HSFA2 Functions in the Physiological Adaptation of Undifferentiated Plant Cells to Spaceflight
Abstract
1. Introduction
2. Results
2.1. The HsfA2 Expression Levels across Samples
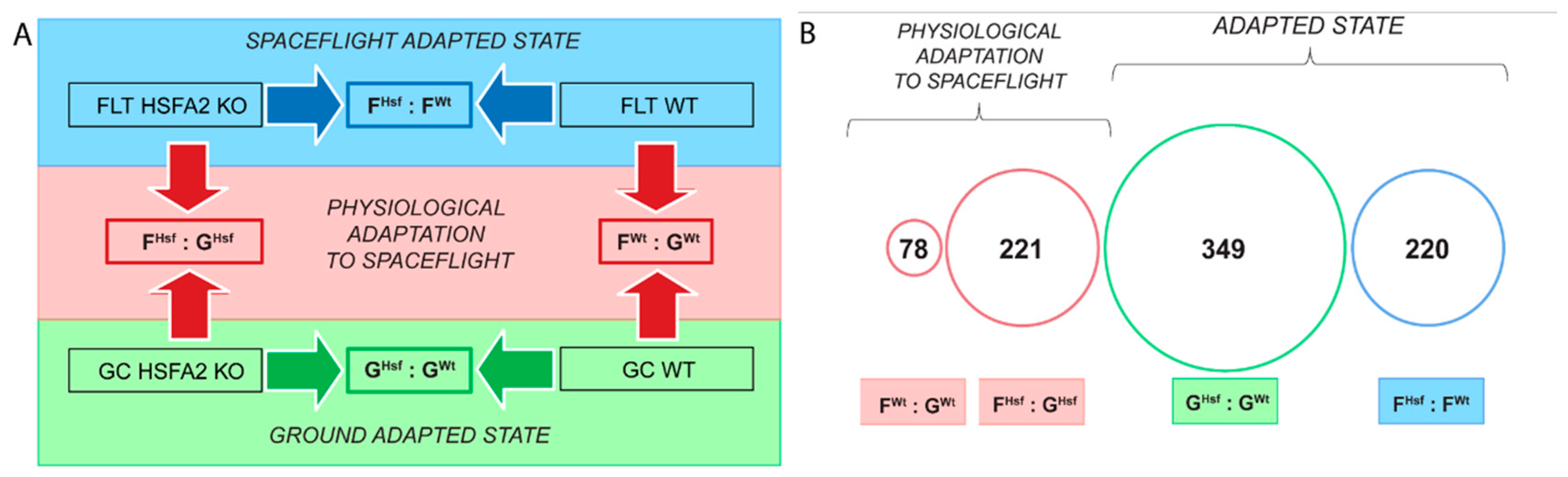
2.2. Different Genes Characterize the Physiological Adaptation of the WT and HSFA2 KO Cells to Spaceflight
2.2.1. The Genes Characterizing the Physiological Adaptation of WT Cells to Spaceflight—FWt : GWt
2.2.2. HSFA2 KO Cells Changed the Expression of Three Times More Genes than Seen in WT Cells—FHsf : GHsf
2.3. WT and HSFA2 KO Show More Difference in Gene Expression Profiles in Their Ground-Adapted State than in Spaceflight-Adapted State
2.3.1. Genotype Specific Genes of the Ground-Adapted State—GHsf : GWt
2.3.2. Genotype Specific Genes of the Spaceflight-Adapted State—FHsf : FWt
2.4. Occurrence of Individual Genes and Gene Function Across Collective Comparisons of Physiological Adaptation, Ground and Spaceflight-Adapted States
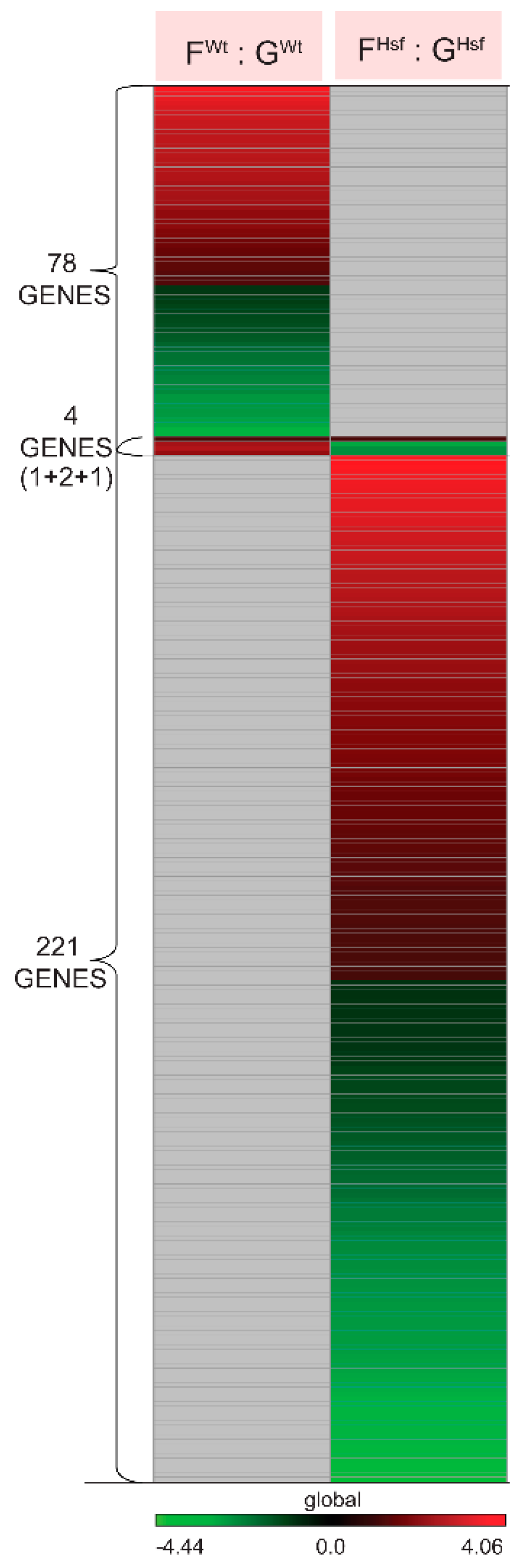
2.4.1. In the Response to Spaceflight, There Were Only Four Differentially Expressed Genes Represented in Both the WT and HSFA2 KO Comparisons (FWt : GWt to FHsf : GHsf)
2.4.2. Only 28 Genes Differentially Expressed Between the Two Genotypes in the Ground-Adapted State were also Differentially Expressed in the Spaceflight-Adapted State (GHsf : GWt to FHsf : FWt)
2.4.3. Required and Corrected Genes for the Spaceflight-Adapted State
- WT cells changed the expression of the gene in spaceflight compared to ground control.
- WT cells differentially expressed the gene on the ground compared to HSFA2 KO cells on the ground.
- HSFA2 KO cells did not change the gene’s expression in spaceflight compared to ground control.
- However, the level of expression of the gene in WT spaceflight was similar to the expression of the gene in HSFA2 KO in spaceflight.
- HSFA2 KO cells changed the expression of the gene in spaceflight compared to ground control.
- HSFA2 KO cells differentially expressed the corrected gene on the ground compared to WT cells on the ground.
- WT cells did not change the corrected gene expression in spaceflight compared to ground control.
- However, the level of expression of the gene in HSFA2 KO in spaceflight is similar to the expression level of the gene in WT in spaceflight.
