerides |
| Howes JB | 2003 | Red-clover isoflavones | 4 weeks | Type 2 diabetes mellitus | 19 | 62 ± 2 | 19 | 62 ± 2 | Blood pressure |
| Jayagopal V | 2002 | Isoflavone | 12 weeks | Postmenopausal, type 2 diabetes mellitus | 32 | 62.5 ± 6.8 | 32 | 62.5 ± 6.8 | HDL-C, LDL-C, triglycerides |
| Lockyer S | 2017 | Phenolic-rich olive leaf extract | 12 weeks | Healthy subjects | 30 | 45.3 ± 12.7 | 30 | 45.3 ± 12.7 | HDL-C, LDL-C, triglycerides, blood pressure |
| Mainini G | 2013 | Isoflavone | 12 weeks | Postmenopausal | 67 | 54.6 ± 5 | 61 | 54.6 ± 5 | HDL-C, LDL-C, triglycerides, apolipoprotein A-I |
| Nestel P | 2007 | Trans-tetrahydrodaidzein | 5 weeks | Postmenopausal, overweight | 25 | 57 ± 7 | 25 | 57 ± 7 | Blood pressure, HDL-C, LDL-C, triglycerides |
| Nogueira LP | 2017 | Green tea extract | 4 weeks | Hypertension, overweight | 20 | 41.1 ± 8.4 | 20 | 41.1 ± 8.4 | Blood pressure, HDL-C, LDL-C, triglycerides |
| Panahi Y | 2015 | Curcuminoid-piperine combination | 8 weeks | Metabolic syndrome | 59 | 44.8 ± 8.7 | 58 | 43.5 ± 9.7 | hs-CRP |
| Pipe EA | 2009 | Soy protein isolate | 4 weeks | Type 2 diabetes mellitus | 29 | 60.1 ± 9.6 | 29 | 60.1 ± 9.6 | HDL-C, LDL-C, triglycerides, apolipoprotein A-I |
| Saarenhovi M | 2017 | Apple polyphenol extract | 4 weeks | Hypertension | 13 | 55.6 ± 7.9 | 13 | 54.9 ± 6.4 | FMD |
| Teede HJ | 2003 | Biochanin | 6 weeks | Healthy subjects | 80 | 54 ± 0.7 | 80 | 54 ± 0.7 | Blood pressure, FMD, arterial stiffness |
| Tomé-Carneiro J | 2013 | Resveratrol | 26 weeks | cardiovascular risk increased | 25 | 60 ± 12 | 25 | 58 ± 9 | Apolipoprotein A-I, HDL-C, LDL-C, triglycerides |
| Trautwein EA | 2010 | Black tea flavonoids | 12 weeks | Healthy subjects | 31 | 50.1 ± 3.6 | 35 | 46.8 ± 7.1 | HDL-C, LDL-C, triglycerides |
| Valls RM | 2016 | Oligopin | 5 weeks | Hypertension | 21 | NA | 21 | NA | HDL-C, LDL-C, triglycerides, blood pressure |
| Verhoeven MO | 2007 | Isoflavone | 12 weeks | Postmenopausal | 56 | 54.1 ± 4.6 | 59 | 53.8 ± 4.4 | HDL-C, LDL-C, triglycerides, lipoprotein, hs-CRP |
| Weseler AR | 2011 | Flavanols | 8 weeks | Smokers | 15 | 46 (30–58) | 13 | (30–60) | HDL-C, LDL-C, triglycerides, hs-CRP |
| Xie L | 2017 | Berry polyphenol | 12 weeks | Smokers | 25 | 32.6 ± 2.6 | 24 | 37.4 ± 3.0 | HDL-C, LDL-C, triglycerides |
| Study Group | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Compound or Extract | Duration | Inclusion Criteria | n | Age ± SD | n | Age ± SD | Neurocognitive Endpoints |
| Dai CX | 2018 | Ginkgo biloba extract | 12 weeks | Depression | 68 | 66..48 ± 4.12 | 68 | 66.82 ± 3.35 | HAMD, WCST |
| Anton SD | 2017 | Resveratrol | 12 weeks | Healthy subjects | 12 | 73.17 ± 2.08 | 10 | 73.3 ± 2.06 | Trail Making, Digits Forward and Backward, Erikson-Flanker, Controlled Oral Word Association, Hopkins Verbal Learning Test-Revised, and Task Switching |
| Evans HM | 2017 | Resveratrol | 14 weeks | post-menopausal women | 39 | 61.5 ± 1.1 | 40 | 61.5 ± 1.2 | RAVLT, trail making test, visual spatial working memory, semantic memory tests, Profile of Mood States |
| Lee J | 2016 | Grape formulation | 24 weeks | Cognitive impairment | 5 | 71.2 ± 2.06 | 5 | 73.2 ± 5.02 | ADAS-Cog, Hopkins VLT, FAS, trail making test, WCST-64, WAIS-IIIWATR, MMSE, HDRS, HARS, CIBIC-Plus |
| Gleason CE | 2015 | Soy isoflavones | 25 weeks | Healthy subjects | 32 | 75.7 ± 7.7 | 33 | 76.8 ± 6.8 | MMSE, verbal memory, Mazes, trail making test, stroop color word test, fluency, visual memory, visual motor, GDS, POMS |
| Witte AV | 2014 | Resveratrol | 26 weeks | Healthy subjects | 23 | 64.8 ± 6.8 | 23 | 63.7 ± 5.3 | Auditory Verbal Learning Test, MRI |
| Gavrilova SI | 2014 | Ginkgo biloba extract | 24 weeks | Cognitive impairment | 80 | 65 ± 7 | 79 | 63 ± 7 | CAMCOG, MMSE, Neuropsychiatric Inventory, State-Trait Anxiety Inventory, Geriatric Depression Scale, Trail-Making Test |
