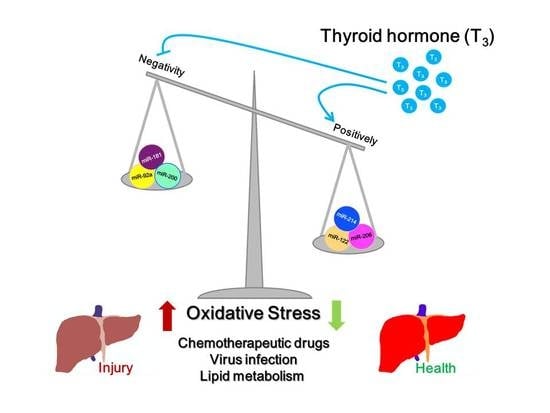
Roles of Thyroid Hormone-Associated microRNAs Affecting Oxidative Stress in Human Hepatocellular Carcinoma
Abstract
1. Introduction
2. Effect of Thyroid Hormone on the Role of Oxidative Stress-Related microRNAs in Liver
2.1. Oxidative Stress Promotes HCC Progression
2.2. Roles of microRNAs Correlated with Oxidative Stress in HCC
2.3. Role of Thyroid Hormone and Its Receptor in HCC
2.4. Thyroid Hormone Induces an Anti-Oxidative Stress Effect in Hepatocytes Mediated by microRNAs
2.5. Thyroid Hormone Promotes Oxidative Stress in Hepatocytes by Regulating microRNAs
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bishayee, A. The role of inflammation and liver cancer. Adv. Exp. Med. Biol. 2014, 816, 401–435. [Google Scholar] [PubMed]
- Balogh, J.; Victor, D., 3rd; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Research. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Krashin, E.; Piekielko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Curro, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Kim, B.Y.; Cho, M.H.; Kim, K.J.; Cho, K.J.; Kim, S.W.; Kim, H.S.; Jung, W.W.; Lee, B.H.; Lee, B.H.; Lee, S.G. Effects of PRELI in Oxidative-Stressed HepG2 Cells. Ann. Clin. Lab. Sci. 2015, 45, 419–425. [Google Scholar]
- Huang, F.Y.; Wong, D.K.; Tsui, V.W.; Seto, W.K.; Mak, L.Y.; Cheung, T.T.; Lai, K.K.; Yuen, M.F. Targeted genomic profiling identifies frequent deleterious mutations in FAT4 and TP53 genes in HBV-associated hepatocellular carcinoma. BMC Cancer 2019, 19, 789. [Google Scholar] [CrossRef]
- Feld, J. Update on the Risk of Primary and Recurrent HCC With the Use of DAA Therapy for HCV Infection. Gastroenterol. Hepatol. 2019, 15, 303–306. [Google Scholar]
- Vandenbulcke, H.; Moreno, C.; Colle, I.; Knebel, J.F.; Francque, S.; Serste, T.; George, C.; de Galocsy, C.; Laleman, W.; Delwaide, J.; et al. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: A prospective study. J. Hepatol. 2016, 65, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Ahmed, F.; Mara, K.C.; Addissie, B.D.; Allen, A.M.; Gores, G.J.; Roberts, L.R. Diabetes is Associated with Increased Risk of Hepatocellular Carcinoma in Cirrhosis Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2019. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbeck’s Arch. Surg. 2006, 391, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wu, S.M.; Lin, Y.H.; Chi, H.C.; Lin, S.L.; Yeh, C.T.; Chuang, W.Y.; Lin, K.H. Induction of nuclear protein-1 by thyroid hormone enhances platelet-derived growth factor A mediated angiogenesis in liver cancer. Theranostics 2019, 9, 2361–2379. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chung, F.L. Oxidative stress and hepatocarcinogenesis. Hepatoma Res. 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Ma-On, C.; Sanpavat, A.; Whongsiri, P.; Suwannasin, S.; Hirankarn, N.; Tangkijvanich, P.; Boonla, C. Oxidative stress indicated by elevated expression of Nrf2 and 8-OHdG promotes hepatocellular carcinoma progression. Med. Oncol. 2017, 34, 57. [Google Scholar] [CrossRef]
- Bisht, S.; Dada, R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. 2017, 9, 420–447. [Google Scholar]
- Veskoukis, A.S.; Tsatsakis, A.M.; Kouretas, D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones 2012, 17, 11–21. [Google Scholar] [CrossRef]
- Fu, N.; Yao, H.; Nan, Y.; Qiao, L. Role of Oxidative Stress in Hepatitis C Virus Induced Hepatocellular Carcinoma. Curr. Cancer Drug Targets 2017, 17, 498–504. [Google Scholar] [CrossRef]
- Marra, M.; Sordelli, I.M.; Lombardi, A.; Lamberti, M.; Tarantino, L.; Giudice, A.; Stiuso, P.; Abbruzzese, A.; Sperlongano, R.; Accardo, M.; et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: An overview. J. Transl. Med. 2011, 9, 171. [Google Scholar] [CrossRef]
- Simic, M.G.; Bergtold, D.S.; Karam, L.R. Generation of oxy radicals in biosystems. Mutat. Res. 1989, 214, 3–12. [Google Scholar] [CrossRef]
- Fujita, N.; Sugimoto, R.; Ma, N.; Tanaka, H.; Iwasa, M.; Kobayashi, Y.; Kawanishi, S.; Watanabe, S.; Kaito, M.; Takei, Y. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J. Viral Hepat. 2008, 15, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Nishina, S.; Sasaki, K.; Hara, Y. Mitochondrial damage and iron metabolic dysregulation in hepatitis C virus infection. Free Radic. Biol. Med. 2019, 133, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nature reviews. Endocrinology 2018, 14, 259–269. [Google Scholar]
- Chi, H.C.; Chen, S.L.; Lin, S.L.; Tsai, C.Y.; Chuang, W.Y.; Lin, Y.H.; Huang, Y.H.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover. Oncogene 2017, 36, 5274–5284. [Google Scholar] [CrossRef]
- Spahis, S.; Delvin, E.; Borys, J.M.; Levy, E. Oxidative Stress as a Critical Factor in Nonalcoholic Fatty Liver Disease Pathogenesis. Antioxid. Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef]
- Cardin, R.; Piciocchi, M.; Bortolami, M.; Kotsafti, A.; Barzon, L.; Lavezzo, E.; Sinigaglia, A.; Rodriguez-Castro, K.I.; Rugge, M.; Farinati, F. Oxidative damage in the progression of chronic liver disease to hepatocellular carcinoma: An intricate pathway. World J. Gastroenterol. 2014, 20, 3078–3086. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Wan, Y.; Cui, R.; Gu, J.; Zhang, X.; Xiang, X.; Liu, C.; Qu, K.; Lin, T. Identification of Four Oxidative Stress-Responsive MicroRNAs, miR-34a-5p, miR-1915-3p, miR-638, and miR-150-3p, in Hepatocellular Carcinoma. Oxidative Med. Cell. Longev. 2017, 2017, 5189138. [Google Scholar] [CrossRef]
- Tian, Y.; Kuo, C.F.; Sir, D.; Wang, L.; Govindarajan, S.; Petrovic, L.M.; Ou, J.H. Autophagy inhibits oxidative stress and tumor suppressors to exert its dual effect on hepatocarcinogenesis. Cell Death Differ. 2015, 22, 1025–1034. [Google Scholar] [CrossRef]
- Cardin, R.; Piciocchi, M.; Sinigaglia, A.; Lavezzo, E.; Bortolami, M.; Kotsafti, A.; Cillo, U.; Zanus, G.; Mescoli, C.; Rugge, M.; et al. Oxidative DNA damage correlates with cell immortalization and mir-92 expression in hepatocellular carcinoma. BMC Cancer 2012, 12, 177. [Google Scholar] [CrossRef]