2.4.4. Genotypic-Specific Spaceflight-Adapted State
HsfA2-Dependent Genes in the WT Genotypic Spaceflight Adaptation
Compensated Genes for Disabled HsfA2 in the HSFA2 KO Spaceflight Adaptation
3. Discussion
3.1. Universal and Specific Aspects of the HSFA2 Role in the Adaptation to Spaceflight
3.2. HSFA2 is Much More Than a Heat Shock Factor—Things Learned from Space
3.3. Role of HSFA2 in Spaceflight Adaptive Processes
3.4. Genotypic Adaptation to Spaceflight
4. Materials and Methods
4.1. The CEL Experiment of BRIC17
4.2. Tissue Culture Cell Lines
4.3. Preparation of BRIC17 CEL Cell Culture Plates
4.4. RNA Extractions
4.5. Microarrays
4.5.1. Microarray Data Analysis
4.5.2. Comparison Groups
4.6. Functional Gene Categorization—Gene Ontology Annotations
- AgriGO—An integrated web-based GO analysis toolkit for the agricultural community AgriGO was used [115]. AgriGO query criteria were as follows: Singular Enrichment Analysis (SEA), Arabidopsis genemodel (TAIR9) precomputed background, Fisher was selected as the statistical test method of choice with the NOT-adjust multi-test adjustment method; significance level was set at 0.01 or 0.05, minimum number of mapping of entries was set at 5, plant GO slim was selected from other Gene ontology types. For Parametric Analysis of Gene Set Enrichment (PAGE), the selected species was Arabidopsis thaliana, NOT-adjust was selected for multi-test adjustment method, significance level was set at 0.1, minimum number of mapping of entries was set at 10, and Plant GO slim was selected from other Gene ontology types.
- AmiGO—If needed, the GO database was accessed through the AmiGO query tool.
- ATTED-II—The ATTED-II database of coexpressed genes, developed to identify functionally related genes in Arabidopsis, was also used [116]. The make gene function table function was implemented to retrieve organized information on gene function (based on TAIR annotation) and subcellular localization (as predicted by TargetP and WOLF PSORT).
- gProfiler—A web-based toolset for functional profiling of gene lists was used [117]. Arabidopsis thaliana was the selected organism with most of default options except where the Benjamini-Hochberg FDR significance threshold was selected.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Appendix A. Gene Expression Tables
| ONT | GO Term Name | ||||
|---|---|---|---|---|---|
| Transcript ID | Gene Symbol | Gene Description | FWt : GWT FC log2 | FHsf : GHsf FC log2 | |
| BP | defense response to other organism GO:0098542 | ||||
| AT2G03760 | ST1 | sulphotransferase 12 | 1.1 | 1.0 | |
| AT5G38980 | unknown | 3.3 | X | ||
| At3g43250 | unknown in wounding | 2.6 | X | ||
| AT4G02460 | PMS1 | DNA mismatch repair protein, putative | 2.2 | ||
| AT2G44490 | PEN2 | Glycosyl hydrolase superfamily protein | 1.4 | X | |
| AT1G59124 | Disease resistance protein (CC-NBS-LRR class) | X | 2.4 | ||
| AT2G17430 | NTA | Seven transmembrane MLO family protein | X | 1.9 | |
| AT4G35770 | SEN1 | Rhodanese/Cell cycle control phosphatase | X | 1.8 | |
| AT3G61060 | PP2-A13 | phloem protein 2-A13 | X | 1.5 | |
| AT3G09940 | MDHAR | monodehydroascorbate reductase | X | 1.4 | |
| CC | plant-type cell wall GO:0009505 | ||||
| AT1G30600 | Subtilase family protein | 2.5 | X | ||
| At4g30500 | unknown in cellulose biosynthetic process | 2.4 | X | ||
| AT2G44490 | PEN2 | Glycosyl hydrolase superfamily protein | 1.4 | X | |
| AT1G78860 | curculin-like (mannose-binding) lectin | 1.3 | X | ||
| AT5G44130 | FLA13 | FASCICLIN-like arabinogalactan protein 13 precursor | −2.1 | X | |
| AT2G06850 | XTH4 | xyloglucan endotransglucosylase/hydrolase 4 | X | −2.4 | |
| AT5G51550 | EXL3 | EXORDIUM like 3 | X | −2.1 | |
| AT5G13980 | Glycosyl hydrolase family 38 protein | X | −1.6 | ||
| AT3G45970 | EXPL1 | expansin-like A1 | X | −1.4 | |
| AT1G15390 | PDF1A | peptide deformylase 1A | X | −1.4 | |
| AT1G75750 | GASA1 | GAST1 protein homolog 1 | X | −1.4 | |
| AT4G08950 | EXO | EXO Exordium, Phosphate-responsive 1 protein | X | −1.1 | |
| AT1G73590 | PIN1 | Auxin efflux carrier family protein | X | −1.0 | |
| BP | regulation of biological process GO:0050789 regulation of metabolic process GO:0019222 regulation of cellular metabolic process GO:0031323 | ||||
| AT3G62080 | SNF7 family protein | 2.6 | X | ||
| AT5G48560 | basic helix-loop-helix (bHLH) DNA-binding | 2.4 | X | ||
| AT5G01920 | STN8 | Protein kinase superfamily protein | 2.4 | X | |
| AT2G46225 | ABIL1 | ABI-1-like 1 | 2.2 | X | |
| AT5G56270 | WRKY2 | WRKY DNA-binding protein 2 | 1.9 | X | |
| AT1G27370 | SPL10 | squamosa promoter binding protein-like 10 | 1.7 | X | |
| AT5G62710 | Leucine-rich repeat protein kinase family protein | 1.4 | X | ||
| AT5G05130 | DNA/RNA helicase protein | 1.1 | X | ||
| AT5G07580 | Integrase-type DNA-binding superfamily protein | −3.3 | X | ||
| AT4G21380 | RK3 | receptor kinase 3 | −2.9 | X | |
| AT2G35530 | bZIP16 | basic region/leucine zipper transcription factor 16 | −2.7 | X | |
| AT1G78980 | SRF5 | STRUBBELIG-receptor family 5 | −2.4 | X | |
| AT1G78080 | WIND1 | related to AP2 4 | −1.2 | X | |
| AT4G36530 | alpha/beta-Hydrolases superfamily protein | −1.2 | X | ||
| CC | endomembrane system GO:0012505 Golgi apparatus GO:0005794 | ||||
| At3g18260 | Reticulon family protein | 3.1 | X | ||
| At1g77510 | PDIL1-2 | PDI-like 1-2 protein disulfide isomerase-like 1-2 | 1.2 | X | |
| At2g03760 | ST1 | sulphotransferase 12 | 1.1 | X | |
| AT5G19070 | SNARE associated Golgi protein family | −2.6 | X | ||
| At2g22900 | Galactosyl transferase GMA12/MNN10 | −2.4 | X | ||
| At4g36640 | Sec14p-like phosphatidylinositol transferprotein | −1.8 | X | ||
| At2g32720 | CB5-B | cytochrome B5 isoform B | −1.5 | X | |
| AT1G19970 | ER lumen protein retaining receptor protein | −1.4 | X | ||
| AT2G43240 | Nucleotide-sugar transporter family protein | −1.3 | X | ||
| BP | cellular response to sucrose starvation GO:0043617 | ||||
| AT3G06850 | DIN3 | 2-oxoacid dehydrogenases acyltransferases | X | 1.2 | |
| AT3G13450 | DIN4 | branched chain alpha-keto acid dehydrogenase E1 beta | X | 1.3 | |
| AT1G21400 | E1α | Thiamin diphosphate-binding fold (THDP-binding) | X | 1.6 | |
| Valine, leucine and isoleucine degradation GO:0009083 | |||||
| AT3G06850 | DIN3 | 2-oxoacid dehydrogenases acyltransferase family protein | X | 1.2 | |
| AT3G13450 | DIN4 | branched chain alpha-keto acid dehydrogenase E1 beta | X | 1.3 | |
| AT1G21400 | E1α | Thiamin diphosphate-binding fold (THDP-binding) | X | 1.6 | |
| AT2G14170 | ALDH6B2 | aldehyde dehydrogenase 6B2 | X | 1.2 | |
| At4g34030 | MCCB | subunit β of methylcrotonyl-CoA carboxylase (MCCB) | X | 1.1 | |
| CC | plasma membrane GO:0005886 | ||||
| AT5G67130 | PLC-like phosphodiesterases superfamily | X | −2.7 | ||
| AT3G21180 | ATACA9 | autoinhibited Ca(2+)-ATPase 9 | X | −2.7 | |
| AT5G65440 | X | −1.9 | |||
