The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity
Abstract
1. Introduction
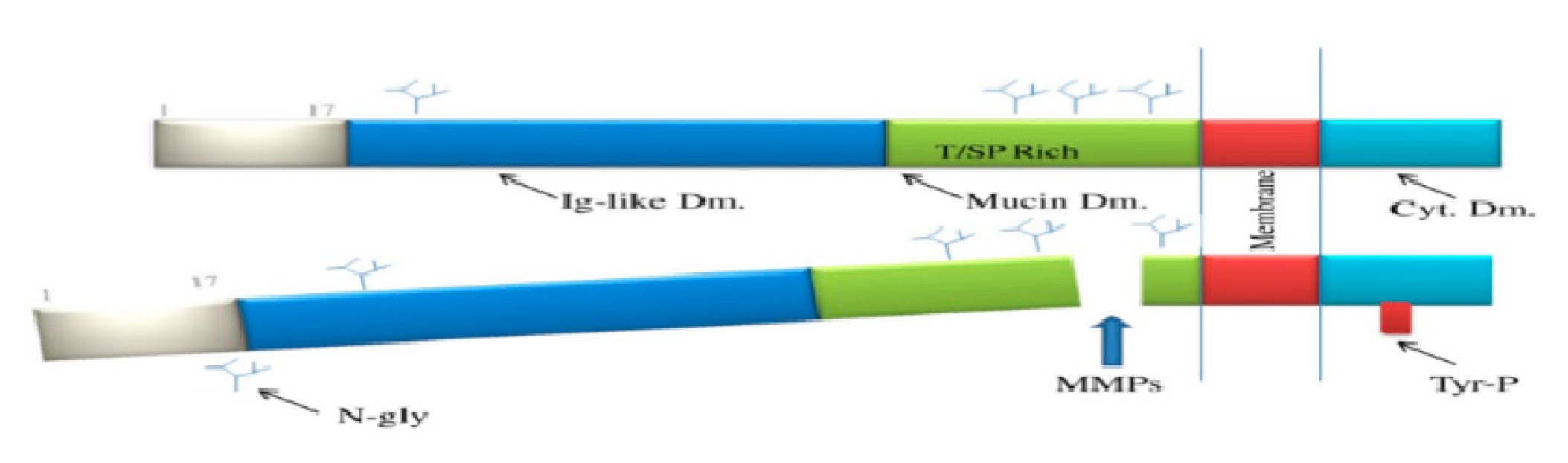
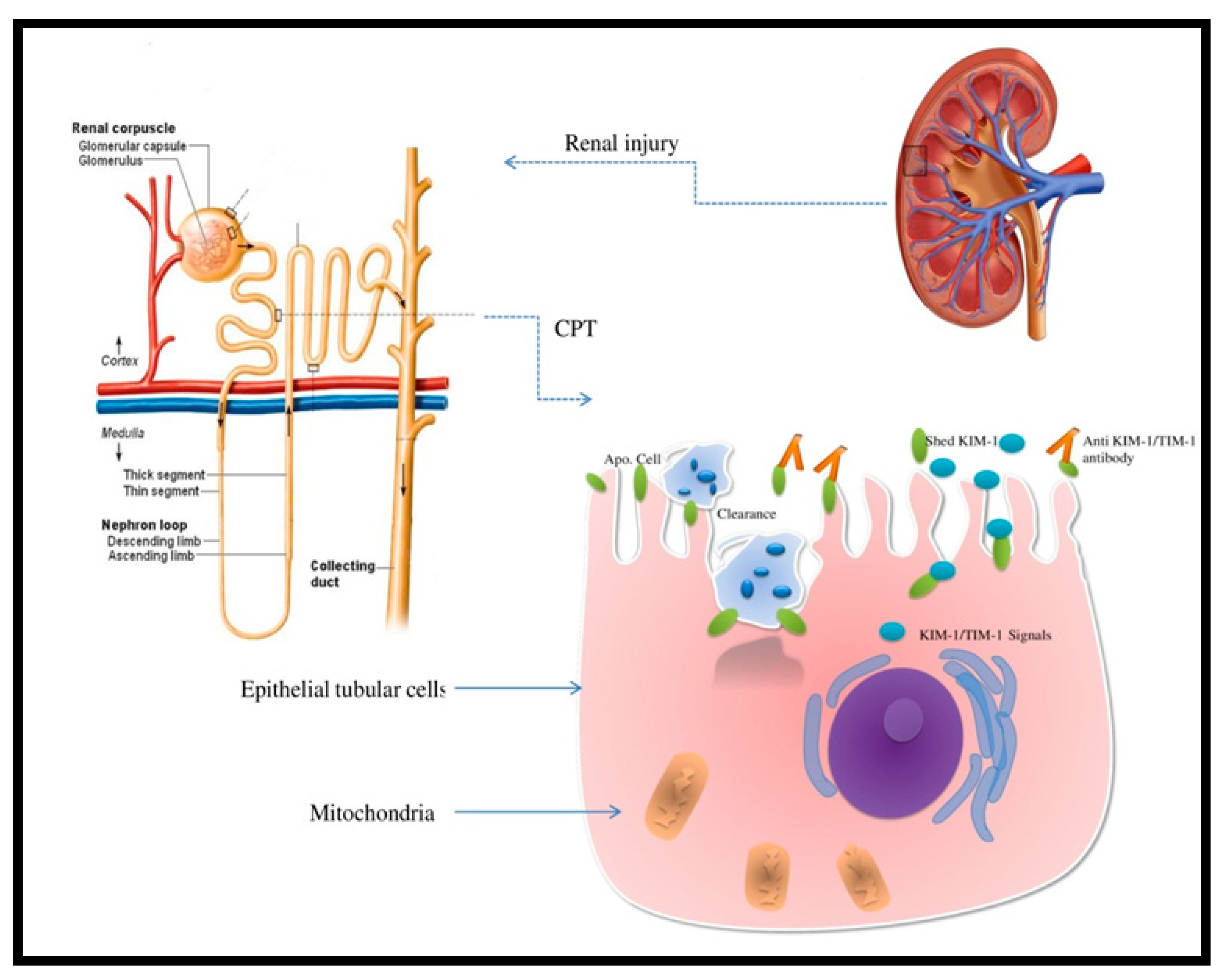
2. Kidney Injury Molecule-1 and AKI
2.1. What Is KIM-1?
2.2. Kidney Molecule-1 in AKI
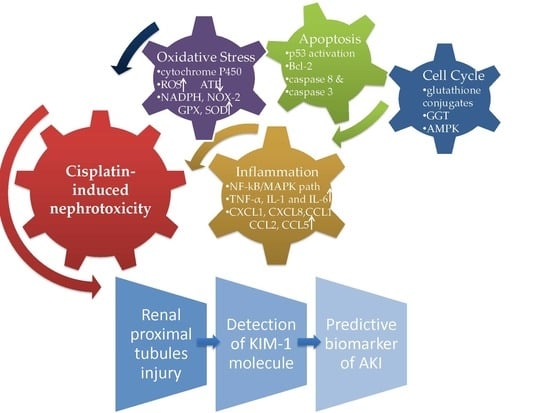
3. Cisplatin-Induced Nephrotoxicity
3.1. Cellular Mechanism
3.2. The Intrinsic and Extrinsic Pathway of Apoptosis
3.3. Oxidative Stress in Cisplatin-Induced AKI
3.4. Inflammation Cytokines and Chemokines in Cellular Damage
4. Assessment of KIM-1 in Cisplatin-Induced Nephrotoxicity
5. Future Directions/Perspective Regarding KIM-1 in Drug-Induced Nephrotoxicity
6. Strategies to Prevent Cisplatin Nephrotoxicity
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CKD | chronic kidney disease |
| AKI | acute kidney injury |
| sCr | serum creatinine |
| GFR | glomerular filtration rate |
| eGFR | estimated glomerular filtration rate |
| BUN | blood urea nitrogen |
| KIM-1 | kidney injury molecule-1 |
| ATN | acute tubular nephritis |
| OCTs | organic cation transporters |
| CTRs | copper transporters |
| ATP | adenosine triphosphate |
| AMPK | AMP-activated protein kinase |
| ER | endoplasmic reticulum |
| Bcl | B-cell lymphoma |
| FasL | Fas ligand |
| TNFα | tumor necrosis factor alpha |
| TNFR | tumor necrosis factor receptor |
| MAPK | mitogen-activated protein kinase |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| DAMPs | damage-associated molecular pattern molecules |
| TLR-4 | toll-like receptor-4 |
| ROS | reactive oxygen species |
| NOX2 | NADPH oxidase 2 |
| GPX | glutathione peroxidase |
| SOD | superoxide dismutase |
| CAT | mitochondria inhibits catalase |
| TNFR | TNF receptor |
| JNK | c-Jun N-terminal kinase |
| ERK | extracellular signal–regulated kinases |
| Tim-1 | T cell immunoglobulin mucin-1 |
| NAG | N-acetyl-β-glucosaminidase |
| DMTU | Dimethylthiourea |
References
