Ceramide and Sphingosine Regulation of Myelinogenesis: Targeting Serine Palmitoyltransferase Using microRNA in Multiple Sclerosis
Abstract
1. Introduction
2. Diversity in Sphingolipid Functions
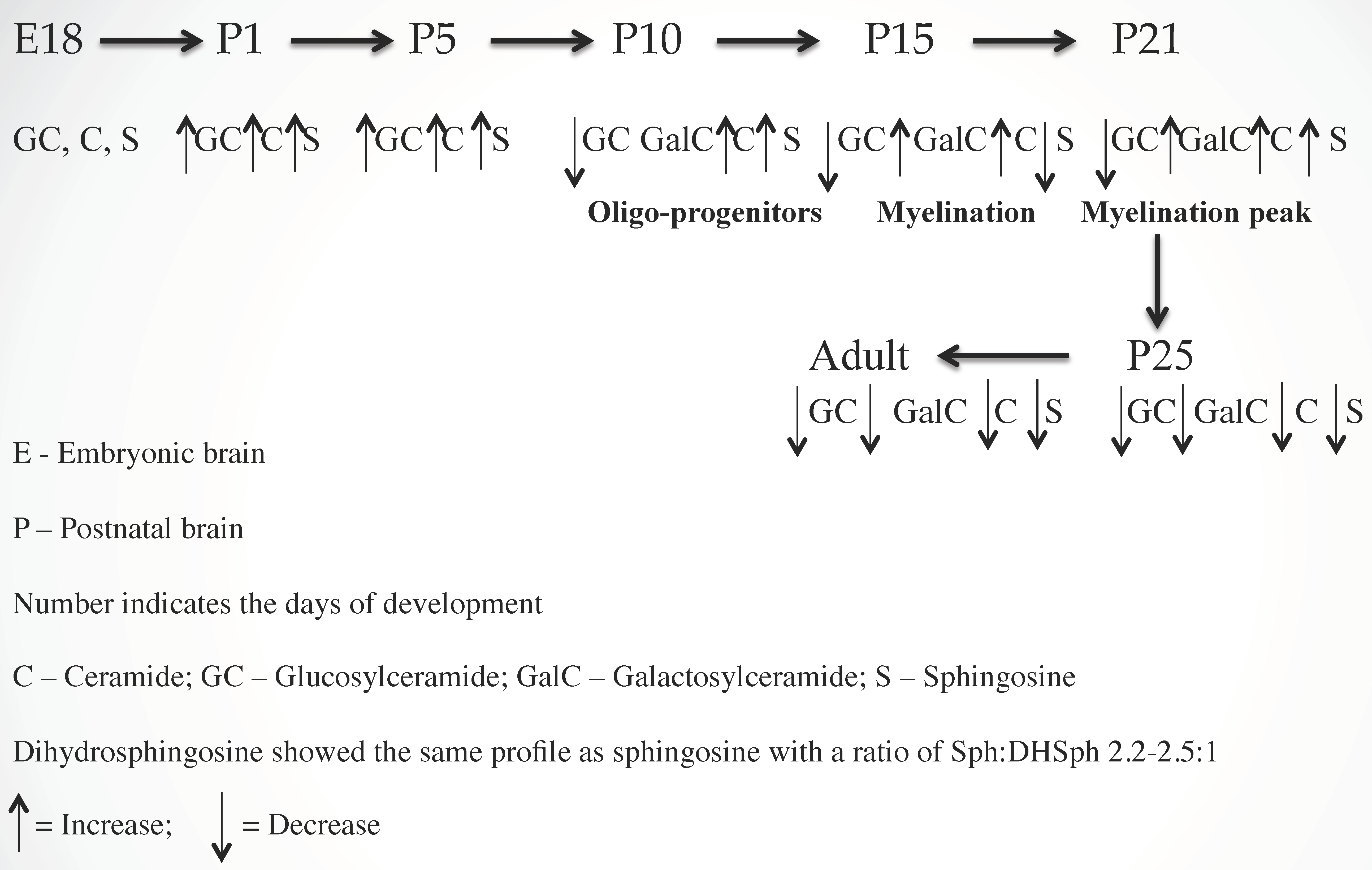
2.1. Ceramide (Cer) and Sphingosine (Sph) Stimulate Myelinogenesis
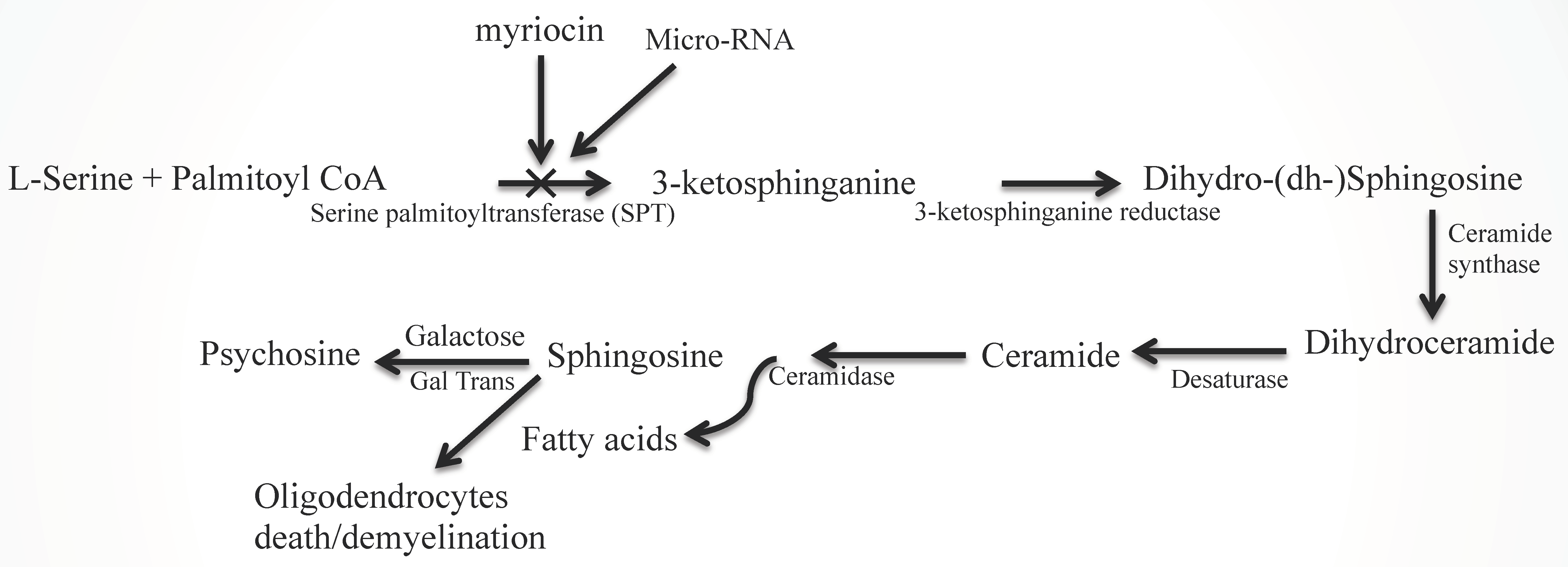
2.2. Sphingolipid Metabolic Disorders
2.3. Aberrant Lipid Metabolism in MS Brains
2.4. Expression of miRNAs
2.4.1. miRNAs in Oligodendrocyte Development and Ceramide Metabolism
2.4.2. miRNAs in MS and Neurodegenerative Disorders
2.4.3. Role of miRNA in Normal and Pathological Functions of Rodent and Human CNS
3. MS Therapy with Conventional, Complementary, and Alternative Medicines and the Perspective of miRNA Targeting
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dasgupta, S.; Ray, S.K. Diverse biological functions of sphingolipids in the CNS: Ceramide and sphingosine regulate myelination in developing brain but stimulate demyelination during pathogenesis of multiple sclerosis. J. Neurol. Psycol. 2017, 5, 1–7. [Google Scholar]
- Miller, L.G., Jr.; Young, J.A.; Ray, S.K.; Wang, G.; Purohit, S.; Banik, N.L. Dasgupta S. Sphingosine toxicity in EAE and MS: Evidence for ceramide generation via serine-palmitoyltransferase activation. Neurochem. Res. 2017, 42, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Kücükali, C.I.; Kürtüncii, M.; Çoban, M.; Tüzün, E. Epigenetics of multiple sclerosis: An updated review. Neuromol. Med. 2015, 17, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Urdinguino, R.G.; Sanchez-Mut, J.V.; Esteller, M. Epigenetic mechanisms in neurological diseases; genes, syndrome, and therapies. Lancet. Neurol. 2009, 8, 1056–1072. [Google Scholar] [CrossRef]
- Lauer, K. Environmental risk factorsin multiple sclerosis. Expert. Rev. Neurthera. 2010, 10, 421–440. [Google Scholar] [CrossRef]
- Oskenburg, J.R.; Baranzini, S.E. Multiple sclerosis genetics-Is the glass half full or half empty? Nat. Rev. Neurol. 2010, 6, 429–437. [Google Scholar]
- Hunter, S.F. Overview and diagnosis of multiple sclerosis. Am. J. Manag. Care 2016, 22, s141–s150. [Google Scholar]
- Compston, A.; Cole, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Lindberg, R.L.; Hoffman, F.; Mehling, M.; Kuhle, J.; Kappos, L. Altered expression of miR17-p in CD+ lymphocytes of relapsing-remitting multiple sclerosis patients. Eur. J. Immunol. 2010, 40, 888–898. [Google Scholar] [CrossRef]
- Thamilarsan, M.; Koczan, D.; Hecker, M.; Brigitte, P.; Zettl, U. MicroRNAs in multiple sclerosis and experimental autoimmune encephalomyeilitis. Autimmune. Rev. 2012, 11, 174–179. [Google Scholar] [CrossRef]
- Aslani, S.; Jafari, N.; Reza, M.; Javan, R.; Karami, J.; Ahmadi, M.; Jafarnejad, M. Epigenetic modifications and therapy in multiple sclerosis. Neuromol. Med. 2017, 19, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Poliani, P.L.; Furlan, R.; Marconi, P.; Glorioso, J.C.; Adorini, L.; Comi, G. Cytokine therapy in immune-mediated demyelinating diseases of the central nervous system: a novel gene therapy approach. J. Neuroimmunol. 2000, 107, 184–190. [Google Scholar] [CrossRef]