- Dai, B.H.; Geng, L.; Wang, Y.; Sui, C.J.; Xie, F.; Shen, R.X.; Shen, W.F.; Yang, J.M. microRNA-199a-5p protects hepatocytes from bile acid-induced sustained endoplasmic reticulum stress. Cell Death Dis. 2013, 4, e604. [Google Scholar] [CrossRef] [PubMed]
- Sendi, H. Dual Role of miR-122 in Molecular Pathogenesis of Viral Hepatitis. Hepat. Mon. 2012, 12, 312–314. [Google Scholar] [CrossRef]
- Wojcik, K.; Piekarska, A.; Szymanska, B.; Jablonowska, E. Hepatic expression of miR-122 and antioxidant genes in patients with chronic hepatitis B. Acta Biochim. Pol. 2016, 63, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Darwish, H.A.; Eldeib, K.M.; Abdel Azim, S.A. miR-26a Potentially Contributes to the Regulation of Fatty Acid and Sterol Metabolism In Vitro Human HepG2 Cell Model of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 8515343. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, N.; Wang, Z.; Ai, D.M.; Cao, Z.Y.; Pan, H.P. Decreased MiR-155 Level in the Peripheral Blood of Non-Alcoholic Fatty Liver Disease Patients may Serve as a Biomarker and may Influence LXR Activity. Cell. Physiol. Biochem. 2016, 39, 2239–2248. [Google Scholar] [CrossRef]
- Huang, P.S.; Lin, Y.H.; Chi, H.C.; Chen, P.Y.; Huang, Y.H.; Yeh, C.T.; Wang, C.S.; Lin, K.H. Thyroid hormone inhibits growth of hepatoma cells through induction of miR-214. Sci. Rep. 2017, 7, 14868. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Chen, Y.; Yin, C.; Chen, J.J.; Liu, S. miR-214 protects erythroid cells against oxidative stress by targeting ATF4 and EZH2. Free Radic. Biol. Med. 2016, 92, 39–49. [Google Scholar] [CrossRef]
- Xiao, Y.; Yan, W.; Lu, L.; Wang, Y.; Lu, W.; Cao, Y.; Cai, W. p38/p53/miR-200a-3p feedback loop promotes oxidative stress-mediated liver cell death. Cell Cycle 2015, 14, 1548–1558. [Google Scholar] [CrossRef]
- Zhou, B.; Li, C.; Qi, W.; Zhang, Y.; Zhang, F.; Wu, J.X.; Hu, Y.N.; Wu, D.M.; Liu, Y.; Yan, T.T.; et al. Downregulation of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic insulin sensitivity. Diabetologia 2012, 55, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Wang, H.B.; Yong, J.K.; Zhong, J.; Li, Q.H. MiR-128-3p overexpression sensitizes hepatocellular carcinoma cells to sorafenib induced apoptosis through regulating DJ-1. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 6667–6677. [Google Scholar] [PubMed]
- Zhao, X.; Jin, Y.; Li, L.; Xu, L.; Tang, Z.; Qi, Y.; Yin, L.; Peng, J. MicroRNA-128-3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting Sirtuin-1. Pharmacol. Res. 2019, 146, 104276. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, C.L.; Fannin, E.E.; Toth, C.L.; Pearson, D.S.; Vickers, K.C.; Sethupathy, P. Inhibition of miR-29 has a significant lipid-lowering benefit through suppression of lipogenic programs in liver. Sci. Rep. 2015, 5, 12911. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qiu, B.; Li, X.; Zhang, H.; Liu, W. p53-p66(shc)/miR-21-Sod2 signaling is critical for the inhibitory effect of betulinic acid on hepatocellular carcinoma. Toxicol. Lett. 2015, 238, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Tian, Q.; Zheng, J.; Bonkovsky, H.L. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology 2010, 51, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, F.; Li, P.; Chen, G.; Tao, Y.; Wang, H. The tumor suppressive miR-26a regulation of FBXO11 inhibits proliferation, migration and invasion of hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 101, 648–655. [Google Scholar]
- Liang, L.; Zeng, J.H.; Wang, J.Y.; He, R.Q.; Ma, J.; Chen, G.; Cai, X.Y.; Hu, X.H. Down-regulation of miR-26a-5p in hepatocellular carcinoma: A qRT-PCR and bioinformatics study. Pathol. Res. Pract. 2017, 213, 1494–1509. [Google Scholar] [CrossRef]
- Li, Y.; Ren, M.; Zhao, Y.; Lu, X.; Wang, M.; Hu, J.; Lu, G.; He, S. MicroRNA-26a inhibits proliferation and metastasis of human hepatocellular carcinoma by regulating DNMT3B-MEG3 axis. Oncol. Rep. 2017, 37, 3527–3535. [Google Scholar] [CrossRef]
- Zhao, W.T.; Lin, X.L.; Liu, Y.; Han, L.X.; Li, J.; Lin, T.Y.; Shi, J.W.; Wang, S.C.; Lian, M.; Chen, H.W.; et al. miR-26a promotes hepatocellular carcinoma invasion and metastasis by inhibiting PTEN and inhibits cell growth by repressing EZH2. Lab. Investig. 2019, 99, 1484–1500. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Hao, M.; Bu, L. Long noncoding RNA Linc01296 promotes hepatocellular carcinoma development through regulation of the miR-26a/PTEN axis. Biol. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Koek, G.H.; Liedorp, P.R.; Bast, A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta 2011, 412, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Dong, B.; Tian, Y.; Lefebvre, P.; Meng, Z.; Wang, X.; Pattou, F.; Han, W.; Wang, X.; Lou, F.; et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J. Clin. Investig. 2015, 125, 2497–2509. [Google Scholar] [CrossRef]
- Greene, M.W.; Burrington, C.M.; Lynch, D.T.; Davenport, S.K.; Johnson, A.K.; Horsman, M.J.; Chowdhry, S.; Zhang, J.; Sparks, J.D.; Tirrell, P.C. Lipid metabolism, oxidative stress and cell death are regulated by PKC delta in a dietary model of nonalcoholic steatohepatitis. PLoS ONE 2014, 9, e85848. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, L.; Xin, G.; Li, S.; Ma, L.; Xu, Y.; Zhuang, M.; Xiong, Q.; Wei, Z.; Xing, Z.; et al. Oxidative stress mediated by lipid metabolism contributes to high glucose-induced senescence in retinal pigment epithelium. Free Radic. Biol. Med. 2019, 130, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wen, H.; Jing, L.; Yang, Y.; Wang, W.; Liang, X.; Nan, K.; Yao, Y.; Tian, T. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway. Cancer Sci. 2017, 108, 620–631. [Google Scholar] [CrossRef]
- Liu, J.H.; Yang, Y.; Song, Q.; Li, J.B. MicroRNA-155regulates the proliferation and metastasis of human breast cancers by targeting MAPK7. J. B. U. 2019, 24, 1075–1080. [Google Scholar]