- World Health Organization. Global Action Plan. for the Prevention and Control. of Noncommunicable Diseases, 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Faught, L.N.; Greff, M.J.; Rieder, M.; Koren, G. Drug-induced acute kidney injury in children. Br. J. Clin. Pharmacol. 2014, 80, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Sinert, R.; Peacock, P.R. Acute kidney injury. In Tintinalli’s Emergency Medicine, 8th ed.; Tintinalli, J.E., Stapczynski, J.S., Ma, A.J., Yealy, D.M., Mecklern, G.D., Cline, D.M., Eds.; McGraw-Hill: New York, NY, USA, 2016; pp. 575–581. [Google Scholar]
- Perazella, M.A. Pharmacology behind common drug nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Dhodi, D.K.; Bhagat, S.B.; Pathak, D.; Patel, S.B.; Dinesh, K. Drug-induced nephrotoxicity. Int. J. Basic Clin. Pharmacol. 2014, 3, 591–597. [Google Scholar] [CrossRef]
- Loghman-Adham, M.; Kiu Weber, C.I.; Ciorciaro, C.; Mann, J.; Meier, M. Detection and management of nephrotoxicity during drug development. Expert Opin. Drug Saf. 2012, 11, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Vaidya, V.S.; Schmouder, R.; Feig, P.; Dieterle, F. Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 2010, 28, 436–440. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Bouchard, J.; Waikar, S.S.; Siew, E.D.; Endre, Z.H.; Goldstein, S.L.; Koyner, J.L.; Macedo, E.; Di Somma, S. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney Injury: Executive summary from the tenth consensus conference of the acute dialysis quality initiative (ADQI). In ADQI Consensus on AKI Biomarkers and Cardiorenal Syndromes; Karger Publishers: Basel, Karger, 2013; Volume 182, pp. 5–12. [Google Scholar]
- Sari, Z. Nephrotoxic Effects of Drugs Poisoning in the Modern World. Ren. Fail. 2019, 41, 576–594. [Google Scholar] [CrossRef]
- Paueksakon, P.; Fogo, A.B. Druginduced nephropathies. Histopathology 2017, 70, 94–108. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Murray, P.T.; Mehta, R.L.; Shaw, A.; Ronco, C.; Endre, Z.; Kellum, J.A.; Chawla, L.S.; Cruz, D.; Ince, C.; Okusa, M.D. Potential use of biomarkers in acute kidney injury: Report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014, 85, 513–521. [Google Scholar] [CrossRef]
