Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3
Abstract
:1. Introduction
2. Results
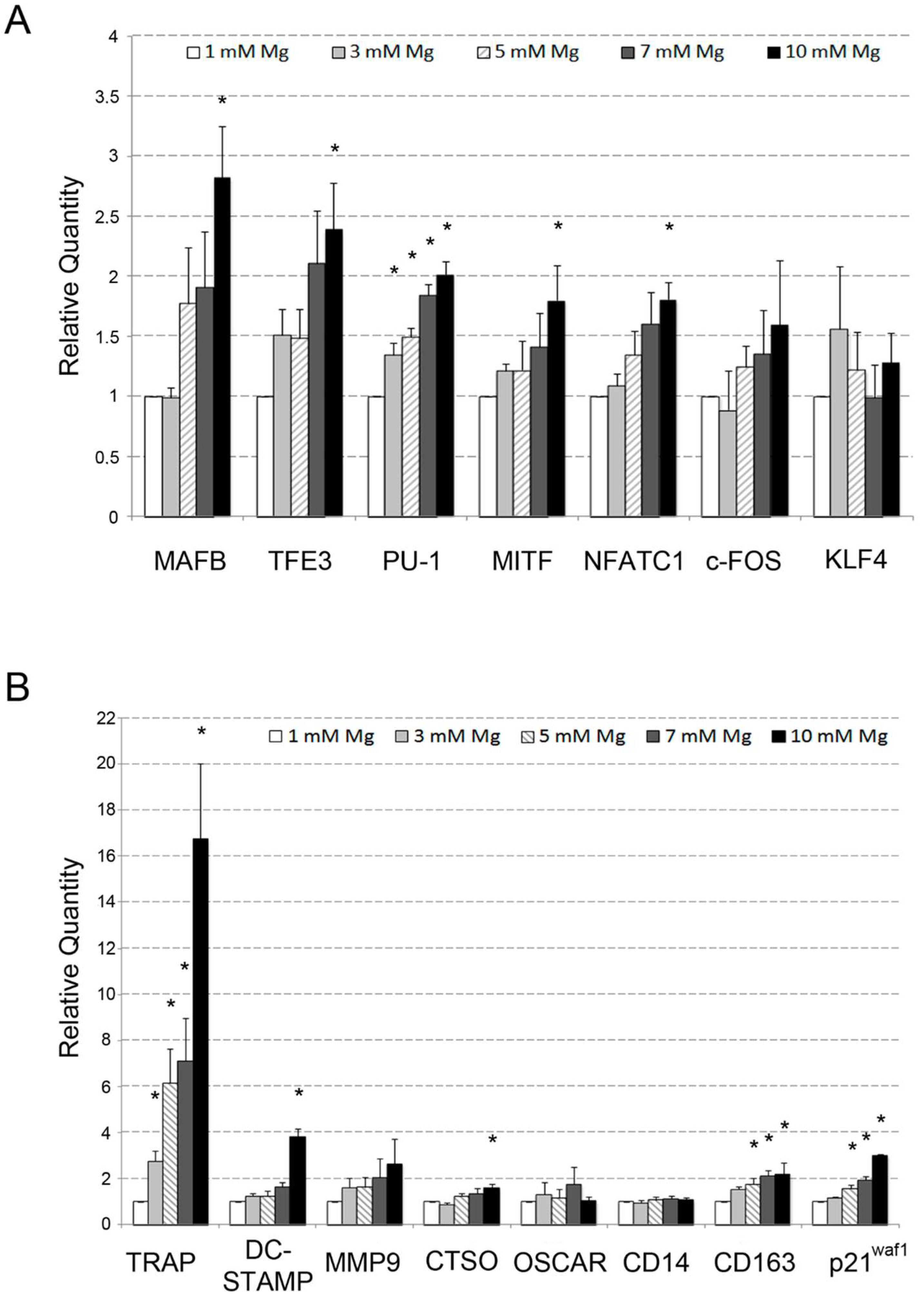
2.1. Analysis of the Effects of High Levels of Mg on the Osteoclastic Differentiation of U937 Cells
2.2. Comparative Analysis of the Effects Determined by High Level of Mg on Monocyte Differentiation of U937 Cells Induced by VD3 and Macrophage Differentiation of the Same Cells Induced by PMA
2.3. Analysis of the Osteoblastic Differentiation of Human bMSCs in Response to VD3
3. Discussion
4. Materials and Methods
4.1. Culture and Differentiation of U937 Cells
4.2. Culture and Differentiation of bMSCs
4.3. Reactive Oxygen Species Evaluation
4.4. Flow Cytometry Analysis
4.5. RNA Extraction and QRT-PCR Reaction
4.6. Quantification of Total Cell Mg by Spectrofluorimetric Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| bMSCs | Bone-marrow mesenchymal stem cells |
| COL1A1 | Collagen type I alpha 1 chain |
| CTSO | Cathepsin o gene |
| DCFH | 20-70-Dichlorofluorescein diacetate |
| DC-STAMP | Dendritic cell-specific transmembrane protein |
| KLF4 | Kruppel-like factor 4 |
| MafB | Transcription factor MafB |
| MMP9 | Matrix metallopeptidase 9 |
| MITF | Microphthalmia-associated transcription factor |
| NFATC1 | Nuclear factor of activated T cells c1 |
| PMA | Phorbol 12-myristate 13-acetate |
| PU.1 | PU.1 transcription factor |
| ROS | Reactive oxygen species |
| RUNX2 | Runt-related transcription factor 2 |
| Tfe3 | Transcription factor E3 |
| TRAP | Tartrate-resistant acid phosphatase |
| VD3 | 25 di-hydroxy vitamin D3 |
References
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasdam, S.M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 73, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. The logic of the Membrane, Magnesium, Mitosis (MMM) model for the regulation of animal cell proliferation. Arch. Biochem. Biophys. 2007, 458, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Brandao, K.; Perraud, A. The channel-kinase TRPM7, revealing the untold story of Mg2+ in cellular signaling. Magnes. Res. 2014, 27, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kuby, S.A.; Noltman, E.A. ATP-Creatine Transphosphorylase. The Enzymes, 2nd ed.; Academic Press: New York, NY, USA, 1962; pp. 515–603. [Google Scholar]
- Ramirez, F.; Marecek, J.F. Coordination of magnesium with adenosine 5V-diphosphate and triphosphate. Biochim. Biophys. Acta 1980, 589, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Iotti, S.; Frassineti, C.; Sabatini, A.; Vacca, A.; Barbiroli, B. Quantitative mathematical expressions for accurate in vivo assessment of cytosolic [ADP] and DeltaG of ATP hydrolysis in the human brain and skeletal muscle. Biochim. Biophys. Acta-Bioenerget. 2005, 1708, 164–177. [Google Scholar] [CrossRef]
- Iotti, S.; Sabatini, A.; Vacca, A. Chemical and biochemical thermodynamics: From ATP hydrolysis to a general reassessment. J. Phys. Chem. B 2010, 114, 1985–1993. [Google Scholar] [CrossRef]