| Henderson VW | 2012 | Isoflavone-rich soy protein | 120 weeks | post-menopausal women | 175 | 61 ± 7 | 175 | 61 ± 7 | Neuropsychological battery, Wechsler Test of Adult Reading, Center for Epidemiological Studies Depression scale (CES-D) |
| Kaschel R | 2011 | Ginkgo biloba extract | 6 weeks | Healthy subjects | 88 | 45–56 | 89 | 45–56 | Memory tests |
| Snitz BE | 2009 | Ginkgo biloba extract | 312 weeks | Healthy subjects | 1545 | 79.1 ± 3.3 | 1524 | 79.1 ± 3.3 | MMSE, ADASCog |
| Gleason CE | 2009 | Isoflavone-rich soy protein | 24 weeks | Healthy subjects | 15 | 73.0 ± 7.9 | 15 | 74.3 ± 6.3 | Buschke Selective Reminding test, VSL, Boston Naming test, FAS, Grooved Pegboard, Stroop Color Word test, Mazes, Trail Making Test |
| Stough C | 2008 | Bacopa monnieri extract | 12 weeks | Healthy subjects | 33 | 41.6 ± 13.4 | 29 | 44.3 ± 11.3 | CDR, RVIP |
| McCarney R | 2008 | Ginkgo biloba extract | 24 weeks | Dementia | 88 | 79.3 ± 7.77 | 88 | 79.7 ± 7.53 | ADAS-Cog, QOL-AD |
| Woelk H | 2007 | Ginkgo biloba extract | 4 weeks | Anxiety disorder | 60 | 47.6 ± 11.7 | 37 | 46.7 ± 13 | HAMA, CGI-C, EAAS |
| Casini ML | 2006 | Isoflavone-rich soy protein | 24 weeks | post-menopausal women | 77 | 49 ± 4.3 | 77 | 50 ± 3.9 | Digit Symbol Test, WAIS, HRSD |
| Kreijkamp-Kaspers S | 2004 | Isoflavone-rich soy protein | 52 weeks | post-menopausal women | 88 | 66.5 ± 4.7 | 87 | 66.7 ± 4.8 | MMSE, RAVLT, WAIS |
| Kanowski S | 2003 | Ginkgo biloba extract | 24 weeks | Degenerative dementia | 106 | 72 ± 10 | 99 | 72 ± 10 | ADAS-Cog, MMSE, Clinical Global Impression of Change |
| Santos RF | 2003 | Ginkgo biloba extract | 32 weeks | Healthy subjects | 23 | 60–70 | 25 | 60–70 | WAIS, Wechsler Memory Scale revised (WMS-R), WCST, Toulouse-Pièron Concentrated Attention |
| Woo J | 2003 | Pueraria lobata (isoflavone) | 12 weeks | post-menopausal women | 45 | 57.4 ± 4.6 | 39 | 57.2 ± 4.8 | SF-36, HKLT, MMSE, WAIS; Trail making test |
| Kritz-Silverstein D | 2003 | Isoflavone-rich soy protein | 24 weeks | post-menopausal women | 27 | 55–74 | 26 | 55–74 | Trails A and B, category fluency, and logical memory and recall |
| Mix JA | 2002 | Ginkgo biloba extract | 6 weeks | Healthy subjects | 131 | 66.97 ± 6.12 | 131 | 68.6 ± 6.96 | SRT, WAIS, WCST |
| Solomon PR | 2002 | Ginkgo biloba extract | 6 weeks | Healthy subjects | 115 | 68.7 ± 4.7 | 108 | 69.9 ± 5.4 | WAIS, WMS, CVLT, Boston Naming test |
| File SE | 2001 | Isoflavone-rich soy protein | 10 weeks | Healthy subjects | 37 | 27.1 ± 3.2 | 37 | 23.9 ± 2.2 | Stocking Cambridge Test, PASAT, WMS |
| Mix JA | 2000 | Ginkgo biloba extract | 6 weeks | Healthy subjects | 24 | 67.5 ± 9.23 | 24 | 68.65 ± 6.95 | MMSE, SCWT, Trail Making Test, WMS |
| Le Bars PL | 2000 | Ginkgo biloba extract | 26 weeks | Degenerative dementia | 154 | 69 ± 10 | 155 | 69 ± 10 | ADAS-Cog, Geriatric Evaluation by Relative’s Rating Instrument(GERRI) and Clinical Global Impression of Change |
| Le Bars PL | 1997 | Ginkgo biloba extract | 52 weeks | Degenerative dementia | 166 | 69 ± 10 | 161 | 69 ± 10 | ADAS-Cog, Geriatric Evaluation by Relative’s Rating Instrument |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potì, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 351. https://doi.org/10.3390/ijms20020351
Potì F, Santi D, Spaggiari G, Zimetti F, Zanotti I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. International Journal of Molecular Sciences. 2019; 20(2):351. https://doi.org/10.3390/ijms20020351
Chicago/Turabian StylePotì, Francesco, Daniele Santi, Giorgia Spaggiari, Francesca Zimetti, and Ilaria Zanotti. 2019. "Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis" International Journal of Molecular Sciences 20, no. 2: 351. https://doi.org/10.3390/ijms20020351
APA StylePotì, F., Santi, D., Spaggiari, G., Zimetti, F., & Zanotti, I. (2019). Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. International Journal of Molecular Sciences, 20(2), 351. https://doi.org/10.3390/ijms20020351







