Current Smoking is Associated with Decreased Expression of miR-335-5p in Parenchymal Lung Fibroblasts
Abstract
1. Introduction
2. Results
2.1. Subject Characteristics
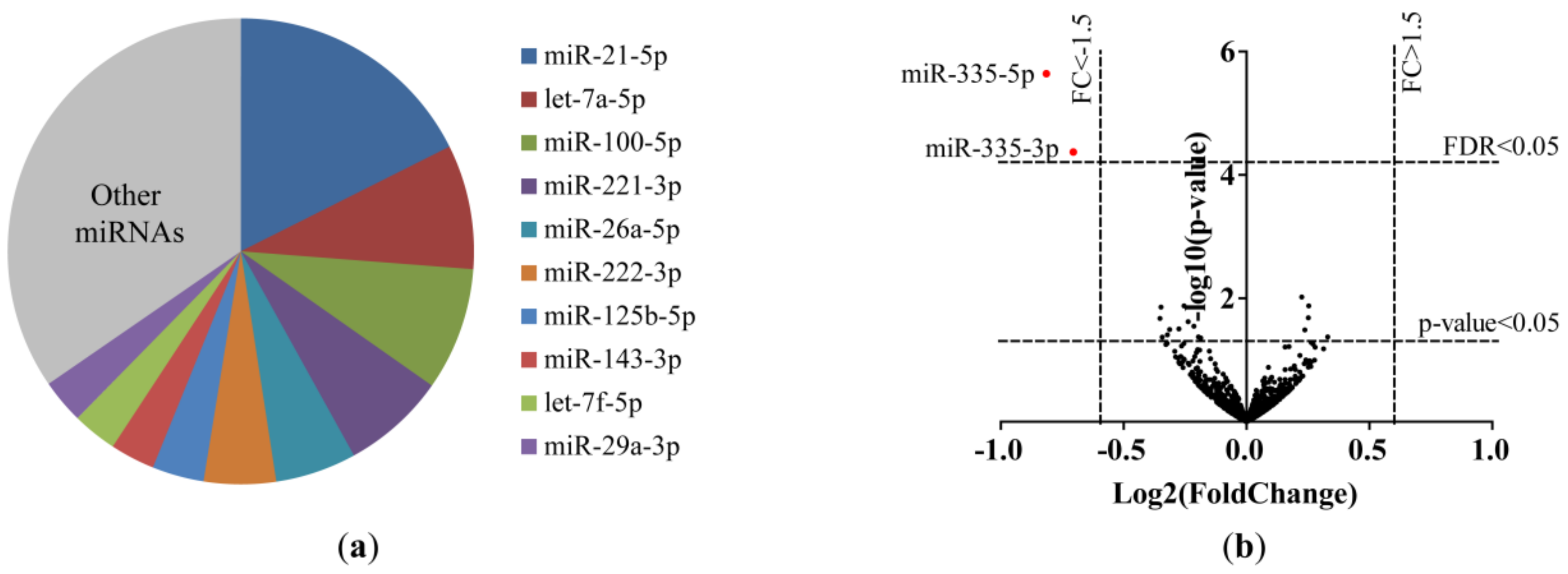
2.2. Differential miRNA Expression in Lung Fibroblasts of Current and Ex-Smokers
2.3. Validation of miR-335-5p Differential Expression in Lung Tissue and Bronchial Biopsies
2.4. No Regional Hypermethylation in miR-335 Host Gene in Lung Tissue of Current Smokers
2.5. Predicted and Experimentally Proven Targets of miR-335-5p in the MiRNA Targetome of Lung Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Isolation, Cell Culture, and CSE Treatment of Primary Lung Fibroblasts
4.3. RNA and DNA Isolation
4.4. Small RNA Sequencing
4.5. RT-qPCR
4.6. Bisulfite Treatment and Methylation-Specific qPCR
4.7. Identification of miR-335-5p Targets Relevant for Lung Fibroblasts
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| miRNA | microRNA |
| CSE | cigarette smoke extract |
| FEV1 | forced expiratory volume in one second |
| FVC | forced vital capacity |
| FC | fold change |
| FDR | false discovery rate |
| MSP | methylation specific qPCR |
| Ago2-IP | argonaute 2-immunoprecipitation |
References
- Goldkorn, T.; Filosto, S.; Chung, S. Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: Molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxid. Redox Signal. 2014, 21, 2149–2174. [Google Scholar] [CrossRef] [PubMed]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.; Beane, J.; Shah, V.; Liu, G.; Schembri, F.; Yang, X.; Palma, J.; Brody, J.S. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc. Natl. Acad. Sci. USA 2004, 101, 10143–10148. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.M.; Jansen, R.; Brooks, A.; Willemsen, G.; van Grootheest, G.; de Geus, E.; Smit, J.H.; Penninx, B.W.; Boomsma, D.I. Differential gene expression patterns between smokers and non-smokers: Cause or consequence? Addict. Biol. 2017, 22, 550–560. [Google Scholar] [CrossRef]
- Li, Z.; Rana, T.M. Therapeutic targeting of microRNAs: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638. [Google Scholar] [CrossRef]
- Schembri, F.; Sridhar, S.; Perdomo, C.; Gustafson, A.M.; Zhang, X.; Ergun, A.; Lu, J.; Liu, G.; Zhang, X.; Bowers, J.; et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 2319–2324. [Google Scholar] [CrossRef]
- Wang, G.; Wang, R.; Strulovici-Barel, Y.; Salit, J.; Staudt, M.R.; Ahmed, J.; Tilley, A.E.; Yee-Levin, J.; Hollmann, C.; Harvey, B.G.; et al. Persistence of smoking-induced dysregulation of miRNA expression in the small airway epithelium despite smoking cessation. PLoS ONE 2015, 10, e0120824. [Google Scholar] [CrossRef]
- Willinger, C.M.; Rong, J.; Tanriverdi, K.; Courchesne, P.L.; Huan, T.; Wasserman, G.A.; Lin, H.; Dupuis, J.; Joehanes, R.; Jones, M.R.; et al. MicroRNA Signature of Cigarette Smoking and Evidence for a Putative Causal Role of MicroRNAs in Smoking-Related Inflammation and Target Organ Damage. Circ. Cardiovasc. Genet. 2017, 10, e001678. [Google Scholar] [CrossRef]