- Navikas, V.; Link, H. Review: cytokines and the pathogenesis of multiple sclerosis. J. Neurosci. Res. 1996, 45, 322–333. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ray, S.K. Insights into abnormal sphingolipid metabolism in multiple sclerosis: targeting ceramide biosynthesis as a unique therapeutic strategy. Ther. Targets Neurol. Dis. 2017, 4, e1598. [Google Scholar] [CrossRef] [PubMed]
- Foster, V.; Macfarlane, E.B. Clear Thinking about Alternate Therapies, Staying Well; National Multiple Sclerosis Society: New York, NY, USA, 2011; pp. 1–22. Available online: www.nationalMSsociety.org.
- Kanno, T.; Nishimoto, T.; Fujita, Y.; Goto, A.; Nakano, T.; Nishizaki, T. Sphingosine induces apoptosis in MKN-28 human gastric cancer cells in an SDK-dependent manner. Cell Physiol. Biochem. 2012, 30, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Ullio, C.; Casas, J.; Brunk, U.T.; Sala, G.; Fabrias, G.; Ghidoni, R.; Bonelli, G.; Baccino, F.M.; Autelli, R. Sphingosine mediates TNFα-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J. Lipid Res. 2012, 53, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.C.; Martin, S.; Doyle, B.T.; Houghton, J.A. Sphingosine-induced apoptosis in rhabdomyosarcoma cell lines is dependent on pre-mitochondrial Bax activation and post-mitochondrial caspases. Cancer Res. 2007, 67, 756–764. [Google Scholar] [CrossRef]
- Stockman-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanism of action of fumonisin B1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef]
- Dasgupta, S.; Hogan, E.L. Chromatographic resolution and quantitative assay of CNS tissue sphingoids and sphingolipids. J. Lipid Res. 2001, 42, 301–308. [Google Scholar]
- Dasgupta, S.; Everhart, M.; Bhat, N.; Hogan, E.L. Neutral monoglycosylceramides in rat brain: occurrence, molecular expression and developmental variation. Dev. Neurosci. 1997, 19, 152–161. [Google Scholar] [CrossRef]
- Dasgupta, S.; Levery, S.B.; Hogan, E.L. 3-O-acetyl-sphingosine-series myelin glycolipids: characterization of novel 3-O-acetyl-sphingosine galactosylceramide. J. Lipid Res. 2002, 43, 751–761. [Google Scholar] [PubMed]
- Hoffmann, A.; Grimm, C.; Kraft, R.; Goldbaum, O.; Wrede, A.; Nolte, C.; Hanisch, U.K.; Richter-Landsberg, C.; Brück, W.; Kettenmann, H.; et al. TRPM3 is expressed in sphingosine-responsive myelinating oligodendrocytes. J. Neurochem. 2010, 114, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Bell, R.M. The sphingomyelin cycle and the second messenger function of ceramide. Science 1989, 243, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Kong, J.; Bieberich, E. Phytoceramide in vertebrate tissues: one step chromatography separation for molecular characterization of ceramide species. PLoS One 2013, 8, e80841. [Google Scholar] [CrossRef] [PubMed]