- Wang, J.; Guo, J.; Fan, H. MiR-155 regulates the proliferation and apoptosis of pancreatic cancer cells through targeting SOCS3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5168–5175. [Google Scholar]
- Liao, W.W.; Zhang, C.; Liu, F.R.; Wang, W.J. Effects of miR-155 on proliferation and apoptosis by regulating FoxO3a/BIM in liver cancer cell line HCCLM3. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1277–1285. [Google Scholar]
- Ning, S.; Liu, H.; Gao, B.; Wei, W.; Yang, A.; Li, J.; Zhang, L. miR-155, miR-96 and miR-99a as potential diagnostic and prognostic tools for the clinical management of hepatocellular carcinoma. Oncol. Lett. 2019, 18, 3381–3387. [Google Scholar] [CrossRef]
- Csak, T.; Bala, S.; Lippai, D.; Kodys, K.; Catalano, D.; Iracheta-Vellve, A.; Szabo, G. MicroRNA-155 Deficiency Attenuates Liver Steatosis and Fibrosis without Reducing Inflammation in a Mouse Model of Steatohepatitis. PLoS ONE 2015, 10, e0129251. [Google Scholar] [CrossRef]
- Bala, S.; Csak, T.; Saha, B.; Zatsiorsky, J.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J. Hepatol. 2016, 64, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Perra, A.; Plateroti, M.; Columbano, A. T3/TRs axis in hepatocellular carcinoma: New concepts for an old pair. Endocr. Relat. Cancer 2016, 23, R353–R369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, S.M.; Cheng, W.L.; Lin, C.D.; Lin, K.H. Thyroid hormone actions in liver cancer. Cell. Mol. Life Sci. 2013, 70, 1915–1936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Yeh, C.T.; Lin, K.H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. [Google Scholar] [CrossRef]
- Manka, P.; Coombes, J.D.; Boosman, R.; Gauthier, K.; Papa, S.; Syn, W.K. Thyroid hormone in the regulation of hepatocellular carcinoma and its microenvironment. Cancer Lett. 2018, 419, 175–186. [Google Scholar] [CrossRef]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 24. [Google Scholar] [CrossRef]
- Huang, F.; Wu, L.; Qiu, Y.; Bu, K.; Huang, H.; Li, B. The role of free triiodothyronine in high-density lipoprotein cholesterol metabolism. Medicine 2019, 98, e17016. [Google Scholar] [CrossRef]
- Orsagova, I.; RoZnovsky, L.; Petrousova, L.; Konecna, M.; Kabieszova, L.; Safarcik, K.; Kloudova, A. Thyroid dysfunction during interferon alpha therapy for chronic hepatitis B and C—Twenty years of experience. Klin. Mikrobiol. A Infekcni Lek. 2014, 20, 92–97. [Google Scholar]
- Mansour-Ghanaei, F.; Mehrdad, M.; Mortazavi, S.; Joukar, F.; Khak, M.; Atrkar-Roushan, Z. Decreased serum total T3 level in hepatitis B and C related cirrhosis by severity of liver damage. Ann. Hepatol. 2012, 11, 667–671. [Google Scholar] [CrossRef]
- Chi, H.C.; Chen, C.Y.; Tsai, M.M.; Tsai, C.Y.; Lin, K.H. Molecular functions of thyroid hormones and their clinical significance in liver-related diseases. Biomed Res. Int. 2013, 2013, 601361. [Google Scholar] [CrossRef] [PubMed]
- Lanni, A.; Moreno, M.; Goglia, F. Mitochondrial Actions of Thyroid Hormone. Compr. Physiol. 2016, 6, 1591–1607. [Google Scholar] [PubMed]
- Videla, L.A.; Fernandez, V.; Cornejo, P.; Vargas, R.; Castillo, I. Thyroid hormone in the frontier of cell protection, survival and functional recovery. Expert Rev. Mol. Med. 2015, 17, e10. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Liao, C.J.; Huang, Y.H.; Wu, M.H.; Chi, H.C.; Wu, S.M.; Chen, C.Y.; Tseng, Y.H.; Tsai, C.Y.; Chung, I.H.; et al. Thyroid hormone receptor represses miR-17 expression to enhance tumor metastasis in human hepatoma cells. Oncogene 2013, 32, 4509–4518. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lin, Y.H.; Chi, H.C.; Liao, C.H.; Liao, C.J.; Wu, S.M.; Chen, C.Y.; Tseng, Y.H.; Tsai, C.Y.; Lin, S.Y.; et al. Thyroid hormone regulation of miR-21 enhances migration and invasion of hepatoma. Cancer Res. 2013, 73, 2505–2517. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wu, M.H.; Liao, C.J.; Huang, Y.H.; Chi, H.C.; Wu, S.M.; Chen, C.Y.; Tseng, Y.H.; Tsai, C.Y.; Chung, I.H.; et al. Repression of microRNA-130b by thyroid hormone enhances cell motility. J. Hepatol. 2015, 62, 1328–1340. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, M.; Tu, J.; Pang, L.; Cai, W.; Liu, X. MicroRNA-122 affects cell aggressiveness and apoptosis by targeting PKM2 in human hepatocellular carcinoma. Oncol. Rep. 2015, 34, 2054–2064. [Google Scholar] [CrossRef]
- Dong, H.; Curran, I.; Williams, A.; Bondy, G.; Yauk, C.L.; Wade, M.G. Hepatic miRNA profiles and thyroid hormone homeostasis in rats exposed to dietary potassium perfluorooctanesulfonate (PFOS). Environ. Toxicol. Pharmacol. 2016, 41, 201–210. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Yang, S.; Wu, Y.; Wang, J.; Yu, W.; Liu, Y. PU.1-deficient mice are resistant to thioacetamide-induced hepatic fibrosis: PU.1 finely regulates Sirt1 expression via transcriptional promotion of miR-34a and miR-29c in hepatic stellate cells. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Yu, Q.; Zhou, J.; Jian, Y.; Xiu, Z.; Xiang, L.; Yang, D.; Zeng, W. MicroRNA-214 suppresses cell proliferation and migration and cell metabolism by targeting PDK2 and PHF6 in hepatocellular carcinoma. Cell Biol. Int. 2019. [Google Scholar] [CrossRef]