- Van Pottelberge, G.R.; Mestdagh, P.; Bracke, K.R.; Thas, O.; van Durme, Y.M.; Joos, G.F.; Vandesompele, J.; Brusselle, G.G. MicroRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 183, 898–906. [Google Scholar] [CrossRef]
- Ambatipudi, S.; Cuenin, C.; Hernandez-Vargas, H.; Ghantous, A.; Le Calvez-Kelm, F.; Kaaks, R.; Barrdahl, M.; Boeing, H.; Aleksandrova, K.; Trichopoulou, A.; et al. Tobacco smoking-associated genome-wide DNA methylation changes in the EPIC study. Epigenomics 2016, 8, 599–618. [Google Scholar] [CrossRef]
- Rennard, S.I.; Togo, S.; Holz, O. Cigarette smoke inhibits alveolar repair: A mechanism for the development of emphysema. Proc. Am. Thorac. Soc. 2006, 3, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, S.; Nakamura, Y.; Mio, T.; Liu, X.; Takigawa, K.; Romberger, D.J.; Spurzem, J.R.; Rennard, S.I. Cigarette smoke extract inhibits fibroblast-mediated collagen gel contraction. Am. J. Physiol. 1998, 274 (Pt 1), L591–L598. [Google Scholar] [CrossRef]
- Miglino, N.; Roth, M.; Lardinois, D.; Sadowski, C.; Tamm, M.; Borger, P. Cigarette smoke inhibits lung fibroblast proliferation by translational mechanisms. Eur. Respir. J. 2012, 39, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Romberger, D.J.; Tate, L.; Ertl, R.F.; Kawamoto, M.; Adachi, Y.; Mio, T.; Sisson, J.H.; Spurzem, J.R.; Rennard, S.I. Cigarette smoke inhibits lung fibroblast proliferation and chemotaxis. Am. J. Respir. Crit. Care Med. 1995, 151, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Nyunoya, T.; Monick, M.M.; Klingelhutz, A.; Yarovinsky, T.O.; Cagley, J.R.; Hunninghake, G.W. Cigarette smoke induces cellular senescence. Am. J. Respir. Cell Mol. Biol. 2006, 35, 681–688. [Google Scholar] [CrossRef]
- Dohi, O.; Yasui, K.; Gen, Y.; Takada, H.; Endo, M.; Tsuji, K.; Konishi, C.; Yamada, N.; Mitsuyoshi, H.; Yagi, N.; et al. Epigenetic silencing of miR-335 and its host gene MEST in hepatocellular carcinoma. Int. J. Oncol. 2013, 42, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Timens, W.; Rajendran, V.; Algra, A.; Spira, A.; Lenburg, M.E.; Campbell, J.D.; van den Berge, M.; Postma, D.S.; van den Berg, A.; et al. Identification of transforming growth factor-beta-regulated microRNAs and the microRNA-targetomes in primary lung fibroblasts. PLoS ONE 2017, 12, e0183815. [Google Scholar] [CrossRef] [PubMed]
- Scarola, M.; Schoeftner, S.; Schneider, C.; Benetti, R. miR-335 directly targets Rb1 (pRb/p105) in a proximal connection to p53-dependent stress response. Cancer Res. 2010, 70, 6925–6933. [Google Scholar] [CrossRef]
- Shi, L.; Jiang, D.; Sun, G.; Wan, Y.; Zhang, S.; Zeng, Y.; Pan, T.; Wang, Z. miR-335 promotes cell proliferation by directly targeting Rb1 in meningiomas. J. Neuro-Oncol. 2012, 110, 155–162. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, R.; Kaul, Z.; Li, L.; Kato, Y.; Zhang, Z.; Groden, J.; Kaul, S.C.; Wadhwa, R. Loss-of-function screening to identify miRNAs involved in senescence: Tumor suppressor activity of miRNA-335 and its new target CARF. Sci. Rep. 2016, 6, 30185. [Google Scholar] [CrossRef]
