Chloroplasts— Beyond Energy Capture and Carbon Fixation: Tuning of Photosynthesis in Response to Chilling Stress
Abstract
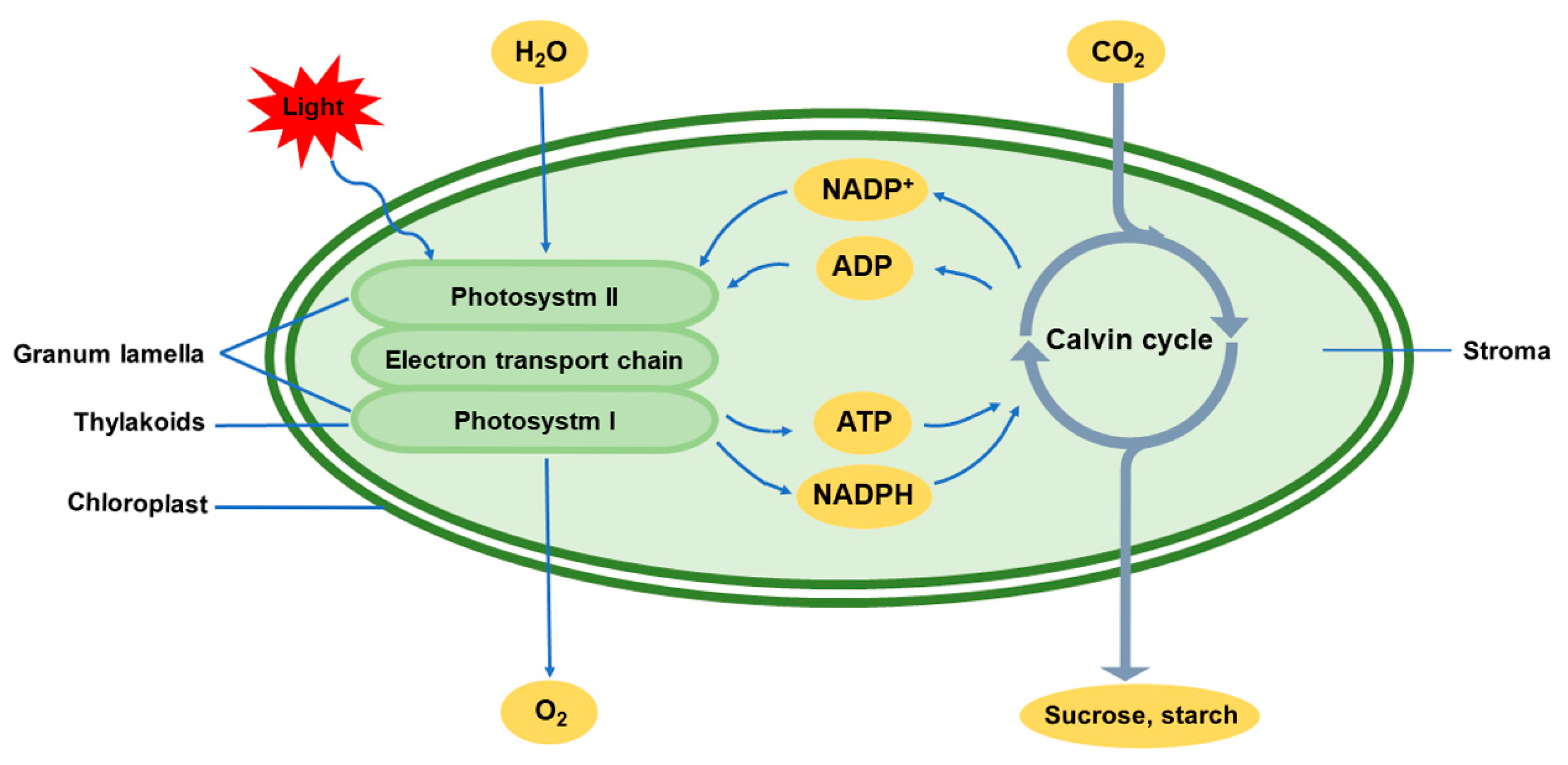
1. Introduction
2. Perception of Chloroplasts to Chilling Stress Signals
3. The Regulatory Response to Chilling Stress Signals in Chloroplasts
3.1. Regulation of the Lipid Membrane State of Chloroplasts
3.2. Regulation of the Photosynthesis-Related Protein Abundance
3.3. Regulation of Dark Reaction-Related Enzyme Activities
3.4. Regulation of the Redox State of Chloroplasts
 ), and singlet oxygen (1O2) are generated due to the highly oxidizing metabolic activity of these compounds and increased electron flow rate [51]. The ROS in plants are in a dynamic equilibrium under optimal conditions and cannot severely damage plants. However, under stressful conditions, with the reduction in the ability of plants to utilize light energy, excessive harvested light energy is converted to ROS, thereby affecting the physiological and biochemical functions of plants [52]. However, plants have developed many strategies to cope with the overaccumulation of ROS and maintain normal photosynthetic efficiency. These processes involve complicated redox reaction chains and ROS-scavenging systems.