- Raymond, G.V. Leukodystrophy: Basic and Clinical. Adv. Neurobiol. 2017, 15, 365–382. [Google Scholar]
- Pavuluri, P.; Vadakedath, S.; Gundu, R.; Uppulety, S.; Kandi, V. Krabbe disease: Report of a rare lipid storage and neurodegenerative disorder. Cureus 2017, 9, e949. [Google Scholar] [CrossRef]
- Potter, G.B.; Petryniak, M.A. Neuroimmune mechanisms in Krabbe’s disease. J. Neurosci. Res. 2016, 94, 1341–1348. [Google Scholar] [CrossRef]
- Saffari, A.; Kölker, S.; Hoffmann, G.F.; Ebrahimi-Fakhari, D. Linking mitochondrial dysfunction to neurodegeneration in lysosomal storage diseases. J. Inherit. Metab. Dis. 2017, 5. [Google Scholar] [CrossRef]
- Lai, M.K.; Chew, W.S.; Torta, F.; Rao, A.; Harris, G.L.; Chun, J.; Herr, D.R. Biological Effects of Naturally Occurring Sphingolipids, Uncommon Variants, and Their Analogs. Neuromolecular Med. 2016, 18, 396–414. [Google Scholar] [CrossRef]
- Chakrabarti, S.S.; Bir, A.; Poddar, J.; Sinha, M.; Ganguly, A.; Chakrabarti, S. Ceramide and Sphingosine-1-Phosphate in Cell Death Pathways: Relevance to the Pathogenesis of Alzheimer Disease. Curr. Alzheimer. Res. 2016, 3, 1232–1248. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Coant, N.; Sakamoto, W.; Mao, C.; Hannun, Y.A. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv. Biol. Regul. 2017, 63, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Sandhoff, K. Neuronal sphingolipidoses: Membrane lipids and sphingolipid activator proteins regulate lysosomal sphingolipid catabolism. Biochimie 2016, 130, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Söderström, M. Clues to the immunopathogenesis of multiple sclerosis by investigating untreated patients during the very early stage of disease. Neurol. Sci. 2001, 22, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Furlan, R.; Brambilla, E.; Bergami, A.; Ruffini, F.; Gironi, M.; Poliani, P.L.; Grimaldi, L.M.; Comi, G. Cytokines and immunity in multiple sclerosis: The dual signal hypothesis. J. Neuroimmunol. 2000, 109, 3–9. [Google Scholar] [CrossRef]
- Deckx, N.; Lee, W.P.; Berneman, Z.N.; Cools, N. Neuroendocrine immunoregulation in multiple sclerosis. Clin. Dev. Immunol. 2013, 2013, 705232. [Google Scholar] [CrossRef] [PubMed]
- Genain, C.P.; Cannella, B.; Hauser, S.L.; Raine, C.S. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 1999, 5, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. Mechanisms of inflammation in MS tissue: Adhesion molecules and chemokines. J. Neuroimmunol. 1999, 98, 57–68. [Google Scholar] [CrossRef]
- Kieseier, B.C.; Storch, M.K.; Archelos, J.J.; Martino, G.; Hartung, H.P. Effector pathways in immune mediated central nervous system demyelination. Curr. Opin. Neurol. 1999, 12, 323–336. [Google Scholar] [CrossRef]