- Yao, L.; Li, M.; Hu, J.; Wang, W.; Gao, M. MiRNA-335-5p negatively regulates granulosa cell proliferation via SGK3 in PCOS. Reproduction 2018. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tong, L.; Meng, H.; Zhu, W.; Guo, L.; Wei, T.; Zhang, J. MiR-335 regulates the chemo-radioresistance of small cell lung cancer cells by targeting PARP-1. Gene 2017, 600, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Heyn, H.; Engelmann, M.; Schreek, S.; Ahrens, P.; Lehmann, U.; Kreipe, H.; Schlegelberger, B.; Beger, C. MicroRNA miR-335 is crucial for the BRCA1 regulatory cascade in breast cancer development. Int. J. Cancer 2011, 129, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, W.; Fu, Q. miR-335 suppresses migration and invasion by targeting ROCK1 in osteosarcoma cells. Mol. Cell. Biochem. 2013, 384, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, M.; Zhang, R.; Wang, Y.; Zang, W.; Ma, Y.; Zhao, G.; Zhang, G. Effect of miR-335 upregulation on the apoptosis and invasion of lung cancer cell A549 and H1299. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2013, 34, 3101–3109. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, F.; Wang, Z.; Song, Y.; Luo, Y.; Zhang, X.; Jiang, L.; Sun, Z.; Miao, Z.; Xu, H. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene 2012, 31, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Zheng, X.; Wu, S.; Lu, H.; Leng, T.; Zhu, W.; Zhou, Y.; Ou, Y.; Lin, X.; Lin, Y.; et al. Targeting oncogenic miR-335 inhibits growth and invasion of malignant astrocytoma cells. Mol. Cancer 2011, 10, 59. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, H.; Yuan, L.; Liu, G.; Zhang, C.; Hong, M.; Liu, Y.; Zhou, M.; Chen, F.; Li, X. Overexpression of miR-335 confers cell proliferation and tumour growth to colorectal carcinoma cells. Mol. Cell. Biochem. 2016, 412, 235–245. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Jiang, A.; Li, R.; Yin, M.; Wang, Y. MicroRNA-335 suppresses the proliferation, migration, and invasion of breast cancer cells by targeting EphA4. Mol. Cell. Biochem. 2018, 439, 95–104. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Zhang, Z.; Lu, M.; Zhao, W.; Zeng, X.; Zhang, W. Long non-coding RNA TUG1 promotes migration and invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells. Cancer Sci. 2017, 108, 859–867. [Google Scholar] [CrossRef]
- Lynch, J.; Meehan, M.H.; Crean, J.; Copeland, J.; Stallings, R.L.; Bray, I.M. Metastasis suppressor microRNA-335 targets the formin family of actin nucleators. PLoS ONE 2013, 8, e78428. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhu, J.; Du, W.; Liu, S.; Zeng, Y.; Ding, Z.; Zhang, Y.; Wang, X.; Liu, Z.; Huang, J. CPNE1 is a target of miR-335-5p and plays an important role in the pathogenesis of non-small cell lung cancer. J. Exp. Clin. Cancer Res. CR 2018, 37, 131. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Fay, J.; Meehan, M.; Bryan, K.; Watters, K.M.; Murphy, D.M.; Stallings, R.L. MiRNA-335 suppresses neuroblastoma cell invasiveness by direct targeting of multiple genes from the non-canonical TGF-beta signalling pathway. Carcinogenesis 2012, 33, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Tome, M.; Lopez-Romero, P.; Albo, C.; Sepulveda, J.C.; Fernandez-Gutierrez, B.; Dopazo, A.; Bernad, A.; Gonzalez, M.A. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2011, 18, 985–995. [Google Scholar] [CrossRef]
- Tavazoie, S.F.; Alarcon, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.D.; Gerald, W.L.; Massague, J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008, 451, 147–152. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, K.; Jin, U.H.; Pfent, C.; Cao, H.; Amendt, B.; Liu, X.; Wilson-Robles, H.; Safe, S. Aryl hydrocarbon receptor agonists induce microRNA-335 expression and inhibit lung metastasis of estrogen receptor negative breast cancer cells. Mol. Cancer Ther. 2012, 11, 108–118. [Google Scholar] [CrossRef]
- Luo, L.; Xia, L.; Zha, B.; Zuo, C.; Deng, D.; Chen, M.; Hu, L.; He, Y.; Dai, F.; Wu, J.; et al. miR-335-5p targeting ICAM-1 inhibits invasion and metastasis of thyroid cancer cells. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 983–990. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Z.; Liu, Z.; Qiu, B.; Liu, K.; Dong, G. MicroRNA-335 inhibits invasion and metastasis of colorectal cancer by targeting ZEB2. Med Oncol. 2014, 31, 982. [Google Scholar] [CrossRef]
- Sandoval-Borquez, A.; Polakovicova, I.; Carrasco-Veliz, N.; Lobos-Gonzalez, L.; Riquelme, I.; Carrasco-Avino, G.; Bizama, C.; Norero, E.; Owen, G.I.; Roa, J.C.; et al. MicroRNA-335-5p is a potential suppressor of metastasis and invasion in gastric cancer. Clin. Epigenet. 2017, 9, 114. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, F.; Wu, J.Y.; Li, H.Y.; Fan, J.J.; Mai, L.; Zhang, J.; Ma, D.M.; Li, Y.; Song, F.Z. MiR-335 inhibits migration of breast cancer cells through targeting oncoprotein c-Met. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 2875–2883. [Google Scholar] [CrossRef]
- Alfano, D.; Gorrasi, A.; Li Santi, A.; Ricci, P.; Montuori, N.; Selleri, C.; Ragno, P. Urokinase receptor and CXCR4 are regulated by common microRNAs in leukaemia cells. J. Cell. Mol. Med. 2015, 19, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wang, P.; Hong, Q.; Liao, Q.; Yan, L.; Xu, W.; Chen, X.; Zheng, Q.; Zhang, L.; Huang, D. MicroRNA-335-5p Plays Dual Roles in Periapical Lesions by Complex Regulation Pathways. J. Endod. 2017, 43, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, H.; Lu, S. MiR-335-5p restores cisplatin sensitivity in ovarian cancer cells through targeting BCL2L2. Cancer Med. 2018, 7, 4598–4609. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, X.; Zhan, Y.; Jiang, W.; Liu, X.; Wang, X.; Wu, B. miR-335 inhibits the proliferation and invasion of clear cell renal cell carcinoma cells through direct suppression of BCL-W. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 6875–6882. [Google Scholar] [CrossRef]