), and singlet oxygen (1O2) are generated due to the highly oxidizing metabolic activity of these compounds and increased electron flow rate [51]. The ROS in plants are in a dynamic equilibrium under optimal conditions and cannot severely damage plants. However, under stressful conditions, with the reduction in the ability of plants to utilize light energy, excessive harvested light energy is converted to ROS, thereby affecting the physiological and biochemical functions of plants [52]. However, plants have developed many strategies to cope with the overaccumulation of ROS and maintain normal photosynthetic efficiency. These processes involve complicated redox reaction chains and ROS-scavenging systems.3.5. Regulation of Retrograde Signals in Chloroplasts
3.6. Regulation of Hormones in Chloroplasts
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| APX | Ascorbate peroxidase |
| ASA | Ascorbic acid |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| CAB | Chlorophyll a/b-binding protein |
| Car | Carotenoid |
| CAT | Catalase |
| CO2 | Carbon dioxide |
| Cytb6f | Cytochrome b6f protein complex |
| DEP | Differentially expressed protein |
| DGDG | Digalactosyldiacylglycerol |
| DHA | Dehydroascorbate |
| DHAR | Dehydroascorbate reductase |
| EX1 | EXECUTER1 |
| EX2 | EXECUTER2 |
| FA | Fatty acid |
| FAD | Fatty acid desaturase |
| FBPase | Fructose-1,6-bisphosphatase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| H2O2 | Hydrogen peroxide |
| ICS | Isochorismate synthase |
| IPL | Isochorismate pyruvate lyase |
| JA | Jasmonic acid |
| LHC | Light-harvesting complex |
| MAPK | Mitogen-activated protein kinase |
| MEP | Methylerythritol phosphate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NADP+ | Oxidized form of NADPH |
| MEcPP | Methylerythritol cyclodiphosphate |
| MGDG | Monogalactosyldiacylglycerol |
| 1O2 | Singlet oxygen |
| O2 | Oxygen |
| OEC | Oxygen-evolving complex |
| OEE | Oxygen-evolving enhancer |
 | Hydroxyl radical |
| O2− | Superoxide anion |
| PAP | 3-Phosphoadenosine 5-phosphate |
| PSI | Photosystem I |
| PSII | Photosystem II |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| RES | Reactive electrophile species |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| Rubisco | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| RuBP | Ribulose-1,5-bisphosphate |
| SA | Salicylic acid |
| SBPase | Sedoheptulose-1,7-bisphosphatase |
| SOD | Superoxide dismutase |
| USFA | Unsaturated fatty acid |
| VE | Vitamin E |
References
- Theocharis, A.; Clement, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Andaya, V.C.; Mackill, D.J. Mapping of QTLs associated with cold tolerance during the vegetative stage in rice. J. Exp. Bot. 2003, 54, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, Y.; Guo, H.; Zhou, L.; Yang, S.; Zhang, Z.; Yang, J.; Zhang, H.; Li, J.; Zeng, Y.; et al. Fine mapping of QTL qCTB10-2 that confers cold tolerance at the booting stage in rice. Theor. Appl. Genet. 2018, 131, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sthapit, B.R.; Witcombe, J.R. Inheritance of tolerance to chilling stress in rice during germination and plumule greening. Crop Sci. 1998, 38, 660–665. [Google Scholar] [CrossRef]
- Pego, J.V.; Kortstee, A.J.; Huijser, C.; Smeekens, S.C. Photosynthesis, sugars and the regulation of gene expression. J. Exp. Bot. 2000, 51, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Neukermans, J.; Queval, G.; Noctor, G.; Harbinson, J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012, 63, 1637–1661. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.O.; Yerramsetty, P.; Zielinski, A.M.; Mure, C.M. Photosynthetic gene expression in higher plants. Photosynth. Res. 2013, 117, 91–120. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Ambavaram, M.M.; Basu, S.; Krishnan, A.; Ramegowda, V.; Batlang, U.; Rahman, L.; Baisakh, N.; Pereira, A. Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat. Commun. 2014, 5, 5302. [Google Scholar] [CrossRef]