- Checa, A.; Khademi, M.; Sar, D.G.; Haeggstrom, J.Z.; Lundberg, J.O.; Piehl, F.; Olsson, T.; Wheelock, C.E. Hexosylceramides as intrathecal markers of worsening disability in multiple sclerosis. Mult. Scler. 2015, 21, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.; Bandaru, V.V.; Calabresi, P.A.; Nath, A.; Haughey, N.J. A defect of sphingolipid metabolism modifies properties of normal appearing whote matter in multiple sclerosis. Brain 2008, 131, 3092–3102. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, O.G.; Haines, J.D.; Katz, S.I.; Adula, K.P.; Huynh, J.L.; McGraw, C.A.; Zhang, F.; Varghese, M.; Sotirchos, E.; Bhargava, P.; et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impairs neuronal bioenergetics. Brain 2014, 137, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- Gakyung, L.; Hasan, H.; Kwon, O.-S.; Jung, B.H. Identification of altered metabolic pathways during disease progression in EAE mice via metabolics and lipidomics. Neuroscience 2019, 416, 74–87. [Google Scholar]
- Slezak-Prochazka, I.; Durmus, S.; Kroesen, B.J.; van den Berg, A. MicroRNAs, macrocontrol: regulation of miRNA processing. RNA 2010, 16, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, J.; Bakillah, A.; Iqbal, J. Regulation of sphingolipid metabolism by micro-RNAs: A potential approach to alleviate aetherosclerosis. Diseases 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Verrier, J.D.; Nielsen, J.A.; Johnson, K.R.; Notterpek, L.; Hudson, L.D. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J. Neurosci. 2008, 28, 11720–11730. [Google Scholar] [CrossRef] [PubMed]
- Geekiyanage, H.; Chan, C. Micro-RNA137/181c regulates serine palmitoyltransferase and in turn amyloid beta, novel targets in sporadic Alzheimer’s disease. J. Neurosci. 2011, 31, 14820–14830. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Medrano, J.; Yang, B.; Garza, N.T.; Segura-Ulate, I.; Perez, R.Z. Up-regulation of protective neuronal microRNAs by FTY720 and novel FTY720-derivatives. Neurosci. Lett. 2019, 690, 178–190. [Google Scholar] [CrossRef]
- Wang, C.; Ji, B.; Cheng, B.; Bai, B. Neuroprtection of microRNA in Neurological disorders (review). Biomed. Rep. 2014, 2, 611–619. [Google Scholar] [CrossRef]
- Quinlan, S.; Kenny, A.; Medina, M.; Engel, T.; Jimenez-Mateos, E.M. MicroRNAs in neurodegenerative diseases. Int. Rev. Cell Mol. Biol. 2017, 334, 309–343. [Google Scholar]
- Brenan, S.; Keon, M.; Liu, B.; Su, Z.; Saksena, M.K. Panoramic visualization of circulating microRNAs across neurodegenerative diseases in humans. Mol. Neurobiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chuturgoon, A.A.; Phulukdaree, A.; Moodley, D. Fumonisin B1 modulates expression of human cytochrome P450 1b1 in human hepatoma (Hepg2) cells by repressing mir-27b. Toxicol. Lett. 2014, 227, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.K.; Xu, X.M. MicroRNA in central nervous system trauma and degenerative disorders. Physiol. Genomics 2011, 43, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, S.S.; Rajanikant, G.K. miR-9 upregulation integrates post-ischemic neuronal survival and regeneration in vitro. Cell Mol. Neurobiol. 2019, 39, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Schratt, G.M.; Tuebing, F.; Nigh, E.A.; Kane, C.G.; Sabatini, M.E.; Kiebler, M.; Greenberg, M.E. A brain-specific micro RNA regulates dendritic spine development. Nature 2006, 439, 283–289. [Google Scholar] [CrossRef]