- Molla, G.S.; Himmelspach, A.; Wohlgemuth, R.; Haupt, E.T.K.; Liese, A. Mechanistic and kinetics elucidation of Mg2+/ATP molar ratio effect on glycerol kinase. J. Mol. Catal. A-Chem. 2018, 445, 36–42. [Google Scholar] [CrossRef]
- Mellis, D.J.; Itzstein, C.; Helfrich, M.H.; Crockett, J.C. The skeleton: A multi-functional complex organ. The role key signalling pathways in osteoclast differentiation and in bone resorption. J. Endocrinol. 2011, 211, 131–143. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Nakashima, T.; Takayanagi, H. Osteocyte Control of Osteoclastogenesis. Bone 2013, 54, 258–263. [Google Scholar] [CrossRef]
- Romani, A.M. Magnesium in health and disease. Met. Ions Life Sci. 2013, 13, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Jaquez-Chairez, F.O.; Rodríguez-Morán, M. Magnesium in metabolic syndrome: A review based on randomized, double-blind clinical trials. Magnes. Res. 2016, 29, 146–153. [Google Scholar] [CrossRef]
- Seo, J.W.; Park, T.J. Magnesium Metabolism. Electrolyte Blood Press. 2008, 6, 86–95. [Google Scholar] [CrossRef]
- Burmester, A.; Luthringer, B.; Willumeit, R.; Feyerabend, F. Comparison of the reaction of bone-derived cells to enhanced MgCl2-salt concentrations. Biomatter 2014, 4, e967616. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Belluci, M.M.; Schoenmaker, T.; Rossa-Junior, C.; Orricoa, S.R.; de Vriesb, T.J.; Everts, V. Magnesium deficiency results in an increased formation of osteoclasts. J. Nutr. Biochem. 2013, 24, 1488–1498. [Google Scholar] [CrossRef]
- Wu, L.; Luthringer, B.J.C.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- Rude, R.K.; Kirchen, M.E.; Gruber, H.E.; Meyer, M.H.; Luck, J.S.; Crawford, D.L. Magnesium deficiency-induced osteoporosis in the rat: Uncoupling of bone formation and bone resorption. Magnes. Res. 1999, 12, 257–267. [Google Scholar]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone 2005, 37, 211–219. [Google Scholar] [CrossRef]
- Rosanoff, A.; Dai, Q.; Shapses, S.A. Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv. Nutr. 2016, 7, 25–43. [Google Scholar] [CrossRef] [Green Version]
- Trautvetter, U.; Neef, N.; Leiterer, M.; Kiehntopf, M.; Kratzsch, J.; Jahreis, G. Effect of calcium phosphate and vitamin D3 supplementation on bone remodelling and metabolism of calcium, phosphorus, magnesium and iron. Nutr. J. 2014, 13, 6. [Google Scholar] [CrossRef] [Green Version]
- Allgrove, J. Physiology of Calcium, Phosphate, Magnesium and Vitamin D. Endocr. Dev. 2015, 28, 7–32. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Katsumata, S.; Kajita, Y.; Miwa, M. Magnesium deficiency regulates vitamin D metabolizing enzymes and type II sodium-phosphate cotransporter mRNA expression in rats. Magnes. Res. 2013, 26, 83–86. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [Green Version]
- Nieves, J.W. Bone. Maximizing bone health—Magnesium, BMD and fractures. Nat. Rev. Endocrinol. 2014, 10, 255–256. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Laukkanen, J.A. Low serum magnesium levels are associated with increased risk of fractures: A long-term prospective cohort study. Eur. J. Epidemiol. 2017, 32, 593–603. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Ding, W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodeling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef]