| AT5G15350 | ENODL17 | early nodulin-like protein 17 | X | −1.7 | |
| AT5G13980 | Glycosyl hydrolase family 38 protein | X | −1.6 | ||
| AT3G54920 | PMR6 | Pectin lyase-like superfamily protein | X | −1.5 | |
| AT1G17620 | Late embryogenesis abundant (LEA) | X | −1.2 | ||
| AT5G03700 | D-mannose binding lectin protein | X | −1.1 | ||
| AT1G73590 | PIN1 | Auxin efflux carrier family protein | X | −1.0 | |
| BP | developmental process GO:0032502 anatomical structure morphogenesis GO:0009653 | ||||
| AT2G35340 | MEE29 | helicase domain-containing protein | X | −3.2 | |
| AT3G63290 | 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase | X | −3.1 | ||
| AT5G65930 | ZWI | kinesin-like calmodulin-binding protein (ZWICHEL) | X | −3.0 | |
| AT4G02460 | PMS1 | DNA mismatch repair protein, putative | X | −2.9 | |
| AT3G47450 | RIF1 | P-loop containing nucleoside triphosphate hydrolase | X | −2.8 | |
| AT3G21180 | ATACA9 | autoinhibited Ca2+-ATPase 9 | X | −2.7 | |
| AT2G06850 | XTH4 | xyloglucan endotransglucosylase/hydrolase 4 | X | −2.4 | |
| AT4G15570 | MAA3 | P-loop containing nucleoside triphosphate hydrolase | X | −1.8 | |
| AT3G54220 | SCR | SCR Scarecrow alias SGR1 Shoot Gravitropism | X | −1.7 | |
| AT3G45970 | EXPL1 | expansin-like A1 | X | −1.4 | |
| AT1G75750 | GASA1 | GAST1 protein homolog 1 | X | −1.4 | |
| AT4G03190 | GRH1 | GRR1-like protein 1 | X | −1.1 | |
| AT1G73590 | PIN1 | Auxin efflux carrier family protein | X | −1.0 | |
| ONT | GO Term Name | ||||
|---|---|---|---|---|---|
| Transcript ID | Gene Symbol | Gene Description | GHsf : GWt FC log2 | FHsf : FWt FC log2 | |
| BP | response to stimulus GO:0050896 defense response to other organism GO:0098542 | ||||
| AT2G24570 | WRKY17 | WRKY DNA-binding protein 17 | 2.1 | X | |
| AT2G44490 | PEN2 | Glycosyl hydrolase superfamily protein | 1.7 | X | |
| AT1G66760 | MATE efflux family protein | 1.6 | X | ||
| AT1G72900 | Toll-Interleukin-Resistance (TIR) domain-containing protein | 1.2 | X | ||
| AT1G64790 | ILA | ILITYHIA | 1.2 | X | |
| AT2G43535 | Scorpion toxin-like knottin superfamily protein | 1.1 | X | ||
| AT5G07010 | ST2A | sulfotransferase 2A | 1.1 | X | |
| AT1G58360 | NAT2 | amino acid permease 1 | 1.0 | X | |
| AT1G71140 | MATE efflux family protein | 1.1 | 1.7 | ||
| AT1G19020 | X | 1.1 | |||
| AT1G50740 | Transmembrane proteins 14C | X | 1.1 | ||
| AT3G49350 | Ypt/Rab-GAP domain of gyp1p superfamily protein | X | 2.4 | ||
| AT3G60420 | Phosphoglycerate mutase family protein | X | 1.5 | ||
| AT4G02380 | SAG21 | senescence-associated gene 21 | X | 1.2 | |
| AT5G25930 | Protein kinase family protein with LRR domain | X | 1.0 | ||
| AT5G48380 | BIR1 | BAK1-interacting receptor-like kinase 1 | X | 1.2 | |
| response to water deprivation GO:0009414 (high salinity) | |||||
| AT3G05880 | RCI2A | Low temperature and salt responsive protein family | 1.5 | X | |
| AT3G11020 | DREB2B | DRE/CRT-binding protein 2B | 1.1 | X | |
| AT1G54410 | dehydrin family protein | 1.3 | X | ||
| response to endoplasmic reticulum stress (ER stress) GO:0034976 response to unfolded protein response (UPR) GO:0006986 response to misfolded protein GO:0051788 response to topologically incorrect protein GO:0035966 | |||||
| At1g72280 | AERO1 | disulfide bond formation protein | 2.9 | X | |
| AT5G38900 | Thioredoxin superfamily protein | 2.8 | X | ||
| AT1G77510 | PDIL1-2 | PDI-like 1-2 disulfide isomerase | 2.0 | X | |
| AT2G47470 | PDIL2-1/UNE5 | thioredoxin family protein | 1.1 | X | |
| AT2G29470 | GSTU3 | glutathione S-transferase tau 3 | 1.7 | 1.8 | |
| AT2G29490 | GSTU1 | glutathione S-transferase TAU 1 | 1.4 | 1.5 | |
| AT1G17170 | GSTU24 | glutathione S-transferase TAU 24 | 1.2 | 1.4 | |
| At2g38470 | WRKY33 | WRKY-type DNA binding protein | 1.2 | 1.2 | |
| At3g49350 | --- | Ypt/Rab-GAP domain of gyp1p protein | X | 2.4 | |
| At2g32920 | PDIL2-3 | PDI-like 2-3 disulfide isomerase | X | 1.5 | |
| At4g02380 | SAG21 | senescence-associated gene 21 | X | 1.2 | |
| At1g50740 | --- | Transmembrane proteins 14C | X | 1.1 | |
| At3g24050 | AtGATA-1 | GATA transcription factor 1 (AtGATA-1) | X | 1.1 | |
| At1g19020 | --- | Expressed protein | X | 1.1 | |
| endoplasmic reticulum associated degradation (ERAD) GO:0036503 | |||||
| AT1G18260 | HRD3A | HCP-like superfamily protein | X | 1.2 | |
| protein localization GO:0008104 establishment of protein localization GO:0045184 protein transport GO:0015031 vesicle-mediated transport GO:0016192 | |||||
| AT1G29310 | SecY protein transport family protein | 1.0 | 1.7 | ||
| AT3G44340 | CEF | clone eighty-four | 1.0 | 1.1 | |
| AT1G18830 | Transducin/WD40 repeat-like superfamily protein | X | 1.2 | ||
| AT1G29060 | Target SNARE coiled-coil domain protein | X | 1.5 | ||
| AT1G50740 | Transmembrane proteins 14C | X | 1.1 | ||
| AT1G70490 | ATARFA1D | Ras-related small GTP-binding family protein | X | 1.7 | |
| AT3G15980 | Coatomer, beta’ subunit | X | 1.1 | ||
| AT3G49350 | Ypt/Rab-GAP domain of gyp1p superfamily protein | X | 2.4 | ||
| AT4G02380 | SAG21 | senescence-associated gene 21 | X | 1.2 | |
| AT5G25930 | Protein kinase family protein with LRR domain | X | 1.0 | ||
| localization GO:0051179 transport GO:0006810 establishment of localization GO:0051234 | |||||
| AT4G24120 | YSL1 | YELLOW STRIPE like 1 | 2.5 | X | |
| AT2G44490 | PEN2 | Glycosyl hydrolase superfamily protein | 1.7 | X | |
| AT5G39040 | TAP2 | transporter assoc. with antigen processing protein 2 | 1.6 | X | |
| AT1G66760 | MATE efflux family protein | 1.6 | X | ||
| AT1G30400 | MRP1 | multidrug resistance-associated protein 1 | 1.5 | X | |
| AT3G53480 | PIS1 | pleiotropic drug resistance 9 | 1.5 | X | |
| AT2G15880 | Leucine-rich repeat (LRR) family protein | 1.3 | X | ||
| AT3G48970 | Heavy metal transport/detoxification protein | 1.3 | X | ||
| AT3G12520 | SULTR4;2 | sulfate transporter 4;2 | 1.2 | X | |
| AT1G58360 | NAT2 | amino acid permease 1 | 1.0 | X | |
| AT2G41700 | AtABCA1 | ATP-binding cassette A1 | 1.0 | X | |
| AT5G27150 | NHX1 | Na+/H+ exchanger 1 | 1.0 | X | |
| AT1G71330 | NAP5 | non-intrinsic ABC protein 5 | 1.5 | 1.2 | |
| AT1G78570 | ROL1 | rhamnose biosynthesis 1 | 1.4 | 1.1 | |
| AT1G71140 | MATE efflux family protein | 1.1 | 1.7 | ||
| AT5G54860 | Major facilitator superfamily protein | 1.1 | 1.5 | ||
| AT2G45180 | Bifunctional inhibitor/lipid-transfer protein | X | 2.9 | ||
| AT4G27840 | SNARE-like superfamily protein | X | 2.7 | ||
| AT5G19070 | SNARE associated Golgi protein family | X | 2.7 | ||
| AT2G29940 | PDR3 | pleiotropic drug resistance 3 | X | 2.4 | |
| AT5G47730 | Sec14p-like phosphatidylinositol transfer protein | X | 2.0 | ||
| AT2G43240 | Nucleotide-sugar transporter family protein | X | 1.6 | ||
| AT2G36300 | Integral membrane Yip1 family protein | X | 1.2 | ||
| AT1G07030 | Mitochondrial substrate carrier family protein | X | 1.2 | ||
| secondary metabolic process GO:0019748 | |||||