- Mix, E.; Meyer-Rienecker, H.; Hartung, H.P.; Zettl, U.K. Animal models of multiple sclerosis-potentials and limitations. Prog. Neurobiol. 2010, 92, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.; Bruck, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Comabella, M.; Sastre-Garriga, J.; Montalban, X. Precision medicine in multiple sclerosis: biomarkers for diagnosis, prognosis, and treatment response. Curr. Opin. Neurol. 2016, 29, 254–262. [Google Scholar] [CrossRef]
- Morris, G.; Reschke, C.R.; Henshall, D.C. Targeting microRNA-134 for seizure control and disease modification in epilepsy. EBioMedicine 2019, 45, 646–654. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, S.; Mayr, C.; Bartel, D.P.; Lodish, H.F. Mir-150, a microRNA expressed in mature B and T cells, blocks early B cell develpoment when expressed prematurely. Proc. Natl. Acad. Sci. USA 2007, 107, 7080–7085. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Kahn, D.; Gibson, W.S.; Round, J.L.; Scholz, A.L.R.l.; Cahudhuri, A.A.; Kahn, M.E.; Rao, D.S.; Baltimore, D. MicrRNA-155 promotes autoimmune inflammation by enhancing inflammatory T-cell development. Immunity 2010, 33, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.F.; Boldin, M.P.; Chaudhury, A.; Lin, L.L.; Taganov, K.D.; Hanada, T.; Yoshimura, A.; Baltimore, D.; Rudensky, A.Y. Function of miR-146a in controlling Treg cell-mediated regulation of Th 1 response. Cell 2010, 142, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Niimoto, T.; Nakasa, T.; Ishikawa, M.; Okuhara, A.; Izumi, B.; Deie, M.; Suzuki, O.; Adachi, N.; Ochi, M. Micro-RNA-146a expresses in interleukin-17 producing T-cells in rheumatoid arthritis patients. BMC Muscuoskelet Disord 2010, 11, 209. [Google Scholar]
- Octaegui, D.; Mostafavi, S.; Bernard, C.C.; Lopez de Munain, A.; Mousavi, P.; Oksenberg, J.R.; Baranzini, S.E. Increased transcriptional activity of milk-related genes following the active phase of experimental autoimmune encephalomyelitis and multiple sclerosis. J. Immunol. 2007, 179, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- Garo, L.P.; Murugaiyan, G. Contribution of microRNAs to autoimmune diseases. Cell Mol. Life Sci. 2016, 73, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, Y.; Cui, W.; Li, M.; Li, B.; Guo, L. MicroRNA-155 modulates Th1 and Th17 cell differentiation and is associated with multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014, 266, 56–63. [Google Scholar] [CrossRef]
- Du, C.; Liu, C.; Kang, J.; Zhao, G.; Ye, Z.; Huang, S.; Li, Z.; Wu, Z.; Pei, G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 2009, 10, 1252–1259. [Google Scholar] [CrossRef]