- Witte, F.; Ulrich, H.; Palm, C.; Willbold, E. Biodegradable magnesium scaffolds: Part II: Peri-implant bone remodeling. J. Biomed. Mat. Res. Part A 2007, 81, 757–765. [Google Scholar] [CrossRef]
- Zhang, X.; Zu, H.; Zhao, D.; Yang, K.; Tian, S.; Yu, X.; Lu, F.; Liu, B.; Yu, X.; Wang, B.; et al. Ion channel functional protein kinase TRPM7 regulates Mg ions to promote the osteoinduction of human osteoblast via PI3K pathway: In vitro simulation of the bone-repairing effect of Mg-based alloy implant. Acta Biomater. 2017, 63, 369–382. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.Y.; Feng, Y.F.; Ma, Z.S.; Ma, T.C.; Zhang, Y.; Li, X.; Wang, L.; Lei, W. Magnesium Ions Promote the Biological Behaviour of Rat Calvarial Osteoblasts by Activating the PI3K/Akt Signalling Pathway. Biol. Trace Elem. Res. 2017, 179, 284–293. [Google Scholar] [CrossRef]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J.C. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef]
- Martinez Sanchez, A.H.; Luthringer, B.J.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Terashima, A.; Takayanagi, H. Overview of Osteoimmunology. Calcif. Tissue Int. 2018, 102, 503–551. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with b-tricalcium phosphate. Biomaterials 2014, 35, 8553–8565. [Google Scholar] [CrossRef]
- Huehnerschulte, T.A.; Reifenrath, J.; von Rechenberg, B.; Dziuba, D.; Seitz, J.M.; Bormann, D.; Windhage, H.; Meyer-Lindenberg, A. In vivo assessment of the host reactions to the biodegradation of the two novel magnesium alloys ZEK100 and AX30 in an animal model. Biomed. Eng. Online 2012, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Maradze, D.; Musson, D.; Zheng, Y.; Cornish, J.; Lewis, M.; Liu, Y. High Magnesium Corrosion Rate has an Effect on Osteoclast and Mesenchymal Stem Cell Role during Bone Remodelling. Sci. Rep. 2018, 8, 10003. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Sargenti, A.; Castiglioni, S.; Olivi, I.; Bianchi, F.; Cazzaniga, A.; Farruggia, G.; Cappadone; Merolle, C.; Malucelli, E.; Ventura, C.; et al. Magnesium deprivation potentiates human mesenchymal stem cell transcriptional remodeling. Int. J. Mol. Sci. 2018, 19, 1410. [Google Scholar] [CrossRef]
- Amoui, M.; Suhr, S.M.; Baylink, D.J.; Lau, K.H.W. An osteoclastic protein-tyrosine phosphatase may play a role in differentiation and activity of human monocytic U-937 cell-derived, osteoclast-like cells. Am. J. Physiol. Cell Physiol. 2004, 287, C874–C884. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Tong, X.; Chen, G.; Wang, D.; Chen, Y.; Yuan, Y.; Liu, X.; Bian, J.; Liu, Z. Effects of 1a,25-(OH)2D3 on the formation and activity of osteoclasts in RAW264.7 cells. J. Steroid Biochem. Mol. Biol. 2015, 152, 25–33. [Google Scholar] [CrossRef]
- Zarei, A.; Morovat, A.; Javaid, K.; Brown, C.P. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res. 2016, 4, 16030. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Li, X.; Wan, Y. Minireview: Nuclear Receptor Regulation of Osteoclast and Bone Remodeling. Mol. Endocr. 2015, 29, 172–186. [Google Scholar] [CrossRef] [Green Version]