| AT2G29470 | GSTU3 | glutathione S-transferase tau 3 | 1.7 | 1.8 | |
| AT1G78570 | ROL1 | rhamnose biosynthesis 1 | 1.4 | 1.1 | |
| AT2G29490 | GSTU1 | glutathione S-transferase TAU 1 | 1.4 | 1.5 | |
| AT1G17170 | GSTU24 | glutathione S-transferase TAU 24 | 1.2 | 1.4 | |
| AT2G38470 | WRKY33 | WRKY DNA-binding protein 33 | 1.2 | 1.2 | |
| AT4G03070 | AOP1.1 | 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase | X | 3.7 | |
| AT1G17180 | GSTU25 | glutathione S-transferase TAU 25 | X | 1.9 | |
| AT5G48180 | NSP5 | nitrile specifier protein 5 | X | 1.4 | |
| AT1G17020 | SRG1 | senescence-related gene 1 | X | 1.1 | |
| CC | plasma membrane GO:0005886 (plasma membrane receptor kinases) | ||||
| AT1G78980 | SRF5 | STRUBBELIG-receptor family 5 | X | 2.2 | |
| AT5G65240 | Leucine-rich repeat protein kinase family protein | X | 1.9 | ||
| AT5G48380 | BIR1 | BAK1-interacting receptor-like kinase 1 | X | 1.2 | |
| AT5G25930 | Protein kinase family protein with LRR domain | X | 1.0 | ||
| BP | response to stimulus GO:0050896 response to stress GO:0006950 | ||||
| protein folding (chaperones) | |||||
| AT2G26150 | HSFA2 | heat shock transcription factor A2 | −7.3 | −6.8 | |
| At3g08910 | DNAJ heat shock family protein | −3.2 | X | ||
| At4g26780 | MGE2 | MGE2 Co-chaperone GrpE family protein | −2.5 | X | |
| At1g80030 | DnaJ protein | −1.4 | X | ||
| At5g37670 | low-molecular-weight heat shock protein | −1.2 | X | ||
| At4g28480 | DNAJ heat shock family protein | −1.1 | X | ||
| protein folding (non-chaperones) | |||||
| At1g44414 | unknown | −1.5 | X | ||
| At1g03070 | Bax inhibitor-1 family protein | −1.3 | X | ||
| At3g15180 | ARM repeat superfamily protein | −1.3 | X | ||
| At5g48580 | FKBP15-2 | peptidyl-prolyl cis-trans isomerase-like protein | −1.1 | X | |
| At4g23493 | unknown | −1.1 | X | ||
| response to oxidative stress GO:0006979 response to reactive oxygen species GO:0000302 response to hydrogen peroxide GO:0042542 | |||||
| AT5G19875 | −3.0 | X | |||
| AT1G76080 | CDSP32 | chloroplastic drought-induced stress protein 32 kD | −2.3 | X | |
| AT4G08920 | OOP2 | cryptochrome 1 | −2.0 | X | |
| AT3G16500 | PAP1 | phytochrome-associated protein 1 | −1.9 | X | |
| AT4G34020 | DJ1C | Class I glutamine amidotransferase-like protein | −1.7 | X | |
| AT4G35770 | SEN1 | Rhodanese/Cell cycle control phosphatase | −1.6 | X | |
| AT2G47180 | GolS1 | galactinol synthase 1 | −1.5 | X | |
| AT3G20340 | −1.4 | X | |||
| AT4G37530 | Peroxidase superfamily protein | −1.3 | X | ||
| AT4G27670 | HSP21 | heat shock protein 21 | −1.3 | X | |
| AT3G25530 | GR1 | glyoxylate reductase 1 | −1.2 | X | |
| AT5G61640 | PMSR1 | peptidemethionine sulfoxide reductase 1 | −1.2 | X | |
| AT2G14170 | ALDH6B2 | aldehyde dehydrogenase 6B2 | −1.1 | X | |
| AT3G53260 | PAL2 | phenylalanine ammonia-lyase 2 | −1.1 | X | |
| AT1G52760 | LysoPL2 | lysophospholipase 2 | −1.0 | X | |
| response to sucrose stimulus GO:0009744 | |||||
| AT2G17880 | Chaperone DnaJ-domain superfamily protein | −3.0 | X | ||
| At3g61060 | PP2-A13 | phloem protein 2-A13 | −2.1 | X | |
| AT4G37220 | Cold acclimation protein WCOR413 family | −1.9 | X | ||
| At3g24190 | Protein kinase superfamily protein | −1.7 | X | ||
| AT3G06850 | DIN3/LTA1 | 2-oxoacid dehydrogenases acyltransferase | −1.2 | X | |
| cellular amino acid and derivative metabolic process GO:0006519 | |||||
| At5g65010 | ASN2 | asparagine synthetase 2 | −2.9 | X | |
| At2g02400 | NAD(P)-binding Rossmann-fold superfamily protein | −1.6 | X | ||
| At3g07630 | AtADT2 | arogenate dehydratase 2 | −1.4 | X | |
| At5g24530 | DMR6 | 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase | −1.4 | X | |
| At1g73500 | MKK9 | MAP kinase kinase 9 | −1.2 | X | |
| At4g34030 | MCCB | 3-methylcrotonyl-CoA carboxylase | −1.1 | X | |
| At5g48220 | Aldolase-type TIM barrel family protein | −1.1 | X | ||
| CC | cell wall GO:0005618 external encapsulating structure GO:0030312 | ||||
| AT1G15020 | QSOX1 | quiescin-sulfhydryl oxidase 1 | X | −3.1 | |
| AT1G76020 | Thioredoxin superfamily protein | X | −2.8 | ||
| At2g30010 | TBL45 | trichome birefringence-like 45 | X | −2.4 | |
| AT3G25140 | QUA1/GAUT8 | galacturanosyl transferase 8 | X | −2.0 | |
| AT2G06850 | XTH4 | xyloglucan endotransglucosylase/hydrolase 4 | X | −2.0 | |
| At4g07960 | CSLC12 | CSL12 Cellulose-synthase-like C12 | X | −2.0 | |
| At5g50030 | Plant invertase/pectin methylesterase inhibitor protein | X | −1.9 | ||
| At1g16530 | ASL9 | assymetric leaves 2 like 9 | X | −1.7 | |
| AT1G75830 | PDF1.1 | low-molecular-weight cysteine-rich 67 | X | −1.1 | |
| AT2G02100 | PDF2.2 | low-molecular-weight cysteine-rich 69 | X | −1.1 | |
| AT5G64260 | EXL2 | EXORDIUM like 2 | X | −1.1 | |
| plastid GO:0009536 | |||||
| AT3G19120 | PIF / Ping-Pong family of plant transposases | X | −3.4 | ||
| AT5G04810 | pentatricopeptide (PPR) repeat-containing protein | X | −3.0 | ||
| AT5G16810 | Protein kinase superfamily protein | X | −2.6 | ||
| AT5G47870 | RAD52-2B | X | −2.4 | ||
| AT1G54350 | ABCD2 | ABC transporter family protein | X | −2.2 | |
| AT1G32440 | PKp3 | plastidial pyruvate kinase 3 | X | −2.2 | |
| AT1G14410 | WHY1 | ssDNA-binding transcriptional regulator | X | −1.8 | |
| AT3G52150 | RNA-binding (RRM/RBD/RNP motifs) family protein | X | −1.6 | ||
| AT3G20930 | RNA-binding (RRM/RBD/RNP motifs) family protein | X | −1.5 | ||
| AT2G20690 | lumazine-binding family protein | X | −1.0 | ||
| AT2G45990 | X | −1.0 | |||
| energy reserve metabolic process GO:0006112 (starch biosynthetic process GO:0019252) | |||||
| AT3G47450 | RIF1 | P-loop containing nucleoside triphosphate hydrolase | X | −2.6 | |
| AT5G58260 | NdhN | oxidoreductase | X | −2.4 | |
| At1g78050 | PGM | phosphoglycerate/bisphosphoglyce | X | −2.2 | |
| AT2G39930 | ISA1 | isoamylase 1 | X | −1.7 | |
| AT4G39550 | Galactose oxidase/kelch repeat superfamily protein | X | −1.3 | ||
| Category | Transcript ID | Gene Symbol | Gene Description | Functional Annotation | FWt : GWT FC log2 | FHsf : GHsf FC log2 | GHsf : GWt FC log2 | FHsf : FHsf FC log2 |
|---|---|---|---|---|---|---|---|---|
| Required | At1g02980 | CUL2 | cullin 2 | proteolytic turnover | 2.5 | X | 3.0 | X |
| At3g18260 | --- | Reticulon family protein | ER | 3.1 | X | 3.7 | X | |
| At1g77510 | ATPDIL1-2 | PDI-like 1-2 | ER stress, UPR | 1.2 | X | 2.0 | X | |
| At1g30600 | Subtilase family protein | cell wall | 2.5 | X | 2.7 | X | ||
| At2g44490 | PEN2 | Penetration 2, BGLU26 Beta Glucosidase 26 | defense response, response to wounding | 1.4 | X | 1.7 | X | |
| Corrected | At5g40470 | RNI-like superfamily protein | response to wounding, mechanical stress | X | 2.1 | −2.1 | X | |
| At1g44350 | ILL6 | ILL6 IAA-leucine resistant (ILR)-like gene 6 | response to wounding, mechanical stress | X | 1.9 | −2.0 | X | |
| At2g17430 | MLO7 | Seven transmembrane MLO family protein | response to wounding, mechanical stress | X | 1.9 | −1.7 | X | |