- Mycko, M.P.; Cichalewska, M.; Cwiklinska, H.; Selmaj, K.W. miR-155-3p Drives the development of autoimmune demyelination by regulation of heat shock protein 40. J. Neurosci. 2015, 35, 16504–16515. [Google Scholar] [CrossRef]
- Huang, X.; Schwind, S.; Yu, B.; Santhanam, R.; Wang, H.; Hoeller-bauer, P.; Mims, A.; Klisovic, R.; Walker, A.R.; Chan, K.K.; et al. Targeted delivery of microRNA-29b by transferrin-conjugated anionic lipopolyplex nanoparticles: a novel therapeutic strategy in acute myeloid leukemia. Clin. Cancer Res. 2013, 19, 2355–2367. [Google Scholar] [CrossRef]
- Babar, I.A.; Cheng, C.J.; Booth, C.J.; Liang, X.; Weidhass, J.B.; Saltzman, W.M.; Slack, F.J. Nano-particles based therapy in an in vivo microRNA-155 (miR-155) dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. USA 2009, 109, E1695–E1704. [Google Scholar] [CrossRef]
- Wang, H.; Moyano, A.L.; Ma, Z.; Deng, Y.; Lin, Y.; Zhao, C.; Zhang, L.; Jiang, M.; He, X.; Ma, Z.; et al. miR-219 Cooperates with miR-338 in myelination and promotes myelin repair in the CNS. Dev. Cell 2017, 40, 566–582. [Google Scholar] [CrossRef]
- Chun, J.; Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Cao, K.; Kato, S.; Komizu, Y.; Mizutani, N.; Tanaka, K.; Arima, C.; Tai, M.C.; Yanagisawa, K.; Togawa, N.; et al. Targeting ceramide synthase 6-dependent metastasis-prone phenotype in lung cancer cells. J. Clinic. Invest. 2016, 126, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Sajja, V.S.S.; Jablonska, A.; Haughey, N.; Bulte, J.W.M.; Stevens, R.D.; Long, J.B.; Walczak, P.; Janowski, M. Sphingolipids and micrRNA changes in blood following blast traumatic brain injury. J. Neurotrauma. 2018, 35, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Piket, E.; Zheleznyakova, G.Y.; Kular, L.; Jagodic, M. Small non-coding RNAsas important players, biomarkers and therapeutic targets in multiple sclerosis: A comprehensive overview. J. Autoimmun. 2019, 101, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, C.; De Riz, M.; Pietroboni, A.M.; Calvi, A.; Serpente, M.; Cioffi, S.M.; Arcaro, M.; Oldoni, E.; Scarpini, E.; Galimberti, D. Effect of fingolimod treatment on circulating miR-15b, miR23a and miR-223 levels in patients with multiple sclerosis. J. Neuroimmunol. 2016, 299, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Eftekharian, M.M.; Komaki, A.; Mazdeh, M.; Arsang-Jang, S.; Taheri, M.; Ghafouri-Fard, S. Expression profile of selected microRNAs in the peripheral blood of Multiple Sclerosis patients: a multivariate statistical analysis with ROC curve to find new biomarkers for fingolimod. J. Mol. Neursci. 2019, 68, 153–161. [Google Scholar] [CrossRef]
| Medications | Administration | Purpose | Therapeutic Approach |
|---|---|---|---|
| Methylprednisolon | Intravenous | Reduce inflammation | Lowest tolerance dose |
| Prednisolon | Oral | Managing the relapse | Lowest tolerance dose |
| ACTH | Injection | Acute exacerbation | 0.75 U/m2 twice daily for 2 weeks |