- Pfannschmidt, T.; Yang, C. The hidden function of photosynthesis: A sensing system for environmental conditions that regulates plant acclimation responses. Protoplasma 2012, 249, S125–S136. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Kim, C.; Landgraf, F.; Apel, K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 10270–10275. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of chilling on the structure, function and development of chloroplasts. Front. Plant Sci. 2018, 9, 1715. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.; Orvar, B.L.; Beyerly, J.; Hirt, H.; Dhindsa, R.S. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathway. Plant J. 2002, 31, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; Ollas, C.; Gomez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Jung, J.H.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.K.; Box, M.S.; Charoensawan, V.; Cortijo, S.; et al. Phytochromes function as thermosensors in Arabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanaka, H.; Konno, N.; Ogasawara, Y.; Hamashima, N.; Tamura, S.; Hasegawa, S.; Hayasaki, Y.; Okajima, K.; Kodama, Y. Phototropin perceives temperature based on the lifetime of its photoactivated state. Proc. Natl. Acad. Sci. USA 2017, 114, 9206–9211. [Google Scholar] [CrossRef]
- Kodama, Y.; Tsuboi, H.; Kagawa, T.; Wada, M. Low temperature-induced chloroplast relocation mediated by a blue light receptor, phototropin 2, in fern gametophytes. J. Plant Res. 2008, 121, 441–448. [Google Scholar] [CrossRef]
- Kutík, J.; Holá, D.; Kočová, M.; Rothová, O.; Haisel, D.; Wilhelmová, N.; Tichá, I. Ultrastructure and dimensions of chloroplasts in leaves of three maize (Zea mays L.) inbred lines and their F1 hybrids grown under moderate chilling stress. Photosynthetica 2004, 42, 447–455. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, B.; Yang, J.H.; Sui, N.; Yang, X.M.; Meng, Q.W. Overexpression of tomato chloroplast omega-3 fatty acid desaturase gene alleviates the photoinhibition of photosystems 2 and 1 under chilling stress. Photosynthetica 2008, 42, 185–192. [Google Scholar] [CrossRef]
- Duan, M.; Feng, H.L.; Wang, L.Y.; Li, D.; Meng, Q.W. Overexpression of thylakoidal ascorbate peroxidase shows enhanced resistance to chilling stress in tomato. J. Plant Physiol. 2012, 169, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Narise, T.; Sonoike, K.; Hashimoto, H.; Sato, N.; Kondo, M.; Nishimura, M.; Sato, M.; Toyooka, K.; Sugimoto, K.; et al. Role of galactolipid biosynthesis in coordinated development of photosynthetic complexes and thylakoid membranes during chloroplast biogenesis in Arabidopsis. Plant J. 2013, 73, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Skupien, J.; Wojtowicz, J.; Kowalewska, L.; Mazur, R.; Garstka, M.; Gieczewska, K.; Mostowska, A. Dark-chilling induces substantial structural changes and modifies galactolipid and carotenoid composition during chloroplast biogenesis in cucumber (Cucumis sativus L.) cotyledons. Plant Physiol. Biochem. 2017, 111, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Ishizaki-Nishizawa, O.; Higashi, S.; Hayashi, H.; Tasaka, Y.; Nishida, I. Genetically engineered alteration in the chilling sensitivity of plants. Nature 1992, 356, 710–713. [Google Scholar] [CrossRef]