- Tian, C.; Wu, H.; Li, C.; Tian, X.; Sun, Y.; Liu, E.; Liao, X.; Song, W. Downreguation of FoxM1 by miR-214 inhibits proliferation and migration in hepatocellular carcinoma. Gene Ther. 2018, 25, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Wang, S.; Liu, Z.; Xiu, M. MiR-29a suppresses cell proliferation by targeting SIRT1 in hepatocellular carcinoma. Cancer Biomark. 2018, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.C.; Zeng, F.; Le, Y.G.; Xin, L. LncRNA CASC2 inhibited the viability and induced the apoptosis of hepatocellular carcinoma cells through regulating miR-24-3p. J. Cell. Biochem. 2018, 119, 6391–6397. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yang, P.; Guo, Z.; Yang, R.; Yang, H.; Yang, F.; Li, L.; Xiang, B. Overexpression of microRNA-21 strengthens stem cell-like characteristics in a hepatocellular carcinoma cell line. World J. Surg. Oncol. 2016, 14, 278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Wang, Y.; Zhen, P.; Luo, X.; Zhang, C.; Zhou, L.; Lu, Y.; Yang, Y.; Zhang, W.; Wan, J. Genome-wide analysis of miRNA signature differentially expressed in doxorubicin-resistant and parental human hepatocellular carcinoma cell lines. PLoS ONE 2013, 8, e54111. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.M.; Rodrigues, C.M.P.; Castro, R.E. Modulation of liver steatosis by miR-21/PPARalpha. Cell Death Discov. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shentu, T.P.; Wen, L.; Johnson, D.A.; Shyy, J.Y. Regulation of SIRT1 by oxidative stress-responsive miRNAs and a systematic approach to identify its role in the endothelium. Antioxid. Redox Signal. 2013, 19, 1522–1538. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, Q.; Zhang, Q.; Lin, S.Y.; Zhu, Y.; Yang, X.; Guo, A.Y. Gene expression, regulation of DEN and HBx induced HCC mice models and comparisons of tumor, para-tumor and normal tissues. BMC Cancer 2017, 17, 862. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Urtasun, R.; Elizalde, M.; Azkona, M.; Latasa, M.U.; Garcia-Irigoyen, O.; Uriarte, I.; Fernandez-Barrena, M.G.; Vicent, S.; Alonso, M.M.; Muntane, J.; et al. Splicing regulator SLU7 preserves survival of hepatocellular carcinoma cells and other solid tumors via oncogenic miR-17-92 cluster expression. Oncogene 2016, 35, 4719–4729. [Google Scholar] [CrossRef]
- Peng, R.; Men, J.; Ma, R.; Wang, Q.; Wang, Y.; Sun, Y.; Ren, J. miR-214 down-regulates ARL2 and suppresses growth and invasion of cervical cancer cells. Biochem. Biophys. Res. Commun. 2017, 484, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Guo, K.; Chen, B.; Wen, Y.; Xu, Y. miR-214-5p inhibits human prostate cancer proliferation and migration through regulating CRMP5. Cancer Biomark. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Ma, L.; Hou, Y.; Pan, J.; Sun, C.; Yang, Y.; Zhang, J. MiR-214 suppressed ovarian cancer and negatively regulated semaphorin 4D. Tumour Biol. 2016, 37, 8239–8248. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.C.; Tien, Y.J.; Wen, C.J.; Yeh, T.S.; Yu, M.C.; Huang, C.H.; Lee, Y.S.; Yen, T.C.; Hsieh, S.Y. MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J. Hepatol. 2012, 57, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wong, Y.S.; Goh, B.K.P.; Chan, C.Y.; Cheow, P.C.; Chow, P.K.H.; Lim, T.K.H.; Goh, G.B.B.; Krishnamoorthy, T.L.; Kumar, R.; et al. Circulating microRNAs as Potential Diagnostic and Prognostic Biomarkers in Hepatocellular Carcinoma. Sci. Rep. 2019, 9, 10464. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, Y.; Qin, J.; Lu, S. lncRNA MIAT promotes proliferation and invasion of HCC cells via sponging miR-214. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G559–G565. [Google Scholar] [CrossRef]
- Gou, X.; Zhao, X.; Wang, Z. Long noncoding RNA PVT1 promotes hepatocellular carcinoma progression through regulating miR-214. Cancer Biomark. 2017, 20, 511–519. [Google Scholar] [CrossRef]
- Park, D.H.; Shin, J.W.; Park, S.K.; Seo, J.N.; Li, L.; Jang, J.J.; Lee, M.J. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. Toxicol. Lett. 2009, 191, 321–326. [Google Scholar] [CrossRef]
- Zhou, F.; Shen, T.; Duan, T.; Xu, Y.Y.; Khor, S.C.; Li, J.; Ge, J.; Zheng, Y.F.; Hsu, S.; De Stefano, J.; et al. Antioxidant effects of lipophilic tea polyphenols on diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis in rats. In Vivo 2014, 28, 495–503. [Google Scholar]
- Unsal, V.; Belge-Kurutas, E. Experimental Hepatic Carcinogenesis: Oxidative Stress and Natural Antioxidants. Maced. J. Med. Sci. 2017, 5, 686–691. [Google Scholar] [CrossRef]
- Chi, H.C.; Chen, S.L.; Tsai, C.Y.; Chuang, W.Y.; Huang, Y.H.; Tsai, M.M.; Wu, S.M.; Sun, C.P.; Yeh, C.T.; Lin, K.H. Thyroid hormone suppresses hepatocarcinogenesis via DAPK2 and SQSTM1-dependent selective autophagy. Autophagy 2016, 12, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lu, L.; Yan, S.; Yi, H.; Yao, H.; Wu, D.; He, G.; Tao, X.; Deng, X. Autophagy and doxorubicin resistance in cancer. Anti-Cancer Drugs 2018, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ozlu, B.; Kabay, G.; Bocek, I.; Yilmaz, M.; Piskin, A.K.; Shim, B.S.; Mutlu, M. Controlled release of doxorubicin from polyethylene glycol functionalized melanin nanoparticles for breast cancer therapy: Part I. Production and drug release performance of the melanin nanoparticles. Int. J. Pharm. 2019, 570, 118613. [Google Scholar] [CrossRef] [PubMed]
- Damiani, V.; Falvo, E.; Fracasso, G.; Federici, L.; Pitea, M.; De Laurenzi, V.; Sala, G.; Ceci, P. Therapeutic Efficacy of the Novel Stimuli-Sensitive Nano-Ferritins Containing Doxorubicin in a Head and Neck Cancer Model. Int. J. Mol. Sci. 2017, 18, 1555. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.M.; Chen, W.B.; Qin, T.; Lv, L.N.; Feng, B.; Lu, Y.L.; Li, Z.Q.; Wang, X.C.; Tao, L.J.; Li, H.W.; et al. ACOX1 destabilizes p73 to suppress intrinsic apoptosis pathway and regulates sensitivity to doxorubicin in lymphoma cells. BMB Rep. 2019, 52, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, W.; Cheng, L.; Meng, F.; Zhang, J.; Zhong, Z. EGFR-targeted multifunctional polymersomal doxorubicin induces selective and potent suppression of orthotopic human liver cancer in vivo. Acta Biomater. 2017, 64, 323–333. [Google Scholar] [CrossRef]
- Akolkar, G.; da Silva Dias, D.; Ayyappan, P.; Bagchi, A.K.; Jassal, D.S.; Salemi, V.M.C.; Irigoyen, M.C.; De Angelis, K.; Singal, P.K. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H795–H809. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Kilany, O.E.; Khalifa, H.A.; Ahmed, A.A.M. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother. Pharmacol. 2017, 80, 745–753. [Google Scholar] [CrossRef]
- Govender, J.; Loos, B.; Marais, E.; Engelbrecht, A.M. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: A review of the protective role of melatonin. J. Pineal Res. 2014, 57, 367–380. [Google Scholar] [CrossRef]