- Nagai, J.; Takano, M. Molecular-targeted approaches to reduce renal accumulation of nephrotoxic drugs. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Moon, A. Drug-Induced Nephrotoxicity and Its Biomarkers. Biomol. Ther 2012, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Labaye, J.; Sarret, D.; Duvic, C.; Hérody, M.; Didelot, F.; Nédélec, G.; Noël, L.H. Renal toxicity of oxaliplatin. Nephrol. Dial. Transplant. 2005, 20, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Ulusakarya, A.; Misra, S.; Haydar, M.; Habert, H.; Castagne, V.; Gumus, Y.; Delmas-Marsalet, B.; Machover, D. Acute renal failure related to oxaliplatin-induced intravascular hemolysis. Med. Oncol. 2010, 27, 1425–1426. [Google Scholar] [CrossRef] [PubMed]
- Yaghobi Joybari, A.; Sarbaz, S.; Azadeh, P.; Mirafsharieh, S.A.; Rahbari, A.; Farasatinasab, M.; Mokhtari, M. Oxaliplatin-induced renal tubular vacuolization. Ann. Pharmacother. 2014, 48, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Isnard-Bagnis, C.; Launay-Vacher, V.; Karie, S.; Deray, G. Anticancer drugs. In Clinical Nephrotoxins Renal Injury from Drug and Chemicals, 3rd ed.; De Broe, M., Porter, G., Bennett, W., Deray, G., Eds.; Springer Scientific: New York, NY, USA, 2008. [Google Scholar]
- Feigelstock, D.; Thompson, P.; Mattoo, P.; Kaplan, G.G. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J. Virol. 1998, 72, 6621–6628. [Google Scholar]
- Chiusolo, A.; Defazio, R.; Zanetti, E.; Mongillo, M.; Mori, N.; Cristofori, P.; Trevisan, A. Kidney injury molecule-1 expression in rat proximal tubule after treatment with segment-specific nephrotoxicants: A tool for early screening of potential kidney toxicity. Toxicol. Pathol. 2010, 38, 338–345. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ozer, J.S.; Dieterle, F.; Collings, F.B.; Ramirez, V.; Troth, S. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 2010, 28, 478–485. [Google Scholar] [CrossRef]
- Sasaki, D.; Yamada, A.; Umeno, H. Comparison of the course of biomarker changes and kidney injury in a rat model of drug-induced acute kidney injury. Biomarkers 2011, 16, 553–566. [Google Scholar] [CrossRef]
- [Internet] FDA. Available online: http://www.fda.gov/bbs/topics/NEWS/2008 /NEW01850.html (accessed on 26 July 2019).