- Stein, G.S.; Lian, J.B.; van Wijnen, A.J.; van Wijnen, A.J.; Stein, J.L.; Montecino, M.; Javed, A.; Zaidi, S.K.; Young, D.W.; Choi, J.Y.; et al. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 2004, 23, 4315–4329. [Google Scholar] [CrossRef] [Green Version]
- Zanocco-Marani, T.; Vignudelli, T.; Gemelli, C.; Pirondi, S.; Testa, A.; Montanari, M.; Parenti, S.; Tenedini, E.; Grande, A.; Ferrari, S. Tfe3 expression is closely associated to macrophage terminal differentiation of human hematopoietic myeloid precursors. Exp. Cell Res. 2006, 312, 4079–4089. [Google Scholar] [CrossRef]
- Zanocco-Marani, T.; Vignudelli, T.; Parenti, S.; Gemelli, C.; Condorelli, F.; Martello, A.; Selmi, T.; Grande, A.; Ferrari, S. TFE3 transcription factor regulates the expression of MAFB during macrophage differentiation. Exp. Cell Res. 2009, 315, 1798–1808. [Google Scholar] [CrossRef]
- Ishiyama, K.; Yashiro, T.; Nakano, N.; Kasakura, K.; Miura, R.; Hara, M.; Kawai, F.; Maeda, K.; Tamura, N.; Okumura, K.; et al. Involvement of PU.1 in NFATc1 promoter function in osteoclast development. Allergol. Int. 2015, 64, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [Green Version]
- Gemelli, C.; Zanocco Marani, T.; Bicciato, S.; Mazza, E.M.C.; Boraschi, D.; Salsi, V.; Zappavigna, V.; Parenti, S.; Selmi, T.; Tagliafico, E.; et al. A MafB is a downstream target of the IL-10/STAT3 signaling pathway, involved in the regulation of macrophage de-activation. Biochim. Biophys. Acta 2014, 1843, 955–964. [Google Scholar] [CrossRef]
- Sørensen, M.G.; Henriksen, K.; Schaller, S.; Henriksen, D.B.; Nielsen, F.C.; Dziegiel, M.H.; Karsdal, M.A. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J. Bone Miner. Metab. 2007, 25, 36–45. [Google Scholar] [CrossRef]
- Montanari, M.; Gemelli, C.; Tenedini, E.; Zanocco Marani, T.; Vignudelli, T.; Siena, M.; Zini, R.; Salati, S.; Chiossi, G.; Tagliafico, E.; et al. Correlation between differentiation plasticity and mRNA expression profiling of CD34þ-derived CD14 and CD14þ human normal myeloid precursors. Cell Death Differ. 2005, 12, 1588–1600. [Google Scholar] [CrossRef]
- Gemelli, C.; Martello, A.; Montanari, M.; Zanocco Marani, T.; Salsi, V.; Zappavigna, V.; Parenti, S.; Vignudelli, T.; Selmi, T.; Ferrari, S.; et al. The Orosomucoid 1 protein is involved in the vitamin D—Mediated macrophage de-activation process. Exp. Cell Res. 2013, 319, 3201–3213. [Google Scholar] [CrossRef]
- Gemelli, C.; Montanari, M.; Tenedini, E.; Zanocco Marani, T.; Vignudelli, T.; Siena, M.; Zini, R.; Salati, S.; Tagliafico, E.; Manfredini, R.; et al. Virally mediated MafB transduction induces the monocyte commitment of human CD34þ hematopoietic stem/progenitor cells. Cell Death Differ. 2006, 13, 1686–1696. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Maier, J.A.M.; Castiglioni, S. Impact of simulated microgravity on human bone stem cells: New hints for space medicine. Biochem. Biophys. Res. Commun. 2016, 473, 181–186. [Google Scholar] [CrossRef]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium deficiency and oxidative stress: An update. BioMedicine 2016, 6. [Google Scholar] [CrossRef]