| Interferon beta | Injection | Modify the course Immunosuppression | 30–250 g alternate day |
| Glatiramer acetate | Injection | Modify the course Immunomodulator | 20–40 mg/day |
| Teriflunomide | Oral | Delay the progression | 7–14 mg once daily |
| Fingolimod | Oral | Delay the progression | 0.25 mg then 0.5 mg |
| Dimethyl fumerate | Oral | Delay the progression | 120 mg twice a day/1 week 240 mg twice a day |
| Alemtuzumab | IV infusion | Inhibit immune cell to cross BBB | 10 mg/mL once for 5 days |
| Natalizumab | Infusion | Inhibit immune cell to cross BBB | 20 mg/mL for 2 weeks |
| Mitoxantrone | Infusion | Inhibit immune cell to cross BBB | 140 mg/m2 |
| Ocrelizumab | Intravenous infusion | Block CD20 + ve lymphocytes | 300 mg 1st week 300 mg 2nd week 600 mg every 6 months |
| Hematopoietic stem cells | Transplantation surgery (chronic progressive stage) | De novo generation of naïve lymphocytes to reduce autoimmunity | Once |
| Autoimmune Disease | Alteration in Specific miRNA Expression | Outcomes of Targeting Specific miRNA | Reference |
|---|---|---|---|
| MS, EAE | Upregulation of miR-326 occurred in MS patients and EAE mice. | Ets-1 expression was down regulated by miR-326 in relapsing MS patients. Disease severity was reversed in EAE mice by expressing Ets1 with a mutated 3′ UTR. | [67] |
| MS, EAE | Overexpression of miR-155 highly correlated with disease severity in MS patients and EAE mice. | Knockdown of miR-155 resulted in low Th1 and Th17 cells and mild EAE. | [66] |
| EAE | Deletion of miR-338 enhances the miR-219 mutant hypomyelination phenotype in EAE. | miR-219 mimics cooperate with miR-338 for myelin repair in EAE. | [71] |
| MS | Circulating miR-15b, miR-23a and miR-223 levels were decreased in relapsing remitting MS patients. | Fingolimod (FTY720) treatment recovered levels of miRNAs and reduced the frequency of exacerbations in MS patients. | [73] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgupta, S.; Ray, S.K. Ceramide and Sphingosine Regulation of Myelinogenesis: Targeting Serine Palmitoyltransferase Using microRNA in Multiple Sclerosis. Int. J. Mol. Sci. 2019, 20, 5031. https://doi.org/10.3390/ijms20205031
Dasgupta S, Ray SK. Ceramide and Sphingosine Regulation of Myelinogenesis: Targeting Serine Palmitoyltransferase Using microRNA in Multiple Sclerosis. International Journal of Molecular Sciences. 2019; 20(20):5031. https://doi.org/10.3390/ijms20205031
Chicago/Turabian StyleDasgupta, Somsankar, and Swapan K. Ray. 2019. "Ceramide and Sphingosine Regulation of Myelinogenesis: Targeting Serine Palmitoyltransferase Using microRNA in Multiple Sclerosis" International Journal of Molecular Sciences 20, no. 20: 5031. https://doi.org/10.3390/ijms20205031
APA StyleDasgupta, S., & Ray, S. K. (2019). Ceramide and Sphingosine Regulation of Myelinogenesis: Targeting Serine Palmitoyltransferase Using microRNA in Multiple Sclerosis. International Journal of Molecular Sciences, 20(20), 5031. https://doi.org/10.3390/ijms20205031