- D’Angeli, S.; Altamura, M.M. Unsaturated lipids change in olive tree drupe and seed during fruit development and in response to cold-stress and acclimation. Int. J. Mol. Sci. 2016, 17, 1889. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, Y. Adaptive regulation of membrane lipids and fluidity during thermal acclimation in Tetrahymena. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 450–462. [Google Scholar] [CrossRef]
- Tovuu, A.; Zulfugarov, I.S.; Wu, G.; Kang, I.S.; Kim, C.; Moon, B.Y.; An, G.; Lee, C.H. Rice mutants deficient in omega-3 fatty acid desaturase (FAD8) fail to acclimate to cold temperatures. Plant Physiol. Biochem. 2016, 109, 525–535. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; McAvoy, R.; Peters, J.; Wu, H.; Li, Y. Enhanced cold tolerance in transgenic tobacco expressing a chloroplast omega-3 fatty acid desaturase gene under the control of a cold-inducible promoter. Planta 2006, 223, 1090–1100. [Google Scholar] [CrossRef]
- Popov, V.N.; Antipina, O.V.; Pchelkin, V.P.; Tsydendambaev, V.D. Changes in fatty acid composition of lipids in chloroplast membranes of tobacco plants during cold hardening. Russ. J. Plant Physiol. 2017, 64, 156–161. [Google Scholar] [CrossRef]
- Sakamoto, A.; Sulpice, R.; Hou, C.-X.; Kinoshita, M.; Higashi, S.-I.; Kanaseki, T.; Nonaka, H.; Moon, B.Y.; Murata, N. Genetic modification of the fatty acid unsaturation of phosphatidylglycerol in chloroplasts alters the sensitivity of tobacco plants to cold stress. Plant Cell Environ. 2003, 27, 99–105. [Google Scholar] [CrossRef]
- Cronan, J.E.; Roughan, P.G. Fatty acid specificity and selectivity of the chloroplast sn-glycerol 3-phosphate acyltransferase of the chilling sensitive plant, Amaranthus lividus. Plant Physiol. 1987, 83, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Guo, T.; Ren, M.; Wang, Z.; Tang, K.; Zhang, W.; Wang, M. Constitutive expression of chloroplast glycerol-3-phosphate acyltransferase from Ammopiptanthus mongolicus enhances unsaturation of chloroplast lipids and tolerance to chilling, freezing and oxidative stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2019, 143, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Cen, W.; Liu, J.; Lu, S.; Jia, P.; Yu, K.; Han, Y.; Li, R.; Luo, J. Comparative proteomic analysis of QTL CTS-12 derived from wild rice (Oryza rufipogon Griff.), in the regulation of cold acclimation and de-acclimation of rice (Oryza sativa L.) in response to severe chilling stress. BMC Plant Biol. 2018, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.M.; Jing, L.; Li, L.B.; Kuang, T.Y. Recent advances of studies on the structure and function of the light harvesting chlorophyll a/b protein complex. Chin. Bull. Bot. 2000, 17, 289–301. [Google Scholar]
- Liu, J.M.; Zhao, Q.; Yin, Z.P.; Xu, C.X.; Wang, Q.H.; Dai, S.J. Heat-responsive mechanisms in plants revealed by proteomic analysis: A review. J. Appl. Ecol. 2015, 26, 2561–2570. [Google Scholar]
- Neilson, K.A.; Mariani, M.; Haynes, P.A. Quantitative proteomic analysis of cold-responsive proteins in rice. Proteomics 2011, 11, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Samemath, G.F.; Ort, D.R.; Portis, A.R. Impaired reductive activation of stromal bisphosphatases in tomato leaves following low-temperature exposure at high light. Arch. Biochem. Biophys. 1990, 282, 302–308. [Google Scholar]