- Dieterle, F.; Sistare, F.; Goodsaid, M.; Papaluca, J.S.; Ozer, C.P.; Webb, W.; Baer, A.; Senagore, M.J.; Walker, E.; Sultana, S.; et al. Renal biomarker qualification submission: A dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat. Biotechnol. 2010, 28, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Harpur, E.; Ennulat, D.; Hoffman, D.; Betton, G.; Gautier, J.C.; Riefke, B.; Bounous, D.; Schuster, K.; Beushausen, S.; Guffroy, M.; et al. Nephrotoxicity. Toxicol. Sci. 2011, 122, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Hubank, M.; Schatz, D.G. Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucl. Acids Res. 1994, 22, 5640–5648. [Google Scholar] [CrossRef] [PubMed]
- Kuchroo, V.K.; Meyers, J.H.; Umetsu, D.T.; DeKruyff, R.H. TIM family of genes in immunity and tolerance. Adv. Immunol. 2006, 91, 227–249. [Google Scholar] [PubMed]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1), “A urinary biomarker and much more”. Nephrol. Dial. Transplant. 2009, 24, 3265–3268. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, M.M.; van den Heuvel, M.C.; Bailly, V.; Bakker, S.J.; van Goor, H.; Stegeman, C.A. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J. Pathol. 2007, 212, 209–217. [Google Scholar] [CrossRef]
- Bailly, V.; Zhang, Z.; Meier, W.; Cate, R.; Sanicola, M.; Bonventre, J.V. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J. Biol. Chem. 2002, 277, 39739–39748. [Google Scholar] [CrossRef]
- Lim, A.I.; Tang, S.C.; Lai, K.N.; Leung, J.C. Kidney injury molecule-1: More than just an injury marker of tubular epithelial cells? J. Cell. Physiol. 2013, 228, 917–924. [Google Scholar] [CrossRef]
- Visnagri, A.; Kandhare, A.D.; Bodhankar, S.L. Renoprotectiveeffect of berberine via intonation on apoptosis andmitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren. Fail. 2015, 37, 482–493. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Kidney injury molecule-1. Curr. Opin. Crit. Care 2010, 16, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.R.; Bonventre, J.V. KIM-1/TIM-1 in proximal tubular cell immune response. Oncotarget 2015, 6, 44059. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.R.; Yeung, M.Y.; Brooks, Y.S.; Chen, H.; Ichimura, T.; Henderson, J.M.; Bonventre, J.V. KIM-1-/TIM-1-mediated phagocytosis links ATG5-/ULK1-dependent clearance of apoptotic cells to antigen presentation. EMBO J. 2015, 34, 2441–2464. [Google Scholar] [CrossRef] [PubMed]