- An, Y.; Cai, H.; Zhang, Y.; Liu, S.; Duan, Y.; Sun, D.; Chen, X.; He, X. circZMYM2 Competed Endogenously with miR-335-5p to Regulate JMJD2C in Pancreatic Cancer. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 2224–2236. [Google Scholar] [CrossRef]
- Meng, Y.; Zou, Q.; Liu, T.; Cai, X.; Huang, Y.; Pan, J. microRNA-335 inhibits proliferation, cell-cycle progression, colony formation, and invasion via targeting PAX6 in breast cancer cells. Mol. Med. Rep. 2015, 11, 379–385. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, F.; Fu, Y. microRNA-335 inhibits colorectal cancer HCT116 cells growth and epithelial-mesenchymal transition (EMT) process by targeting Twist1. Die Pharm. 2017, 72, 475–481. [Google Scholar]
- Yang, B.; Huang, J.; Liu, H.; Guo, W.; Li, G. miR-335 directly, while miR-34a indirectly modulate survivin expression and regulate growth, apoptosis, and invasion of gastric cancer cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 1771–1779. [Google Scholar] [CrossRef]
- Gong, M.; Ma, J.; Guillemette, R.; Zhou, M.; Yang, Y.; Yang, Y.; Hock, J.M.; Yu, X. miR-335 inhibits small cell lung cancer bone metastases via IGF-IR and RANKL pathways. Mol. Cancer Res. MCR 2014, 12, 101–110. [Google Scholar] [CrossRef]
- Gao, L.; Yang, Y.; Xu, H.; Liu, R.; Li, D.; Hong, H.; Qin, M.; Wang, Y. MiR-335 functions as a tumor suppressor in pancreatic cancer by targeting OCT4. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 8309–8318. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, C.; Guan, H.; Liu, J.; Cui, Y. LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335-5p. J. Cell. Physiol. 2019, 234, 7266–7278. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Lei, Y.; Hu, B.; Yang, J.; Fang, S.; Wang, Q.; Li, M.; Guo, L. WW domain binding protein 5 induces multidrug resistance of small cell lung cancer under the regulation of miR-335 through the Hippo pathway. Br. J. Cancer 2016, 115, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.T.; Nakamura, K.; Davies, R.; Nahas, S.A.; Brown, C.; Tunuguntla, R.; Gatti, R.A.; Hu, H. ATM-dependent MiR-335 targets CtIP and modulates the DNA damage response. PLoS Genet. 2013, 9, e1003505. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bian, T.; Feng, J.; Qian, L.; Zhang, J.; Jiang, D.; Zhang, Q.; Li, X.; Liu, Y.; Shi, J. miR-335 inhibited cell proliferation of lung cancer cells by target Tra2beta. Cancer Sci. 2018, 109, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Sun, R.; Luo, D.H.; Sun, J.; Zhang, M.Y.; Wang, M.H.; Yang, Y.; Wang, H.Y.; Mai, S.J. Upregulated TRIM29 promotes proliferation and metastasis of nasopharyngeal carcinoma via PTEN/AKT/mTOR signal pathway. Oncotarget 2016, 7, 13634–13650. [Google Scholar] [CrossRef]