- Kingston-Smith, A.H.; Harbinson, J.; Williams, J.; Foyer, C.H. Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiol. 1997, 114, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Portis, A.R. Mechanism of light regulation of Rubisco: A specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proc. Natl. Acad. Sci. USA 1999, 96, 9438–9443. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.S.; Groom, Q.; Ort, D.R. Differential effects of chilling-induced photooxidation on the redox regulation of photosynthetic enzymes. Biochemistry 2000, 39, 6679–6688. [Google Scholar] [CrossRef] [PubMed]
- Streb, P.; Shang, W.; Feierabend, J.; Bligny, R. Divergent strategies of photoprotection in high-mountain plants. Planta 1998, 207, 313–324. [Google Scholar] [CrossRef]
- Byrd, G.T.; Ort, D.R.; Ogren, W. The Effects of chilling in the light on ribulose-1,5 bisphosphate carboxylase/oxygenase activation in tomato (Lycopersicon esculentum Mil.). Plant Physiol. 1995, 107, 585–591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guy, C.L.; Huber, J.L.A.; Huber, S.C. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol. 1992, 100, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Holaday, A.S.; Martindale, W.; Aired, R.; Brooks, A.L.; Leegood, R.C. Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol. 1992, 98, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Hurry, V.M.; Malmberg, G.; Cardestrom, P.; Oquist, G. Effects of a short-term shift to low temperature and of long-term cold hardening on photosynthesis and ribulose-l,5-bisphosphate carboxylase/oxygenase and sucrose phosphate synthase activity in leaves of winter rye (Secale cereale 1. Plant Physiol. 1994, 106, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Strand, A.; Hurry, V.; Gustafsson, P.; Gardestrom, P. Developmen of Arabidopsis thaliana leaves at low temperatures releases the suppression of photosynthesis and photosynthetic gene expression despite the accumulation of soluble carbohydrates. Plant J. 1997, 12, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Strand, A.; Hurry, V.; Henkes, S.; Huner, N.; Gustafsson, P.; Gardestrom, P.; Stitt, M. Acclimation of Arabidopsis leaves developing at low temperatures. increasing cytoplasmic volume accompanies increased activities of enzymes in the calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol. 1999, 119, 1387–1397. [Google Scholar] [CrossRef]
- Savitch, L.V.; Barker-Åstrom, J.; Ivanov, A.G.; Hurry, V.; Öquist, G.; Huner, N.P.; Gardeström, P. Cold acclimation of Arabidopsis thaliana results in incomplete recovery of photosynthetic capacity, associated with an increased reduction of the chloroplast stroma. Planta 2001, 214, 295–303. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idanheimo, N.; Hoeberichts, F.A.; Muhlenbock, P.; Brosche, M.; Van Breusegem, F.; Kangasjarvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [PubMed]
- Mittovaa, V.; Talb, M.; Volokitaa, M.; Guya, M. Salt stress induces up-regulation of an efficient chloroplast antioxidant system in the salt-tolerant wild tomato species Lycopersicon pennellii but not in the cultivated species. Physiol. Plant. 2002, 115, 393–400. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Zhong, B.; Liu, X.; Chan, Z. Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J. Integr. Plant Biol. 2014, 56, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.Y.; Yan, L.; Weng, B.Q. Review on physiology of chilling stress and chilling resistance of plants. Fujian J. Agri. Sci. 2002, 17, 190–195. [Google Scholar]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torresk, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jonesk, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Laloi, C.; Apel, K.; Danon, A. Reactive oxygen signalling: The latest news. Curr. Opin. Plant Biol. 2004, 7, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox. Signal 2006, 8, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox. Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Van Buer, J.; Cvetkovic, J.; Baier, M. Cold regulation of plastid ascorbate peroxidases serves as a priming hub controlling ROS signaling in Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, A.J.; Pawelzik, E. Apoplastic antioxidative system responses to ozone stress in strawberry leaves. J. Plant Physiol. 2008, 165, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; ElSayed, A.I.; Moore, M.; Dietz, K.J. Redox and reactive oxygen species network in acclimation for salinity tolerance in sugar beet. J. Exp. Bot. 2017, 68, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Kaniuga, Z.; Ząbek, J.; Michalski, W.P. Photosynthetic apparatus in chilling-sensitive plants: VI. cold and dark-induced changes in chloroplast superoxide dismutase activity in relation to loosely-bound manganese content. Planta 1979, 145, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Jeong, S.W.; Jeong, W.J.; Kwon, S.Y.; Chow, W.S.; Park, Y.I. Chloroplast Cu/Zn-superoxide dismutase is a highly sensitive site in cucumber leaves chilled in the light. Planta 2002, 216, 315–324. [Google Scholar] [CrossRef]
- Hu, W.H.; Song, X.S.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Changes in electron transport, superoxide dismutase and ascorbate peroxidase isoenzymes in chloroplasts and mitochondria of cucumber leaves as influenced by chilling. Photosynthetica 2008, 46, 581–588. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kwon, S.Y.; Bang, J.W.; Kwak, S.S. Photosynthetic efficiency in transgenic tobacco plants expressing both Cu/Zn SOD and APX in chloroplasts against oxidative stress caused by highlight and chilling. Korean J. Plant Biotechnol. 2003, 30, 399–403. [Google Scholar]
- Lim, S.; Kim, Y.H.; Kim, S.H.; Kwon, S.Y.; Lee, H.S.; Kim, J.S.; Cho, K.Y.; Paek, K.Y.; Kwak, S.S. Enhanced tolerance of transgenic sweet potato plants that express both Cu/Zn SOD and APX in chloroplasts to methyl viologen-mediated oxidative stress and chilling. Mol. Breeding 2007, 19, 227–239. [Google Scholar] [CrossRef]
- Shu, D.F.; Wang, L.Y.; Duan, M.; Deng, Y.S.; Meng, Q.W. Antisense-mediated depletion of tomato chloroplast glutathione reductase enhances susceptibility to chilling stress. Plant Physiol. Biochem. 2011, 49, 1228–1237. [Google Scholar] [CrossRef]
- Ding, S.; Lei, M.; Lu, Q.; Zhang, A.; Yin, Y.; Wen, X.; Zhang, L.; Lu, C. Enhanced sensitivity and characterization of photosystem II in transgenic tobacco plants with decreased chloroplast glutathione reductase under chilling stress. Biochim. Biophys. Acta. 2012, 1817, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Guo, Y.K.; Li, Q.; Zhang, J.; Wang, X.J.; Bai, J.G. The pretreatment of cucumber with methyl jasmonate regulates antioxidant enzyme activities and protects chloroplast and mitochondrial ultrastructure in chilling-stressed leaves. Sci. Hortic. 2012, 143, 135–143. [Google Scholar] [CrossRef]
- Wu, X.X.; Ding, H.D.; Chen, J.L.; Zhu, Z.W.; Zha, D.S. Exogenous spray application of 24-epibrassinolide induced changes in photosynthesis and anti-oxidant defences against chilling stress in eggplant (Solanum melongenaL.) seedlings. J. Hortic. Sci. Biotech. 2015, 90, 217–225. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Alayafi, A.A.M.; El Kelish, A.A.; Abu-Elsaoud, A.M. Acetylsalicylic acid enhance tolerance of Phaseolus vulgaris L. to chilling stress, improving photosynthesis, antioxidants and expression of cold stress responsive genes. Bot. Stud. 2018, 59, 6. [Google Scholar] [CrossRef] [PubMed]