| At3g61060 | AtPP2-A13 | PP2-A13 phloem protein 2-A13 | response to wounding, mechanical stress | X | 1.5 | −2.1 | X | |
| At4g38550 | phospholipase-like protein (PEARLI 4) family | response to wounding, mechanical stress | X | 1.4 | −1.3 | X | ||
| At1g61340 | FBS1 F-box stress induced 1 | response to wounding, mechanical stress | X | 1.0 | −1.8 | X | ||
| At2g02400 | NAD(P)-binding Rossmann-fold superfamily protein | cell wall | X | 2.0 | −1.6 | X | ||
| At1g53840 | ATPME1 | PME1 pectin methylesterase 1 | cell wall | X | 1.1 | −1.4 | X | |
| At5g15350 | ENODL17 | early nodulin-like protein 17 | cell wall | X | −1.7 | 1.0 | X | |
| At2g36830 | GAMMA-TIP | GAMMA-TIP gamma tonoplast intrinsic protein | transmembrane transporter genes | X | 3.1 | −3.5 | X | |
| At1g50400 | porin family protein | transmembrane transporter genes | X | 2.2 | −2.0 | X | ||
| At2g40090 | ATATH9 | ABC2 homolog 9 | transmembrane transporter genes | X | 1.1 | −1.0 | X | |
| At5g14570 | ATNRT2.7 | NRT2.7 high affinity nitrate transporter | nitrate transporters genes | X | 2.4 | −2.6 | X | |
| At4g40080 | ENTH/ANTH/VHS superfamily protein | nitrate transporters genes | X | 2.1 | −2.3 | X | ||
| At3g06850 | DIN3 | DIN3 dark inducible 3, BCE2 | valine, leucine and isoleucine degradation | X | 1.2 | −1.2 | X | |
| At2g14170 | ALDH6B2 | ALDH6B2 aldehyde dehydrogenase 6B2 | valine, leucine and isoleucine degradation | X | 1.2 | −1.1 | X | |
| At4g34030 | MCCB | MCCB 3-methylcrotonyl-CoA carboxylase | valine, leucine and isoleucine degradation | X | 1.1 | −1.1 | X | |
| HsfA2-Dependent WT genotypic adaptation | At4g07960 | ATCSLC12 | Cellulose-synthase-like C12 | cell wall | 1.9 | X | X | −2.0 |
| At1g78980 | SRF5 | SRF5 STRUBBELIG-receptor family 5 | plasma membrane receptor kinase | −2.4 | X | X | 2.2 | |
| At5g19070 | --- | SNARE associated Golgi protein family | vesicle-mediated transport | −2.6 | X | X | 2.7 | |
| At2g43240 | --- | Nucleotide-sugar transporter family protein | transport | −1.3 | X | X | 1.6 | |
| Compensated HSFA2 KO genotypic adaptation | At2g32920 | ATPDIL2-3 | PDI-like 2-3 | ER stress, UPR | X | 1.0 | X | 1.5 |
| At1g17180 | ATGSTU25 | glutathione S-transferase TAU 25 | UPR | X | 1.7 | X | 1.9 | |
| At3g15980 | --- | Coatomer, beta’ subunit | vesicle-mediated transport | X | 1.7 | X | 1.7 | |
| At1g29060 | --- | Target SNARE coiled-coil domain protein | vesicle-mediated transport | X | 1.3 | X | 1.5 | |
| At2g36300 | --- | Integral membrane Yip1 family protein | vesicle-mediated transport | X | 1.0 | X | 1.2 | |
| At3g12180 | --- | Cornichon family protein | intra-Golgi vesicle-mediated transport | X | −2.7 | X | −2.0 | |
| At5g66160 | RMR1 | receptor homology, transmembrane domain ring H2 | intra-Golgi vesicle-mediated transport | X | −1.6 | X | −1.7 | |
| At3g21180 | ACA9 | autoinhibited Ca(2+)-ATPase 9 | calcium pump | X | −2.7 | X | −2.6 | |
| At4g37640 | ACA2 | calcium ATPase 2 | calcium pump | X | −2.2 | X | −1.7 |
References
- Fortunati, A.; Piconese, S.; Tassone, P.; Ferrari, S.; Migliaccio, F. Rha1, a new mutant of Arabidopsis disturbed in root slanting, gravitropism and auxin physiology. Plant Signal. Behav. 2008, 3, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, G.L.; Hilaire, E.M.; Peterson, B.V.; Brown, C.S.; Guikema, J.A. Effects of microgravity and clinorotation on stress ethylene production in two starchless mutants of Arabidopsis thaliana. J. Gravit. Physiol. 1995, 2, P153–P154. [Google Scholar] [PubMed]
- Kriegs, B.; Theisen, R.; Schnabl, H. Inositol 1,4,5-trisphosphate and Ran expression during simulated and real microgravity. Protoplasma 2006, 229, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Zupanska, A.K.; Denison, F.C.; Ferl, R.J.; Paul, A.-L. Spaceflight engages heat shock protein and other molecular chaperone genes in tissue culture cells of Arabidopsis thaliana. Am. J. Bot. 2013, 100, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Martzivanou, M.; Babbick, M.; Cogoli-Greuter, M.; Hampp, R. Microgravity-related changes in gene expression after short-term exposure of Arabidopsis thaliana cell cultures. Protoplasma 2006, 229, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Centis-Aubay, S.; Gasset, G.; Mazars, C.; Ranjeva, R.; Graziana, A. Changes in gravitational forces induce modifications of gene expression in A. thaliana seedlings. Planta 2003, 218, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Babbick, M.; Dijkstra, C.; Larkin, O.J.; Anthony, P.; Davey, M.R.; Power, J.B.; Lowe, K.C.; Cogoli-Greuter, M.; Hampp, R. Expression of transcription factors after short-term exposure of Arabidopsis thaliana cell cultures to hypergravity and simulated microgravity (2-D/3-D clinorotation, magnetic levitation). Adv. Space Res. 2007, 39, 1182–1189. [Google Scholar] [CrossRef]
- Kimbrough, J.M.; Salinas-Mondragon, R.; Boss, W.F.; Brown, C.S.; Sederoff, H.W. The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex. Plant Physiol. 2004, 136, 2790–2805. [Google Scholar] [CrossRef]
- Soh, H.; Auh, C.; Soh, W.Y.; Han, K.; Kim, D.; Lee, S.; Rhee, Y. Gene expression changes in Arabidopsis seedlings during short- to long-term exposure to 3-D clinorotation. Planta 2011, 234, 255–270. [Google Scholar] [CrossRef]
- Soh, H.; Choi, Y.; Lee, T.-K.; Yeo, U.-D.; Han, K.; Auh, C.; Lee, S. Identification of unique cis-element pattern on simulated microgravity treated Arabidopsis by in silico and gene expression. Adv. Space Res. 2012, 50, 397–407. [Google Scholar] [CrossRef]
- Kittang, A.I.; van Loon, J.J.; Vorst, O.; Hall, R.D.; Fossum, K.; Iversen, T.H. Ground based studies of gene expression in Arabidopsis exposed to gravity stresses. J. Gravit. Physiol. 2004, 11, P223–P224. [Google Scholar] [PubMed]
- Chen, B.; Wang, Y. Proteomic and physiological studies provide insight into photosynthetic response of rice (Oryza sativa L.) seedlings to microgravity. Photochem. Photobiol. 2016, 92, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Correll, M.J.; Pyle, T.P.; Millar, K.D.; Sun, Y.; Yao, J.; Edelmann, R.E.; Kiss, J.Z. Transcriptome analyses of Arabidopsis thaliana seedlings grown in space: Implications for gravity-responsive genes. Planta 2013, 238, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Ferl, R.J.; Koh, J.; Denison, F.; Paul, A.-L. Spaceflight induces specific alterations in the proteomes of Arabidopsis. Astrobiology 2015, 15, 32–56. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Subramanian, A.; Pattathil, S.; Correll, M.J.; Kiss, J.Z. Comparative transcriptomics indicate changes in cell wall organization and stress response in seedlings during spaceflight. Am. J. Bot. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Sparks, J.A.; Nakashima, J.; Allen, S.N.; Tang, Y.; Blancaflor, E.B. Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Am. J. Bot. 2015, 102, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Mazars, C.; Briere, C.; Grat, S.; Pichereaux, C.; Rossignol, M.; Pereda-Loth, V.; Eche, B.; Boucheron-Dubuisson, E.; Le Disquet, I.; Medina, F.J.; et al. Microsome-associated proteome modifications of Arabidopsis seedlings grown on board the International Space Station reveal the possible effect on plants of space stresses other than microgravity. Plant Signal. Behav. 2014, 9, e29637. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.-L.; Popp, M.P.; Gurley, W.B.; Guy, C.L.; Norwood, K.L.; Ferl, R.J. Arabidopsis gene expression patterns are altered during spaceflight. Adv. Space Res. 2005, 36, 1175–1181. [Google Scholar] [CrossRef]