- Ong, J.; Woldhuis, R.R.; Boudewijn, I.M.; van den Berg, A.; Kluiver, J.; Kok, K.; Terpstra, M.M.; Guryev, V.; de Vries, M.; Vermeulen, C.J.; et al. Age-related gene and miRNA expression changes in airways of healthy individuals. Sci. Rep. 2019, 9, 3765. [Google Scholar] [CrossRef]
- Herwig, S.; Strauss, M. The retinoblastoma protein: A master regulator of cell cycle, differentiation and apoptosis. Eur. J. Biochem. 1997, 246, 581–601. [Google Scholar] [CrossRef]
- Du, X.; Qi, F.; Lu, S.; Li, Y.; Han, W. Nicotine upregulates FGFR3 and RB1 expression and promotes non-small cell lung cancer cell proliferation and epithelial-to-mesenchymal transition via downregulation of miR-99b and miR-192. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 101, 656–662. [Google Scholar] [CrossRef]
- Tao, X.; West, A.E.; Chen, W.G.; Corfas, G.; Greenberg, M.E. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron 2002, 33, 383–395. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Martin, R.J. Brain-derived neurotrophic factor in the airways. Pharmacol. Ther. 2014, 143, 74–86. [Google Scholar] [CrossRef]
- Rojas, F.; Hernandez, M.E.; Silva, M.; Li, L.; Subramanian, S.; Wilson, M.J.; Liu, P. The Oncogenic Response to MiR-335 Is Associated with Cell Surface Expression of Membrane-Type 1 Matrix Metalloproteinase (MT1-MMP) Activity. PLoS ONE 2015, 10, e0132026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.K.; Li, Y.S.; Zhang, C.D.; Dai, D.Q. Up-regulation of CRKL by microRNA-335 methylation is associated with poor prognosis in gastric cancer. Cancer Cell Int. 2017, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Serra, P.; Esteller, M. DNA methylation-associated silencing of tumor-suppressor microRNAs in cancer. Oncogene 2012, 31, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Yao, H.; Rahman, I. Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid. Redox Signal. 2013, 18, 1956–1971. [Google Scholar] [CrossRef]
- Noordhoek, J.A.; Postma, D.S.; Chong, L.L.; Menkema, L.; Kauffman, H.F.; Timens, W.; van Straaten, J.F.; van der Geld, Y.M. Different modulation of decorin production by lung fibroblasts from patients with mild and severe emphysema. J. Chronic Obstr. Pulm. Dis. 2005, 2, 17–25. [Google Scholar] [CrossRef]
- Noordhoek, J.A.; Postma, D.S.; Chong, L.L.; Vos, J.T.; Kauffman, H.F.; Timens, W.; van Straaten, J.F. Different proliferative capacity of lung fibroblasts obtained from control subjects and patients with emphysema. Exp. Lung Res. 2003, 29, 291–302. [Google Scholar] [CrossRef]
- Hoonhorst, S.J.; ten Hacken, N.H.; Lo Tam Loi, A.T.; Koenderman, L.; Lammers, J.W.; Telenga, E.D.; Boezen, H.M.; van den Berge, M.; Postma, D.S. Lower corticosteroid skin blanching response is associated with severe COPD. PLoS ONE 2014, 9, e91788. [Google Scholar] [CrossRef]
- Brandsma, C.A.; Timens, W.; Jonker, M.R.; Rutgers, B.; Noordhoek, J.A.; Postma, D.S. Differential effects of fluticasone on extracellular matrix production by airway and parenchymal fibroblasts in severe COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L582–L589. [Google Scholar] [CrossRef]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Kluiver, J.; Slezak-Prochazka, I.; van den Berg, A. Studying microRNAs in lymphoma. Methods Mol. Biol. 2013, 971, 265–276. [Google Scholar]