- Boudin, E.; Van Hul, W. Genetics of human bone formation. Eur. J. Endocr. 2017, 177, R69–R83. [Google Scholar] [CrossRef]
- Shahi, M.; Peymani, A.; Sahmani, M. Regulation of Bone Metabolism. Rep. Biochem. Mol. Biol. 2017, 5, 73–82. [Google Scholar]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-Osteoclast Interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Udagawa, N.; Suda, T. Vitamin D endocrine system and osteoclasts. Bonekey Rep. 2014, 3, 495. [Google Scholar] [CrossRef]
- Hou, Y.C.; Wu, C.C.; Liao, M.T.; Shyu, J.F.; Hung, C.F.; Yen, T.H.; Lu, C.L.; Lu, K. Role of nutritional vitamin D in osteoporosis treatment. Clin. Chim. Acta 2018, 484, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Van de Peppel, J.; van Leeuwen, J. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014, 5, 13. [Google Scholar] [CrossRef]
- Paredes, R.; Arriagada, G.; Cruzat, F.; Olate, J.; Van Wijnen, A.; Lian, J.; Stein, G.; Stein, J.; Montecino, M.J. The Runx2 transcription factor plays a key role in the 1alpha,25-dihydroxy Vitamin D3-dependent upregulation of the rat osteocalcin (OC) gene expression in osteoblastic cells. Steroid Biochem. Mol. Biol. 2004, 89–90, 269–271. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Kook, H.; Kim, K.K.; Lee, S.Y.; Kim, N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 2007, 109, 3253–3259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, I.S.; Dirr, E.W.; Ramaswamy, V.; Allen, J.B.; Allen, K.D.; Manuel, M.V. The effect of Mg-Ca-Sr alloy degradation products on human mesenchymal stem cells. J. Biomed. Mater Res. B Appl. Biomater. 2018, 106, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaw, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Li, R.W.; Kirkland, N.T.; Truong, J.; Wang, J.; Smith, P.N.; Birbilis, N.; Nisbet, D.R. The influence of biodegradable magnesium alloys on the osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. A 2014, 102, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- Luthringer, B.J.; Willumeit-Römer, R. Effects of magnesium degradation products on mesenchymal stem cell fate and osteoblastogenesis. Gene 2016, 575, 920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, C.; Li, J.; Zhu, Y.; Zhang, X. High extracellular magnesium inhibits mineralized matrix deposition and modulates intracellular calcium signaling in human bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Romeo, V.; Locatelli, L.; Cazzaniga, A.; Maier, J.A.M. TRPM7 and MagT1 in the osteogenic differentiation of human mesenchymal stem cells in vitro. Sci. Rep. 2018, 8, 16195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Yue, J.; Wang, Y.; Yang, C.; Cui, Q. High magnesium prevents matrix vesicle-mediated mineralization in human bone marrow-derived mesenchymal stem cells via mitochondrial pathway and autophagy. Cell Biol. Int. 2018, 42, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A. High magnesium inhibits human osteoblast differentiation in vitro. Magnes. Res. 2011, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Leidi, M.; Carpanese, E.; Maier, J.A. Extracellular magnesium and in vitro cell differentiation: Different behaviour of different cells. Magnes. Res. 2013, 26, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Grande, A.; Montanari, M.; Tagliafico, E.; Manfredini, R.; Zanocco Marani, T.; Siena, M.; Tenedini, E.; Gallinelli, A.; Ferrari, S. Physiological levels of 1alpha, 25 dihydroxyvitamin D3 induce the monocytic commitment of CD34+ hematopoietic progenitors. J. Leukoc. Biol. 2002, 7, 641–651. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, C.; Orlandi, C.; Zanocco Marani, T.; Martello, A.; Vignudelli, T.; Ferrari, F.; Montanari, M.; Parenti, S.; Testa, A.; Grande, A.; et al. The Vitamin D3/Hox-A10 Pathway Supports MafB Function during the Monocyte Differentiation of Human CD34+ Hemopoietic Progenitors. J. Immunol. 2008, 181, 5660–5672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargenti, A.; Farruggia, G.; Malucelli, E.; Cappadone, C.; Merolle, L.; Marraccini, C.; Andreani, G.; Prodi, L.; Zaccheroni, N.; Sgarzi, M.; et al. A novel fluorescent chemosensor allows the assessment of intracellular total magnesium in small samples. Analyst 2014, 139, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Sargenti, A.; Farruggia, G.; Zaccheroni, N.; Marraccini, C.; Sgarzi, M.; Cappadone, C.; Malucelli, E.; Procopio, A.; Prodi, L.; Lombardo, M.; et al. Synthesis of a highly Mg2+-selective fluorescent probe and its application to quantifying and imaging total intracellular magnesium. Nat. Protoc. 2017, 12, 461–471. [Google Scholar] [CrossRef] [PubMed]






| Mg Extracellular Concentration | Mg Intracellular Concentration (nmol/106 cells) Mean ± SEM |
|---|---|
| Control (1 mM) | 12.2 ± 0.0 |
| Control (10 mM) | 19.1 ± 2.1 |
| PMA + VD3 (1 mM) | 57.2 ± 2.0 |
| PMA + VD3 (10 mM) | 101.6 ± 13.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.M.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. https://doi.org/10.3390/ijms20020385
Mammoli F, Castiglioni S, Parenti S, Cappadone C, Farruggia G, Iotti S, Davalli P, Maier JAM, Grande A, Frassineti C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. International Journal of Molecular Sciences. 2019; 20(2):385. https://doi.org/10.3390/ijms20020385
Chicago/Turabian StyleMammoli, Fabiana, Sara Castiglioni, Sandra Parenti, Concettina Cappadone, Giovanna Farruggia, Stefano Iotti, Pierpaola Davalli, Jeanette A.M. Maier, Alexis Grande, and Chiara Frassineti. 2019. "Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3" International Journal of Molecular Sciences 20, no. 2: 385. https://doi.org/10.3390/ijms20020385
APA StyleMammoli, F., Castiglioni, S., Parenti, S., Cappadone, C., Farruggia, G., Iotti, S., Davalli, P., Maier, J. A. M., Grande, A., & Frassineti, C. (2019). Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. International Journal of Molecular Sciences, 20(2), 385. https://doi.org/10.3390/ijms20020385