- Paul, A.-L.; Zupanska, A.K.; Ostrow, D.T.; Zhang, Y.; Sun, Y.; Li, J.L.; Shanker, S.; Farmerie, W.G.; Amalfitano, C.E.; Ferl, R.J. Spaceflight transcriptomes: Unique responses to a novel environment. Astrobiology 2012, 12, 40–56. [Google Scholar] [CrossRef]
- Paul, A.-L.; Zupanska, A.K.; Schultz, E.R.; Ferl, R.J. Organ-specific remodeling of the Arabidopsis transcriptome in response to spaceflight. BMC Plant Biol. 2013, 13, 112. [Google Scholar] [CrossRef]
- Shi, K.; Gu, J.; Guo, H.; Zhao, L.; Xie, Y.; Xiong, H.; Li, J.; Zhao, S.; Song, X.; Liu, L. Transcriptome and proteomic analyses reveal multiple differences associated with chloroplast development in the spaceflight-induced wheat albino mutant mta. PLoS ONE 2017, 12, e0177992. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Oono, Y.; Gusev, O.; Matsumoto, T.; Yazawa, T.; Levinskikh, M.A.; Sychev, V.N.; Bingham, G.E.; Wheeler, R.; Hummerick, M. Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Vandenbrink, J.P.; Kiss, J.Z. Space, the final frontier: A critical review of recent experiments performed in microgravity. Plant Sci. 2016, 243, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.-L.; Sng, N.J.; Zupanska, A.K.; Krishnamurthy, A.; Schultz, E.R.; Ferl, R.J. Genetic dissection of the Arabidopsis spaceflight transcriptome: Are some responses dispensable for the physiological adaptation of plants to spaceflight? PLoS ONE 2017, 12, e0180186. [Google Scholar] [CrossRef]
- Fengler, S.; Spirer, I.; Neef, M.; Ecke, M.; Nieselt, K.; Hampp, R. A whole-genome microarray study of Arabidopsis thaliana semisolid callus cultures exposed to microgravity and nonmicrogravity related spaceflight conditions for 5 days on board of Shenzhou 8. BioMed Res. Int. 2015, 2015, 547495. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.L.; Roux, S.J. Gene expression changes induced by space flight in single-cells of the fern Ceratopteris richardii. Planta 2008, 229, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Xie, J.; Zheng, H. Differential protein expression profiling of Arabidopsis thaliana callus under microgravity on board the Chinese SZ-8 spacecraft. Planta 2015, 241, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Zupanska, A.K.; Schultz, E.R.; Yao, J.; Sng, N.J.; Zhou, M.; Callaham, J.B.; Ferl, R.J.; Paul, A.-L. ARG1 Functions in the Physiological Adaptation of Undifferentiated Plant Cells to Spaceflight. Astrobiology 2017, 17, 1077–1111. [Google Scholar] [CrossRef]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.H.; Ma, X.; Luo, D.X.; Gong, Z.H.; Lu, M.H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]
- Nover, L.; Bharti, K.; Doring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K.D. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones 2001, 6, 177–189. [Google Scholar] [CrossRef]
- Schoffl, F.; Prandl, R.; Reindl, A. Regulation of the heat-shock response. Plant Physiol. 1998, 117, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Rajan, V.B.; D’Silva, P. Arabidopsis thaliana J-class heat shock proteins: Cellular stress sensors. Funct. Integr. Genomics 2009, 9, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C. Mechanisms of the Hsp70 chaperone system. Biochem. Cell Biol. 2010, 88, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.P.; Weissman, J.S. Oxidative protein folding in eukaryotes: Mechanisms and consequences. J. Cell Biol. 2004, 164, 341–346. [Google Scholar] [CrossRef]
- Riemer, J.; Bulleid, N.; Herrmann, J.M. Disulfide formation in the ER and mitochondria: Two solutions to a common process. Science 2009, 324, 1284–1287. [Google Scholar] [CrossRef]
- Trombetta, E.S.; Parodi, A.J. Quality control and protein folding in the secretory pathway. Annu. Rev. Cell Dev. Biol. 2003, 19, 649–676. [Google Scholar] [CrossRef]
- Howell, S.H. Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 2013, 64, 477–499. [Google Scholar] [CrossRef]
- Martinez, I.M.; Chrispeels, M.J. Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 2003, 15, 561–576. [Google Scholar] [CrossRef]
- Su, W.; Liu, Y.; Xia, Y.; Hong, Z.; Li, J. The Arabidopsis homolog of the mammalian OS-9 protein plays a key role in the endoplasmic reticulum-associated degradation of misfolded receptor-like kinases. Mol. Plant 2012, 5, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Huttner, S.; Veit, C.; Schoberer, J.; Grass, J.; Strasser, R. Unraveling the function of Arabidopsis thaliana OS9 in the endoplasmic reticulum-associated degradation of glycoproteins. Plant Mol. Biol. 2012, 79, 21–33. [Google Scholar] [CrossRef]
- Ceriotti, A. Waste disposal in the endoplasmic reticulum, ROS production and plant salt stress response. Cell Res. 2011, 21, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Howell, S.H. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 2010, 22, 2930–2942. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, A.; Yabuta, Y.; Yoshida, E.; Maruta, T.; Yoshimura, K.; Shigeoka, S. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006, 48, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Q.; Gao, X.; Qi, B.; Chen, N.; Xu, S.; Chen, J.; Wang, X. AtHsfA2 modulates expression of stress responsive genes and enhances tolerance to heat and oxidative stress in Arabidopsis. Sci. China C Life Sci. 2005, 48, 540–550. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.D.; et al. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef]
- Sugio, A.; Dreos, R.; Aparicio, F.; Maule, A.J. The cytosolic protein response as a subcomponent of the wider heat shock response in Arabidopsis. Plant Cell 2009, 21, 642–654. [Google Scholar] [CrossRef]
- Cubano, L.A.; Lewis, M.L. Effect of vibrational stress and spaceflight on regulation of heat shock proteins hsp70 and hsp27 in human lymphocytes (Jurkat). J. Leukoc. Biol. 2001, 69, 755–761. [Google Scholar]
- Nishizawa-Yokoi, A.; Yoshida, E.; Yabuta, Y.; Shigeoka, S. Analysis of the regulation of target genes by an Arabidopsis heat shock transcription factor, HsfA2. Biosci. Biotechnol. Biochem. 2009, 73, 890–895. [Google Scholar] [CrossRef]
- Hahn, J.S.; Hu, Z.; Thiele, D.J.; Iyer, V.R. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell. Biol. 2004, 24, 5249–5256. [Google Scholar] [CrossRef] [PubMed]