- Chi, H.C.; Chen, S.L.; Cheng, Y.H.; Lin, T.K.; Tsai, C.Y.; Tsai, M.M.; Lin, Y.H.; Huang, Y.H.; Lin, K.H. Chemotherapy resistance and metastasis-promoting effects of thyroid hormone in hepatocarcinoma cells are mediated by suppression of FoxO1 and Bim pathway. Cell Death Dis. 2016, 7, e2324. [Google Scholar] [CrossRef]
- Deng, L.; Chen, M.; Tanaka, M.; Ku, Y.; Itoh, T.; Shoji, I.; Hotta, H. HCV upregulates Bim through the ROS/JNK signalling pathway, leading to Bax-mediated apoptosis. J. Gen. Virol. 2015, 96, 2670–2683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.C.; Li, Y.Y.; Wang, H.Y.; Fu, S.; Wang, X.P.; Zeng, M.S.; Zeng, Y.X.; Shao, J.Y. Knockdown of miR-214 promotes apoptosis and inhibits cell proliferation in nasopharyngeal carcinoma. PLoS ONE 2014, 9, e86149. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B.; Mahmoud, A.I.; Luo, X.; Johnson, B.A.; van Rooij, E.; Matsuzaki, S.; Humphries, K.M.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca(2)(+) overload and cell death. J. Clin. Investig. 2012, 122, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ha, T.; Hu, Y.; Lu, C.; Liu, L.; Zhang, X.; Kao, R.; Kalbfleisch, J.; Williams, D.; Li, C. MicroRNA-214 protects against hypoxia/reoxygenation induced cell damage and myocardial ischemia/reperfusion injury via suppression of PTEN and Bim1 expression. Oncotarget 2016, 7, 86926–86936. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, W.; Chang, Z.; Pan, Y.; Zong, H.; Fan, Q.; Wang, L. miR-4417 Targets Tripartite Motif-Containing 35 (TRIM35) and Regulates Pyruvate Kinase Muscle 2 (PKM2) Phosphorylation to Promote Proliferation and Suppress Apoptosis in Hepatocellular Carcinoma Cells. Med. Sci. Monit. 2017, 23, 1741–1750. [Google Scholar] [CrossRef]
- Li, W.; Qiu, Y.; Hao, J.; Zhao, C.; Deng, X.; Shu, G. Dauricine upregulates the chemosensitivity of hepatocellular carcinoma cells: Role of repressing glycolysis via miR-199a:HK2/PKM2 modulation. Food Chem. Toxicol. 2018, 121, 156–165. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Xu, K.; Dong, J. Dynamic regulation of uncoupling protein 2 expression by microRNA-214 in hepatocellular carcinoma. Biosci. Rep. 2016, 36, e0035. [Google Scholar] [CrossRef]
- Yokoyama, C.; Sueyoshi, Y.; Ema, M.; Mori, Y.; Takaishi, K.; Hisatomi, H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol. Lett. 2017, 14, 6066–6070. [Google Scholar] [CrossRef]
- Yang, S.; Fei, X.; Lu, Y.; Xu, B.; Ma, Y.; Wan, H. miRNA-214 suppresses oxidative stress in diabetic nephropathy via the ROS/Akt/mTOR signaling pathway and uncoupling protein 2. Exp. Ther. Med. 2019, 17, 3530–3538. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Wang, R.; Wang, P.; Tao, Z.; Gao, L.; Yan, F.; Liu, X.; Yu, S.; Ji, X.; et al. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke 2015, 46, 513–519. [Google Scholar] [CrossRef]
- Henke, J.I.; Goergen, D.; Zheng, J.; Song, Y.; Schuttler, C.G.; Fehr, C.; Junemann, C.; Niepmann, M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008, 27, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Nabih, H.K. The Significance of HCV Viral Load in the Incidence of HCC: A Correlation Between Mir-122 and CCL2. J. Gastrointest. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L. Regulation of hepatitis C virus by microRNA-122. Biochem. Soc. Trans. 2008, 36, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Rivera-Serrano, E.E.; Chen, X.; Lemon, S.M. TNRC6 proteins modulate hepatitis C virus replication by spatially regulating the binding of miR-122/Ago2 complexes to viral RNA. Nucleic Acids Res. 2019, 47, 6411–6424. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.; Gebert, L.F.R.; Gan, H.H.; Camacho, E.; Gunsalus, K.C.; MacRae, I.J.; Sagan, S.M. miR-122 and Ago interactions with the HCV genome alter the structure of the viral 5’ terminus. Nucleic Acids Res. 2019, 47, 5307–5324. [Google Scholar] [CrossRef]
- Mahmoudian-Sani, M.R.; Asgharzade, S.; Alghasi, A.; Saeedi-Boroujeni, A.; Adnani Sadati, S.J.; Moradi, M.T. MicroRNA-122 in patients with hepatitis B and hepatitis B virus-associated hepatocellular carcinoma. J. Gastrointest. Oncol. 2019, 10, 789–796. [Google Scholar] [CrossRef]
- Rana, M.A.; Ijaz, B.; Daud, M.; Tariq, S.; Nadeem, T.; Husnain, T. Interplay of Wnt beta-catenin pathway and miRNAs in HBV pathogenesis leading to HCC. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 373–386. [Google Scholar] [CrossRef]
- Liu, G.; Ma, X.; Wang, Z.; Wakae, K.; Yuan, Y.; He, Z.; Yoshiyama, H.; Iizasa, H.; Zhang, H.; Matsuda, M.; et al. Adenosine deaminase acting on RNA-1 (ADAR1) inhibits HBV replication by enhancing microRNA-122 processing. J. Biol. Chem. 2019, 294, 14043–14054. [Google Scholar] [CrossRef]
- He, J.; Zhao, K.; Zheng, L.; Xu, Z.; Gong, W.; Chen, S.; Shen, X.; Huang, G.; Gao, M.; Zeng, Y.; et al. Upregulation of microRNA-122 by farnesoid X receptor suppresses the growth of hepatocellular carcinoma cells. Mol. Cancer 2015, 14, 163. [Google Scholar] [CrossRef]
- Nassirpour, R.; Mehta, P.P.; Yin, M.J. miR-122 regulates tumorigenesis in hepatocellular carcinoma by targeting AKT3. PLoS ONE 2013, 8, e79655. [Google Scholar] [CrossRef]
- Ahsani, Z.; Mohammadi-Yeganeh, S.; Kia, V.; Karimkhanloo, H.; Zarghami, N.; Paryan, M. WNT1 Gene from WNT Signaling Pathway Is a Direct Target of miR-122 in Hepatocellular Carcinoma. Appl. Biochem. Biotechnol. 2017, 181, 884–897. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef]
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Yu, W.G.; Liu, W.; Jin, Y.H. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ. Toxicol. Chem. 2009, 28, 990–996. [Google Scholar] [CrossRef]
- Wang, G.; Dong, F.; Xu, Z.; Sharma, S.; Hu, X.; Chen, D.; Zhang, L.; Zhang, J.; Dong, Q. MicroRNA profile in HBV-induced infection and hepatocellular carcinoma. BMC Cancer 2017, 17, 805. [Google Scholar] [CrossRef]
- Sadri Nahand, J.; Bokharaei-Salim, F.; Salmaninejad, A.; Nesaei, A.; Mohajeri, F.; Moshtzan, A.; Tabibzadeh, A.; Karimzadeh, M.; Moghoofei, M.; Marjani, A.; et al. microRNAs: Key players in virus-associated hepatocellular carcinoma. J. Cell. Physiol. 2019, 234, 12188–12225. [Google Scholar] [CrossRef]