- Melchers, L.J.; Clausen, M.J.; Mastik, M.F.; Slagter-Menkema, L.; van der Wal, J.E.; Wisman, G.B.; Roodenburg, J.L.; Schuuring, E. Identification of methylation markers for the prediction of nodal metastasis in oral and oropharyngeal squamous cell carcinoma. Epigenetics 2015, 10, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Lung Fibroblast Donors | Lung Tissue Donors | Bronchial Biopsy Donors | ||||
|---|---|---|---|---|---|---|---|
| Ex-Smokers | Current Smokers | Never-Smokers | Ex-Smokers | Current Smokers | Never-Smokers | Current Smokers | |
| N | 9 | 6 | 14 | 33 | 20 | 42 | 40 |
| Male/Female, n | 6/3 | 1/5 | 6/8 | 21/12 | 7/13 | 23/19 | 23/17 |
| Age, years 1 | 65.0 (55.0–68.0) | 56.5 (48.5–69.0) | 56.0 (48.8–73.8) | 65.0 (54.0–71.5) | 61.0 (51.3–67.8) | 38.1 (21.6–57.8) | 43.0 (23.4–52.4) |
| Pack-years, n 1 | 31.5 (17.9–43.1) (n = 6) | 36.5 (27.8–52.0) (n = 6) | NA | 33.5 (20.0–46.3) (n = 26) | 34.0 (20.3–50.8) (n = 16) | NA | 15.9 (3.9–30.3) (n = 40) |
| FEV1, % pred 1,2 | 96.9 (86.8–97.7) | 92.4 3 | 102.0 (91.2–116.5) | 90.9 (84.2–104.3) | 94.2 (86.1–107.7) | 101.2 (92.0–108.6) | 97.7 (93.3–107.3) |
| FEV1/FVC, % 1,4 | 76.0 (71.4–79.9) | 73.8 (73.1–79.2) | 78.0 (72.8–83.0) | 73.3 (70.0–78.9) | 75.7 (72.6–79.2) | 79.5 (75.0–85.4) | 78.0 (73.9–83.0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, J.; van den Berg, A.; Faiz, A.; Boudewijn, I.M.; Timens, W.; Vermeulen, C.J.; Oliver, B.G.; Kok, K.; Terpstra, M.M.; van den Berge, M.; et al. Current Smoking is Associated with Decreased Expression of miR-335-5p in Parenchymal Lung Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5176. https://doi.org/10.3390/ijms20205176
Ong J, van den Berg A, Faiz A, Boudewijn IM, Timens W, Vermeulen CJ, Oliver BG, Kok K, Terpstra MM, van den Berge M, et al. Current Smoking is Associated with Decreased Expression of miR-335-5p in Parenchymal Lung Fibroblasts. International Journal of Molecular Sciences. 2019; 20(20):5176. https://doi.org/10.3390/ijms20205176
Chicago/Turabian StyleOng, Jennie, Anke van den Berg, Alen Faiz, Ilse M Boudewijn, Wim Timens, Cornelis J Vermeulen, Brian G Oliver, Klaas Kok, Martijn M Terpstra, Maarten van den Berge, and et al. 2019. "Current Smoking is Associated with Decreased Expression of miR-335-5p in Parenchymal Lung Fibroblasts" International Journal of Molecular Sciences 20, no. 20: 5176. https://doi.org/10.3390/ijms20205176
APA StyleOng, J., van den Berg, A., Faiz, A., Boudewijn, I. M., Timens, W., Vermeulen, C. J., Oliver, B. G., Kok, K., Terpstra, M. M., van den Berge, M., Brandsma, C.-A., & Kluiver, J. (2019). Current Smoking is Associated with Decreased Expression of miR-335-5p in Parenchymal Lung Fibroblasts. International Journal of Molecular Sciences, 20(20), 5176. https://doi.org/10.3390/ijms20205176