- Fey, V.; Wagner, R.; Brautigam, K.; Pfannschmidt, T. Photosynthetic redox control of nuclear gene expression. J. Exp. Bot. 2005, 56, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.; Salamini, F.; Leister, D. A prediction of the size and evolutionary origin of the proteome of chloroplasts of Arabidopsis. Trends Plant Sci. 2000, 5, 141–142. [Google Scholar] [CrossRef]
- Wagner, D.; Przybyla, D.; Op den Camp, R.; Kim, C.; Landgraf, F.; Lee, K.P.; Wu¨rsch, M.; Laloi, C.; Nater, M.; Hideg, E.; et al. The genetic basis of singlet oxygen–induced stress responses of Arabidopsis thaliana. Science 2004, 306, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylides, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Davoine, C. Reactive electrophile species. Curr. Opin. Plant Biol. 2007, 10, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Xiaolong, W.; Qiang, Z. Research progress of abiotic stress responsive signal pathway in plant. Chin. J. Mol. Plant Breeding 2018, 16, 614–625. [Google Scholar]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Retrograde signaling between plastid and nucleus: A review. J. Plant Physiol. 2015, 181, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Bohmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Guye, M.; Wilson, J. The effects of chilling and chill-hardening temperatures on stomatal behaviour in a range of chill-sensitive species and cultivars. Plant Physiol. Bioch. 1987, 25, 717–722. [Google Scholar]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate regulates the inducer of CBF expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2011, 414, 562–571. [Google Scholar] [CrossRef]
- Dong, C.J.; Li, L.; Shang, Q.M.; Liu, X.Y.; Zhang, Z.G. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 2014, 240, 687–700. [Google Scholar] [CrossRef]
- Chen, M.; Thelen, J.J. ACYL-LIPID DESATURASE2 Is Required for Chilling and Freezing Tolerance in Arabidopsis. Plant Cell 2013, 25, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Musser, R.L.; Thomas, S.A.; Wise, R.R.; Peeler, T.C.; Naylor, A.W. Chloroplast ultrastructure, chlorophyll fluorescence, and pigment composition in chilling-stressed soybeans. Plant Physiol. 1984, 74, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Karim, S.; Alezzawi, M.; Garcia-Petit, C.; Solymosi, K.; Khan, N.Z.; Lindquist, E.; Dahl, P.; Hohmann, S.; Aronsson, H. A novel chloroplast localized Rab GTPase protein CPRabA5e is involved in stress, development, thylakoid biogenesis and vesicle transport in Arabidopsis. Plant Mol. Biol. 2013, 84, 675–692. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, P.; Liu, F.; Li, R.; Wang, S.; Luo, J. Chloroplasts— Beyond Energy Capture and Carbon Fixation: Tuning of Photosynthesis in Response to Chilling Stress. Int. J. Mol. Sci. 2019, 20, 5046. https://doi.org/10.3390/ijms20205046
Gan P, Liu F, Li R, Wang S, Luo J. Chloroplasts— Beyond Energy Capture and Carbon Fixation: Tuning of Photosynthesis in Response to Chilling Stress. International Journal of Molecular Sciences. 2019; 20(20):5046. https://doi.org/10.3390/ijms20205046
Chicago/Turabian StyleGan, Ping, Fang Liu, Rongbai Li, Shaokui Wang, and Jijing Luo. 2019. "Chloroplasts— Beyond Energy Capture and Carbon Fixation: Tuning of Photosynthesis in Response to Chilling Stress" International Journal of Molecular Sciences 20, no. 20: 5046. https://doi.org/10.3390/ijms20205046
APA StyleGan, P., Liu, F., Li, R., Wang, S., & Luo, J. (2019). Chloroplasts— Beyond Energy Capture and Carbon Fixation: Tuning of Photosynthesis in Response to Chilling Stress. International Journal of Molecular Sciences, 20(20), 5046. https://doi.org/10.3390/ijms20205046