- Shigoka, M.; Tsuchida, A.; Matsudo, T.; Nagakawa, Y.; Saito, H.; Suzuki, Y.; Aoki, T.; Murakami, Y.; Toyoda, H.; Kumada, T.; et al. Deregulation of miR-92a expression is implicated in hepatocellular carcinoma development. Pathol. Int. 2010, 60, 351–357. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Xie, C. miR-92a promotes hepatocellular carcinoma cells proliferation and invasion by FOXA2 targeting. Iran. J. Basic Med. Sci. 2017, 20, 783–790. [Google Scholar]
- Zhang, Y.; Li, T.; Qiu, Y.; Zhang, T.; Guo, P.; Ma, X.; Wei, Q.; Han, L. Serum microRNA panel for early diagnosis of the onset of hepatocellular carcinoma. Medicine 2017, 96, e5642. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, Y.; Cui, K.; Gao, J.; Yu, F.; Chen, L.; Li, S. Expression and significance of PTEN and miR-92 in hepatocellular carcinoma. Mol. Med. Rep. 2013, 7, 1413–1416. [Google Scholar] [CrossRef]
- Yang, W.; Dou, C.; Wang, Y.; Jia, Y.; Li, C.; Zheng, X.; Tu, K. MicroRNA-92a contributes to tumor growth of human hepatocellular carcinoma by targeting FBXW7. Oncol. Rep. 2015, 34, 2576–2584. [Google Scholar] [CrossRef][Green Version]
- Ock, C.Y.; Kim, E.H.; Choi, D.J.; Lee, H.J.; Hahm, K.B.; Chung, M.H. 8-Hydroxydeoxyguanosine: Not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J. Gastroenterol. 2012, 18, 302–308. [Google Scholar] [CrossRef]
- Liu, D.Y.; Peng, Z.H.; Qiu, G.Q.; Zhou, C.Z. Expression of telomerase activity and oxidative stress in human hepatocellular carcinoma with cirrhosis. World J. Gastroenterol. 2003, 9, 1859–1862. [Google Scholar] [CrossRef]
- Saini, N.; Srinivasan, R.; Chawla, Y.; Sharma, S.; Chakraborti, A.; Rajwanshi, A. Telomerase activity, telomere length and human telomerase reverse transcriptase expression in hepatocellular carcinoma is independent of hepatitis virus status. Liver Int. 2009, 29, 1162–1170. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxidative Med. Cell. Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef]
- Frau, C.; Loi, R.; Petrelli, A.; Perra, A.; Menegon, S.; Kowalik, M.A.; Pinna, S.; Leoni, V.P.; Fornari, F.; Gramantieri, L.; et al. Local hypothyroidism favors the progression of preneoplastic lesions to hepatocellular carcinoma in rats. Hepatology 2015, 61, 249–259. [Google Scholar] [CrossRef]
- Pang, C.; Huang, G.; Luo, K.; Dong, Y.; He, F.; Du, G.; Xiao, M.; Cai, W. miR-206 inhibits the growth of hepatocellular carcinoma cells via targeting CDK9. Cancer Med. 2017, 6, 2398–2409. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, L.; Zhong, Y.; Lai, L.; Li, X. miR-206 inhibits cell proliferation, invasion, and migration by down-regulating PTP1B in hepatocellular carcinoma. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Wang, Y.; Tai, Q.; Zhang, J.; Kang, J.; Gao, F.; Zhong, F.; Cai, L.; Fang, F.; Gao, Y. MiRNA-206 inhibits hepatocellular carcinoma cell proliferation and migration but promotes apoptosis by modulating cMET expression. Acta Biochim. Et Biophys. Sin. 2019, 51, 243–253. [Google Scholar] [CrossRef]
- Singh, A.; Happel, C.; Manna, S.K.; Acquaah-Mensah, G.; Carrerero, J.; Kumar, S.; Nasipuri, P.; Krausz, K.W.; Wakabayashi, N.; Dewi, R.; et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Investig. 2013, 123, 2921–2934. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, C.; Zhang, N.; Kang, W.; Lu, R.; Wu, H.; Geng, Y.; Zhao, Y.; Xu, X. Serum microRNA miR-206 is decreased in hyperthyroidism and mediates thyroid hormone regulation of lipid metabolism in HepG2 human hepatoblastoma cells. Mol. Med. Rep. 2018, 17, 5635–5641. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, X.P.; Zhu, J.Y.; Chen, Z.G.; Li, X.J.; Zhang, X.H.; Huang, S.; He, J.B.; Lian, F.; Zhao, Y.N.; et al. miR-128-3p suppresses hepatocellular carcinoma proliferation by regulating PIK3R1 and is correlated with the prognosis of HCC patients. Oncol. Rep. 2015, 33, 2889–2898. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Lv, Z. miR-128 modulates hepatocellular carcinoma by inhibition of ITGA2 and ITGA5 expression. Am. J. Transl. Res. 2015, 7, 1564–1573. [Google Scholar]
- Lee, D.Y.; Kim, H.S.; Won, K.J.; Lee, K.P.; Jung, S.H.; Park, E.S.; Choi, W.S.; Lee, H.M.; Kim, B. DJ-1 regulates the expression of renal (pro)renin receptor via reactive oxygen species-mediated epigenetic modification. Biochim. Et Biophys. Acta 2015, 1850, 426–434. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Bell, B.A.; Rayborn, M.E.; Yang, X.; Kaul, C.; Grossman, G.H.; Samuels, I.S.; Hollyfield, J.G.; Xie, C.; Cai, H.; et al. Loss of DJ-1 elicits retinal abnormalities, visual dysfunction, and increased oxidative stress in mice. Exp. Eye Res. 2015, 139, 22–36. [Google Scholar] [CrossRef]
- Wang, J.; Chu, Y.; Xu, M.; Zhang, X.; Zhou, Y.; Xu, M. miR-21 promotes cell migration and invasion of hepatocellular carcinoma by targeting KLF5. Oncol. Lett. 2019, 17, 2221–2227. [Google Scholar] [CrossRef]
- Musaddaq, G.; Shahzad, N.; Ashraf, M.A.; Arshad, M.I. Circulating liver-specific microRNAs as noninvasive diagnostic biomarkers of hepatic diseases in human. Biomarkers 2019, 24, 103–109. [Google Scholar] [CrossRef]
- Zhan, Y.; Zheng, N.; Teng, F.; Bao, L.; Liu, F.; Zhang, M.; Guo, M.; Guo, W.; Ding, G.; Wang, Q. MiR-199a/b-5p inhibits hepatocellular carcinoma progression by post-transcriptionally suppressing ROCK1. Oncotarget 2017, 8, 67169–67180. [Google Scholar] [CrossRef]
- Lou, Z.; Gong, Y.Q.; Zhou, X.; Hu, G.H. Low expression of miR-199 in hepatocellular carcinoma contributes to tumor cell hyper-proliferation by negatively suppressing XBP1. Oncol. Lett. 2018, 16, 6531–6539. [Google Scholar] [CrossRef]
- Bernstein, H.; Payne, C.M.; Bernstein, C.; Schneider, J.; Beard, S.E.; Crowley, C.L. Activation of the promoters of genes associated with DNA damage, oxidative stress, ER stress and protein malfolding by the bile salt, deoxycholate. Toxicol. Lett. 1999, 108, 37–46. [Google Scholar] [CrossRef]