- Pirkkala, L.; Nykanen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Yamaguchi, K.; Nishiuchi, T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J. Exp. Bot. 2007, 58, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.T.; Christiansen, K.; Knierim, B.; Carroll, A.; Ito, J.; Batth, T.S.; Smith-Moritz, A.M.; Morrison, S.; McInerney, P.; Hadi, M.Z.; et al. Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol. 2012, 159, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Gebbie, L.K.; Burn, J.E.; Hocart, C.H.; Williamson, R.E. Genes encoding ADP-ribosylation factors in Arabidopsis thaliana L. Heyn.; genome analysis and antisense suppression. J. Exp. Bot. 2005, 56, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, A.; Lund, C.H.; Sakuragi, Y.; Scheller, H.V. Golgi-localized enzyme complexes for plant cell wall biosynthesis. Trends Plant Sci. 2013, 18, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Inada, N.; Ueda, T. Membrane trafficking pathways and their roles in plant-microbe interactions. Plant Cell Physiol. 2014, 55, 672–686. [Google Scholar] [CrossRef]
- Imazu, H.; Sakurai, H. Saccharomyces cerevisiae heat shock transcription factor regulates cell wall remodeling in response to heat shock. Eukaryot. Cell 2005, 4, 1050–1056. [Google Scholar] [CrossRef]
- Hsu, P.H.; Chiang, P.C.; Liu, C.H.; Chang, Y.W. Characterization of cell wall proteins in Saccharomyces cerevisiae clinical isolates elucidates Hsp150p in virulence. PLoS ONE 2015, 10, e0135174. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.H.; Chen, F.; Torres-Jerez, I.; Tang, Y.; Wang, M.; Du, Q.; Cheng, X.; Wen, J.; Dixon, R. Transcriptome analysis of secondary cell wall development in Medicago truncatula. BMC Genom. 2016, 17, 23. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.; Losansky, A.; Muller, R.; Plock, A.; Kliebenstein, D.J.; Wittstock, U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in Arabidopsis. Plant Physiol. 2009, 149, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z.; Jamet, E.; Negroni, L.; Arsene der Garabedian, P.; Zivy, M.; Jouanin, L. A sub-proteome of Arabidopsis thaliana mature stems trapped on Concanavalin A is enriched in cell wall glycoside hydrolases. J. Exp. Bot. 2007, 58, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Senechal, F.; Graff, L.; Surcouf, O.; Marcelo, P.; Rayon, C.; Bouton, S.; Mareck, A.; Mouille, G.; Stintzi, A.; Hofte, H.; et al. Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT3.5, a subtilisin-like serine protease. Ann. Bot. 2014, 114, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Raab, T.K.; Schiff, C.; Somerville, S.C. PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 2002, 14, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z.; Jouanin, L. Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiol. Biochem. 2006, 44, 435–449. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, D.; Kende, H. Expansins: Ever-expanding numbers and functions. Curr. Opin. Plant Biol. 2001, 4, 527–532. [Google Scholar] [CrossRef]
- Di Laurenzio, L.; Wysocka-Diller, J.; Malamy, J.E.; Pysh, L.; Helariutta, Y.; Freshour, G.; Hahn, M.G.; Feldmann, K.A.; Benfey, P.N. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 1996, 86, 423–433. [Google Scholar] [CrossRef]
- Boavida, L.C.; Shuai, B.; Yu, H.J.; Pagnussat, G.C.; Sundaresan, V.; McCormick, S. A collection of Ds insertional mutants associated with defects in male gametophyte development and function in Arabidopsis thaliana. Genetics 2009, 181, 1369–1385. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Oppenheimer, D.G. Extragenic suppressors of the arabidopsis zwi-3 mutation identify new genes that function in trichome branch formation and pollen tube growth. Development 1999, 126, 3079–3088. [Google Scholar] [PubMed]
- Schiott, M.; Romanowsky, S.M.; Baekgaard, L.; Jakobsen, M.K.; Palmgren, M.G.; Harper, J.F. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. USA 2004, 101, 9502–9507. [Google Scholar] [CrossRef]
- Lalonde, S.; Sero, A.; Pratelli, R.; Pilot, G.; Chen, J.; Sardi, M.I.; Parsa, S.A.; Kim, D.Y.; Acharya, B.R.; Stein, E.V.; et al. A membrane protein/signaling protein interaction network for Arabidopsis version AMPv2. Front. Physiol. 2010, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.F.; Chen, P.Y.; Nguyen, Q.T.; Huang, L.Y.; Zeng, G.R.; Huang, T.L.; Lin, C.Y.; Huang, H.J. Transcriptome profiling of genes and pathways associated with arsenic toxicity and tolerance in Arabidopsis. BMC Plant Biol. 2014, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Mortley, D.G.; Bonsi, C.K.; Hill, W.A.; Morris, C.E.; Williams, C.S.; Davis, C.F.; Williams, J.W.; Levine, L.H.; Petersen, B.V.; Wheeler, R.M. Influence of microgravity environment on root growth, soluble sugars, and starch concentration of sweetpotato stem cuttings. J. Am. Soc. Hortic. Sci. 2008, 133, 327–332. [Google Scholar] [PubMed]
- Stutte, G.W.; Monje, O.; Hatfield, R.D.; Paul, A.L.; Ferl, R.J.; Simone, C.G. Microgravity effects on leaf morphology, cell structure, carbon metabolism and mRNA expression of dwarf wheat. Planta 2006, 224, 1038–1049. [Google Scholar] [CrossRef]
- Kuznetsov, O.A.; Brown, C.S.; Levine, H.G.; Piastuch, W.C.; Sanwo-Lewandowski, M.M.; Hasenstein, K.H. Composition and physical properties of starch in microgravity-grown plants. Adv. Space Res. 2001, 28, 651–658. [Google Scholar] [CrossRef]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262. [Google Scholar] [CrossRef]
- Liu, H.C.; Charng, Y.Y. Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol. 2013, 163, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Hara, M. The multifunctionality of dehydrins: An overview. Plant Signal. Behav. 2010, 5, 503–508. [Google Scholar] [CrossRef]
- Medina, J.; Rodriguez-Franco, M.; Penalosa, A.; Carrascosa, M.J.; Neuhaus, G.; Salinas, J. Arabidopsis mutants deregulated in RCI2A expression reveal new signaling pathways in abiotic stress responses. Plant J. 2005, 42, 586–597. [Google Scholar] [CrossRef]
- Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Seki, M.; Miura, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration- and high-salinity-responsive gene expression. Plant Mol. Biol. 2000, 42, 6657–6665. [Google Scholar] [CrossRef]
- Miller, G.; Mittler, R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006, 98, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Xing, D.; Gao, C. Characterization of mitochondrial dynamics and subcellular localization of ROS reveal that HsfA2 alleviates oxidative damage caused by heat stress in Arabidopsis. J. Exp. Bot. 2009, 60, 2073–2091. [Google Scholar] [CrossRef]