- Silva, T.M.; Moretto, F.C.F.; Sibio, M.T.; Goncalves, B.M.; Oliveira, M.; Olimpio, R.M.C.; Oliveira, D.A.M.; Costa, S.M.B.; Depra, I.C.; Namba, V.; et al. Triiodothyronine (T3) upregulates the expression of proto-oncogene TGFA independent of MAPK/ERK pathway activation in the human breast adenocarcinoma cell line, MCF7. Arch. Endocrinol. Metab. 2019, 63, 142–147. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Ambrosio, R.; Dentice, M. Thyroid hormone promotes differentiation of colon cancer stem cells. Mol. Cell. Endocrinol. 2017, 459, 84–89. [Google Scholar] [CrossRef]
- Latteyer, S.; Christoph, S.; Theurer, S.; Hones, G.S.; Schmid, K.W.; Fuehrer, D.; Moeller, L.C. Thyroxine promotes lung cancer growth in an orthotopic mouse model. Endocr. Relat. Cancer 2019. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wu, M.H.; Huang, Y.H.; Yeh, C.T.; Chi, H.C.; Tsai, C.Y.; Chuang, W.Y.; Yu, C.J.; Chung, I.H.; Chen, C.Y.; et al. Thyroid hormone negatively regulates tumorigenesis through suppression of BC200. Endocr. Relat. Cancer 2018, 25, 967–979. [Google Scholar] [CrossRef]
- Sap, J.; Munoz, A.; Damm, K.; Goldberg, Y.; Ghysdael, J.; Leutz, A.; Beug, H.; Vennstrom, B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 1986, 324, 635–640. [Google Scholar] [CrossRef]
- Barlow, C.; Meister, B.; Lardelli, M.; Lendahl, U.; Vennstrom, B. Thyroid abnormalities and hepatocellular carcinoma in mice transgenic for v-erbA. EMBO J. 1994, 13, 4241–4250. [Google Scholar] [CrossRef]
- Kabir, A. Decreased serum total T3 level in hepatitis B and C related cirrhosis by severity of liver damage. Ann. Hepatol. 2013, 12, 506–507. [Google Scholar] [CrossRef]
- Hassan, M.M.; Kaseb, A.; Li, D.; Patt, Y.Z.; Vauthey, J.N.; Thomas, M.B.; Curley, S.A.; Spitz, M.R.; Sherman, S.I.; Abdalla, E.K.; et al. Association between hypothyroidism and hepatocellular carcinoma: A case-control study in the United States. Hepatology 2009, 49, 1563–1570. [Google Scholar] [CrossRef]
- Reddy, A.; Dash, C.; Leerapun, A.; Mettler, T.A.; Stadheim, L.M.; Lazaridis, K.N.; Roberts, R.O.; Roberts, L.R. Hypothyroidism: A possible risk factor for liver cancer in patients with no known underlying cause of liver disease. Clin. Gastroenterol. Hepatol. 2007, 5, 118–123. [Google Scholar] [CrossRef]
- Yu, L.X.; Ling, Y.; Wang, H.Y. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. Npj Precis. Oncol. 2018, 2, 6. [Google Scholar] [CrossRef]
- Adler, S.M.; Wartofsky, L. The nonthyroidal illness syndrome. Endocrinol. Metab. Clin. North Am. 2007, 36, 657–672. [Google Scholar] [CrossRef]
- De Groot, L.J. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit. Care Clin. 2006, 22, 57–86. [Google Scholar] [CrossRef]
- Lin, S.L.; Wu, S.M.; Chung, I.H.; Lin, Y.H.; Chen, C.Y.; Chi, H.C.; Lin, T.K.; Yeh, C.T.; Lin, K.H. Stimulation of Interferon-Stimulated Gene 20 by Thyroid Hormone Enhances Angiogenesis in Liver Cancer. Neoplasia 2018, 20, 57–68. [Google Scholar] [CrossRef]
- Baskol, G.; Atmaca, H.; Tanriverdi, F.; Baskol, M.; Kocer, D.; Bayram, F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Exp. Clin. Endocrinol. Diabetes 2007, 115, 522–526. [Google Scholar] [CrossRef]
- Haribabu, A.; Reddy, V.S.; Pallavi, C.; Bitla, A.R.; Sachan, A.; Pullaiah, P.; Suresh, V.; Rao, P.V.; Suchitra, M.M. Evaluation of protein oxidation and its association with lipid peroxidation and thyrotropin levels in overt and subclinical hypothyroidism. Endocrine 2013, 44, 152–157. [Google Scholar] [CrossRef]
- Mourouzis, I.; Politi, E.; Pantos, C. Thyroid hormone and tissue repair: New tricks for an old hormone? J. Thyroid Res. 2013, 2013, 312104. [Google Scholar] [CrossRef]
- D’Espessailles, A.; Dossi, C.; Intriago, G.; Leiva, P.; Romanque, P. Hormonal pretreatment preserves liver regenerative capacity and minimizes inflammation after partial hepatectomy. Ann. Hepatol. 2013, 12, 881–891. [Google Scholar] [CrossRef]
- Fernandez, V.; Castillo, I.; Tapia, G.; Romanque, P.; Uribe-Echevarria, S.; Uribe, M.; Cartier-Ugarte, D.; Santander, G.; Vial, M.T.; Videla, L.A. Thyroid hormone preconditioning: Protection against ischemia-reperfusion liver injury in the rat. Hepatology 2007, 45, 170–177. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Ueno, T.; Komatsu, M. Autophagy in the liver: Functions in health and disease. Nat. Reviews. Gastroenterol. Hepatol. 2017, 14, 170–184. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Ke, P.Y.; Liao, C.J.; Wu, S.M.; Chi, H.C.; Tsai, C.Y.; Chen, C.Y.; Lin, Y.H.; Lin, K.H. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy 2014, 10, 20–31. [Google Scholar] [CrossRef]
- Vargas, R.; Riquelme, B.; Fernandez, J.; Alvarez, D.; Perez, I.F.; Cornejo, P.; Fernandez, V.; Videla, L.A. Docosahexaenoic acid-thyroid hormone combined protocol as a novel approach to metabolic stress disorders: Relation to mitochondrial adaptation via liver PGC-1alpha and sirtuin1 activation. BioFactors 2019, 45, 271–278. [Google Scholar] [CrossRef]
- El Agaty, S.M. Triiodothyronine improves age-induced glucose intolerance and increases the expression of sirtuin-1 and glucose transporter-4 in skeletal muscle of aged rats. Gen. Physiol. Biophys. 2018, 37, 677–686. [Google Scholar]
- Singh, B.K.; Sinha, R.A.; Ohba, K.; Yen, P.M. Role of thyroid hormone in hepatic gene regulation, chromatin remodeling, and autophagy. Mol. Cell. Endocrinol. 2017, 458, 160–168. [Google Scholar] [CrossRef]
- Singh, B.K.; Sinha, R.A.; Zhou, J.; Tripathi, M.; Ohba, K.; Wang, M.E.; Astapova, I.; Ghosh, S.; Hollenberg, A.N.; Gauthier, K.; et al. Hepatic FOXO1 Target Genes Are Co-regulated by Thyroid Hormone via RICTOR Protein Deacetylation and MTORC2-AKT Protein Inhibition. J. Biol. Chem. 2016, 291, 198–214. [Google Scholar] [CrossRef]
- Sinha, R.A.; Singh, B.K.; Zhou, J.; Wu, Y.; Farah, B.L.; Ohba, K.; Lesmana, R.; Gooding, J.; Bay, B.H.; Yen, P.M. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy 2015, 11, 1341–1357. [Google Scholar] [CrossRef]
- Webb, A.E.; Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef]