- Nishizawa-Yokoi, A.; Tainaka, H.; Yoshida, E.; Tamoi, M.; Yabuta, Y.; Shigeoka, S. The 26S proteasome function and Hsp90 activity involved in the regulation of HsfA2 expression in response to oxidative stress. Plant Cell Physiol. 2010, 51, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Loreti, E.; Solfanelli, C.; Novi, G.; Alpi, A.; Perata, P. Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J. Plant Res. 2006, 119, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, Y.; Ito, M.; Nishida, I.; Watanabe, A. Multiple signaling pathways in gene expression during sugar starvation. Pharmacological analysis of din gene expression in suspension-cultured cells of Arabidopsis. Plant Physiol. 2000, 124, 1139–1148. [Google Scholar] [CrossRef]
- Binder, S. Branched-Chain Amino Acid Metabolism in Arabidopsis thaliana. Arabidopsis Book 2010, 8, e0137. [Google Scholar] [CrossRef]
- Kaufman, R.J.; Scheuner, D.; Schroder, M.; Shen, X.; Lee, K.; Liu, C.Y.; Arnold, S.M. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 2002, 3, 411–421. [Google Scholar] [CrossRef]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef]
- McHale, L.; Tan, X.; Koehl, P.; Michelmore, R.W. Plant NBS-LRR proteins: Adaptable guards. Genome Biol. 2006, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.A.; Miklis, M.; Schmelzer, E.; Schulze-Lefert, P.; Panstruga, R. Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proc. Natl. Acad. Sci. USA 2005, 102, 3135–3140. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Pathange, P.; Chung, J.S.; Jiang, J.; Gao, L.; Oikawa, A.; Hirai, M.Y.; Saito, K.; Pare, P.W.; Shi, H. A stress-inducible sulphotransferase sulphonates salicylic acid and confers pathogen resistance in Arabidopsis. Plant Cell Environ. 2010, 33, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Jung, H.J.; Kwak, K.J.; Kim, M.K.; Oh, S.H.; Han, Y.S.; Kang, H. The Arabidopsis U12-type spliceosomal protein U11/U12-31K is involved in U12 intron splicing via RNA chaperone activity and affects plant development. Plant Cell 2010, 22, 3951–3962. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Shu, M.D.; Steitz, J.A. Splicing of U12-type introns deposits an exon junction complex competent to induce nonsense-mediated mRNA decay. Proc. Natl. Acad. Sci. USA 2004, 101, 17976–17981. [Google Scholar] [CrossRef] [PubMed]
- Meiri, D.; Tazat, K.; Cohen-Peer, R.; Farchi-Pisanty, O.; Aviezer-Hagai, K.; Avni, A.; Breiman, A. Involvement of Arabidopsis ROF2 (FKBP65) in thermotolerance. Plant Mol. Biol. 2010, 72, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.P.; Christopher, D.A. Endoplasmic reticulum stress activates the expression of a sub-group of protein disulfide isomerase genes and AtbZIP60 modulates the response in Arabidopsis thaliana. Mol. Genet. Genom. 2008, 280, 199–210. [Google Scholar] [CrossRef]
- Risseeuw, E.P.; Daskalchuk, T.E.; Banks, T.W.; Liu, E.; Cotelesage, J.; Hellmann, H.; Estelle, M.; Somers, D.E.; Crosby, W.L. Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 2003, 34, 753–767. [Google Scholar] [CrossRef]
- Bednarek, P.; Pislewska-Bednarek, M.; Svatos, A.; Schneider, B.; Doubsky, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef]
- Nedukha, E.M. Effects of microgravity on the structure and function of plant cell walls. Int. Rev. Cytol. 1997, 170, 39–77. [Google Scholar]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 2002, 215, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Calderon, M.T.; Sepulveda-Garcia, E.; Rocha-Sosa, M. Characterization of novel F-box proteins in plants induced by biotic and abiotic stress. Plant Sci. 2012, 185, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Widemann, E.; Miesch, L.; Lugan, R.; Holder, E.; Heinrich, C.; Aubert, Y.; Miesch, M.; Pinot, F.; Heitz, T. The amidohydrolases IAR3 and ILL6 contribute to jasmonoyl-isoleucine hormone turnover and generate 12-hydroxyjasmonic acid upon wounding in Arabidopsis leaves. J. Biol. Chem. 2013, 288, 31701–31714. [Google Scholar] [CrossRef] [PubMed]
- Walley, J.W.; Coughlan, S.; Hudson, M.E.; Covington, M.F.; Kaspi, R.; Banu, G.; Harmer, S.L.; Dehesh, K. Mechanical stress induces biotic and abiotic stress responses via a novel cis-element. PLoS Genet. 2007, 3, e172. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Joung, J.G.; Fei, Z.; Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Desprez, T.; Juraniec, M.; Crowell, E.F.; Jouy, H.; Pochylova, Z.; Parcy, F.; Hofte, H.; Gonneau, M.; Vernhettes, S. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 15572–15577. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, D.; Batoux, M.; Fulton, L.; Pfister, K.; Yadav, R.K.; Schellenberg, M.; Schneitz, K. STRUBBELIG defines a receptor kinase-mediated signaling pathway regulating organ development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 9074–9079. [Google Scholar] [CrossRef]
- Antebi, A.; Fink, G.R. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 1992, 3, 633–654. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Lee, G.J.; Hwang, I. AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J. Cell Biol. 2005, 170, 757–767. [Google Scholar] [CrossRef]
- Johnson, C.M.; Subramanian, A.; Edelmann, R.E.; Kiss, J.Z. Morphometric analyses of petioles of seedlings grown in a spaceflight experiment. J. Plant Res. 2015, 128, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, E.; Liu, W.M.; Mei, R. Robust estimators for expression analysis. Bioinformatics 2002, 18, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhou, X.; Ling, Y.; Zhang, Z.; Su, Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38, W64–W70. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, T.; Hayashi, S.; Saeki, M.; Ohta, H.; Kinoshita, K. ATTED-II provides coexpressed gene networks for Arabidopsis. Nucleic Acids Res. 2009, 37, D987–D991. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Kull, M.; Peterson, H.; Hansen, J.; Vilo, J. g:Profiler--a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 2007, 35, W193–W200. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupanska, A.K.; LeFrois, C.; Ferl, R.J.; Paul, A.-L. HSFA2 Functions in the Physiological Adaptation of Undifferentiated Plant Cells to Spaceflight. Int. J. Mol. Sci. 2019, 20, 390. https://doi.org/10.3390/ijms20020390
Zupanska AK, LeFrois C, Ferl RJ, Paul A-L. HSFA2 Functions in the Physiological Adaptation of Undifferentiated Plant Cells to Spaceflight. International Journal of Molecular Sciences. 2019; 20(2):390. https://doi.org/10.3390/ijms20020390
Chicago/Turabian StyleZupanska, Agata K., Collin LeFrois, Robert J. Ferl, and Anna-Lisa Paul. 2019. "HSFA2 Functions in the Physiological Adaptation of Undifferentiated Plant Cells to Spaceflight" International Journal of Molecular Sciences 20, no. 2: 390. https://doi.org/10.3390/ijms20020390
APA StyleZupanska, A. K., LeFrois, C., Ferl, R. J., & Paul, A.-L. (2019). HSFA2 Functions in the Physiological Adaptation of Undifferentiated Plant Cells to Spaceflight. International Journal of Molecular Sciences, 20(2), 390. https://doi.org/10.3390/ijms20020390