- Videla, L.A.; Cornejo, P.; Romanque, P.; Santibanez, C.; Castillo, I.; Vargas, R. Thyroid hormone-induced cytosol-to-nuclear translocation of rat liver Nrf2 is dependent on Kupffer cell functioning. Sci. World J. 2012, 2012, 301494. [Google Scholar] [CrossRef][Green Version]
- Cornejo, P.; Vargas, R.; Videla, L.A. Nrf2-regulated phase-II detoxification enzymes and phase-III transporters are induced by thyroid hormone in rat liver. BioFactors 2013, 39, 514–521. [Google Scholar] [CrossRef]
- Romanque, P.; Cornejo, P.; Valdes, S.; Videla, L.A. Thyroid hormone administration induces rat liver Nrf2 activation: Suppression by N-acetylcysteine pretreatment. Thyroid 2011, 21, 655–662. [Google Scholar] [CrossRef]
- Li, N.; Alam, J.; Venkatesan, M.I.; Eiguren-Fernandez, A.; Schmitz, D.; Di Stefano, E.; Slaughter, N.; Killeen, E.; Wang, X.; Huang, A.; et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: Protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 2004, 173, 3467–3481. [Google Scholar] [CrossRef]

| microRNA | Correlative with Oxidative Stress | Ref. |
|---|---|---|
| miR-34a-5p miR-1915-3p miR-638 miR-150-3p | Associated with oxidative stress-related apoptosis | [30] |
| miR-92 | Correlated positively with telomerase activity, 8-OHdG Target to anti-oxidative gene Sirt1 | [32] |
| miR-199a/b | Prevents the liver cell oxidative stress induced by bile acid Target to Sirt1 | [33] |
| miR-122 | Correlative with HCV/ HBV infection Positive association with antioxidant enzyme NQO1 | [34] [35] |
| miR-26a | Affecting liver lipid metabolism | [36] |
| miR-155 | Affecting liver lipid metabolism | [37] |
| miR-214 | Associated with oxidative stress-related apoptosis Target to ATF4 and EZH2 | [38] [39] |
| miR-200 | Target to p38α and repression anti-oxidative gene Nrf2 | [40] |
| miR-181 | Target to Sirt1 and impair insulin sensitivity | [41] |
| miR-128 | Target to DJ-1 Target to Sirt1 | [42] [43] |
| miR-29a/c | Controls the hepatic lipogenic process | [44] |
| miR-21 | Leading to mitochondrial ROS accumulation | [45] |
| miR-196 | Downregulates Bach1, and inhibition of HCV expression | [46] |
| miRNAs Positively Affected by Thyroid Hormones * | ||||||||
|---|---|---|---|---|---|---|---|---|
| HepG2-TRα1 | HepG2-TRβ1 | |||||||
| TH/microRNAs | MicroRNA | Fold | HCC/ROS Ref. | TH | MicroRNA | Fold | HCC/ROS Ref. | TH |
| Three times repetitive experiments | miR-122 | 10.54 | [35,77] | [78] | miR-29c | 4.21 | [44,79] | |
| miR-152 | 3.42 | miR-214 | 3.50 | [38,80,81] | [38] | |||
| miR-139-5p | 10.38 | miR-202 | 2.41 | |||||
| miR-128a | 71.90 | [43] | ||||||
| miR-139-3p | 3.85 | |||||||
| miR-548d-3p | 3.09 | |||||||
| miR-140-3p | 2.77 | |||||||
| Two times repetitive experiments | miR-143 | 6.20 | miR-193b | 2.99 | ||||
| miR-210 | 5.22 | miR-139-5p | 3.12 | |||||
| miR-365 | 5.53 | miR-210 | 2.52 | |||||
| miR-135b | 4.38 | miR-323-3p | 4.12 | |||||
| miR-148a | 5.16 | miR-22 | 2.54 | |||||
| miR-193b | 3.30 | miR-29a | 2.18 | [44,82] | ||||
| miR-125a-3p | 2.92 | miR-29b-1 * | 3.30 | |||||
| miR-29a | 3.15 | [44,82] | miR-193a-3p | 3.34 | ||||
| miR-24 | 2.40 | [83] | [78] | miR-139-3p | 2.22 | |||
| miR-372 | 3.57 | miR-510 | 2.32 | [75,78] | ||||
| miR-372 | 5.07 | miR-21 * | 2.22 | [45,84,85,86] | ||||
| miR-188-3p | 3.05 | |||||||
| miR-100 | 4.11 | |||||||
| miR-126 | 2.35 | |||||||
| miR-21 | 3.30 | [45,84,85,86] | [75,78] | |||||
| miRNAs Negativity Affected by Thyroid Hormones * | ||||||||
|---|---|---|---|---|---|---|---|---|
| HepG2-TRα1 | HepG2-TRβ1 | |||||||
| TH/microRNAs | MicroRNA | Fold | HCC/ROS Ref. | TH | MicroRNA | Fold | HCC/ROS Ref. | TH |
| Three times repetitive experiments | miR-184 | 0.22 | miR-455-3p | 0.22 | ||||
| miR-455-3p | 0.12 | miR-148a | 0.36 | |||||
| miR-499-3p | 0.20 | miR-425 * | 0.24 | |||||
| miR-221 | 0.33 | miR-187 | 0.27 | |||||
| miR-181b | 0.30 | [87] | miR-429 | 0.41 | ||||
| miR-130b | 0.34 | [76] | ||||||
| miR-149 | 0.35 | |||||||
| miR-17 | 0.34 | [85] | [74] | |||||
| Two times repetitive experiments | miR-425 * | 0.22 | miR-106a | 0.23 | ||||
| miR-20a | 0.31 | miR-199a-5p | 0.22 | [33,87,88] | [38] | |||
| miR-377 | 0.42 | miR-548d-5p | 0.24 | |||||
| miR-15b | 0.43 | miR-146a | 0.31 | |||||
| miR-516a-5p | 0.29 | miR-221 | 0.27 | |||||
| miR-652 | 0.49 | miR-30a * | 0.35 | |||||
| miR-550 | 0.26 | miR-499-3p | 0.32 | |||||
| miR-18a | 0.23 | miR-888 | 0.27 | |||||
| miR-106a | 0.28 | miR-100 | 0.33 | |||||
| miR-628-3p | 0.34 | miR-339-3p | 0.45 | |||||
| miR-146a | 0.36 | miR-18a | 0.39 | |||||
| miR-181c | 0.41 | [87] | miR-18b | 0.24 | ||||
| miR-92a | 0.36 | [32,89,90] | miR-10a | 0.25 | ||||
| miR-106b | 0.38 | miR-421 | 0.30 | |||||
| miR-487b | 0.35 | miR-525-3p | 0.41 | [74,85] | ||||
| miR-570 | 0.40 | miR-17 | 0.37 | [85,90] | [74] | |||
| let-7d | 0.44 | miR-542-5p | 0.33 | [46,85] | ||||
| miR-15b * | 0.44 | miR-196a * | 0.42 | [46] | ||||
| miR-196b | 0.46 | |||||||
| miR-19a | 0.46 | [87] | ||||||
| miR-181d | 0.32 | |||||||
| miR-20b | 0.40 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-S.; Wang, C.-S.; Yeh, C.-T.; Lin, K.-H. Roles of Thyroid Hormone-Associated microRNAs Affecting Oxidative Stress in Human Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 5220. https://doi.org/10.3390/ijms20205220
Huang P-S, Wang C-S, Yeh C-T, Lin K-H. Roles of Thyroid Hormone-Associated microRNAs Affecting Oxidative Stress in Human Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2019; 20(20):5220. https://doi.org/10.3390/ijms20205220
Chicago/Turabian StyleHuang, Po-Shuan, Chia-Siu Wang, Chau-Ting Yeh, and Kwang-Huei Lin. 2019. "Roles of Thyroid Hormone-Associated microRNAs Affecting Oxidative Stress in Human Hepatocellular Carcinoma" International Journal of Molecular Sciences 20, no. 20: 5220. https://doi.org/10.3390/ijms20205220
APA StyleHuang, P.-S., Wang, C.-S., Yeh, C.-T., & Lin, K.-H. (2019). Roles of Thyroid Hormone-Associated microRNAs Affecting Oxidative Stress in Human Hepatocellular Carcinoma. International Journal of Molecular Sciences, 20(20), 5220. https://doi.org/10.3390/ijms20205220