- Tsigou, E.; Psallida, V.; Demponeras, C.; Boutzouka, E.; Baltopoulos, G. Role of new biomarkers: Functional andstructural damage. Crit. Care Res. Pract. 2013, 2013, 361078. [Google Scholar] [PubMed]
- Ichimura, T.; Brooks, C.R.; Bonventre, J.V. Kim-1/ Tim-1 and immune cells: Shifting sands. Kidney Int. 2012, 81, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Gobe, G.C.; Coombes, J.S.; Fassett, R.G.; Endre, Z.H. Biomarkers of drug-induced acute kidney injury in the adult. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1683–1694. [Google Scholar] [CrossRef]
- Kadioglu, T.; Uzunlulu, M.; Yigit Kaya, S.; Oguz, A.; Gonenli, G.; Isbilen, B.; Isman, F.K. Urinary kidney injury molecule-1 levels as a marker of early kidney injury in hypertensive patients. Minerva Urol. Nefrol. 2016, 68, 456–461. [Google Scholar]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef]
- Perco, P.; Oberbauer, R. Kidney injury molecule-1 as a biomarker of acute kidney injury in renal transplant recipients. Nat. Clin. Pract. Nephrol. 2008, 4, 362–363. [Google Scholar] [CrossRef]
- Medic, B.; Rovcanin, B.; Basta Jovanovic, G.; Radojevic-Skodric, S.; Prostran, M. Kidney injury molecule-1 and cardiovascular diseases: From basic science to clinical practice. BioMed Res. Int. 2015, 2015, 854070. [Google Scholar] [CrossRef]
- Shao, X.; Tian, L.; Xu, W.; Zhang, Z.; Wang, C.; Qi, C.; Ni, Z.; Mou, S. Diagnostic Value of Urinary Kidney Injury Molecule 1 for Acute Kidney Injury: A Meta-Analysis. PLoS ONE 2014, 9, e84131. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.A.; Engström, G.; Nilsson, J.; Almgren, P.; Petkovic, M.; Christensson, A.; Nilsson, P.M.; Melander, O.; Melander, M.O. Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol. Dial. Transplant. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Arthur, E.G.; Hill, J.L.; Lewis, E.C.; Neely, B.A.; Janech, M.G.; Tumlin, J.A.; Chawla, L.S.; Shaw, A.D. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014, 85, 431–438. [Google Scholar] [CrossRef]
- Pinches, M.D.; Betts, C.J.; Bickerton, S.J.; Beattie, L.; Burdett, L.D.; Thomas, H.T.; Derbyshire, N.A.; Moores, M.; Price, S.A. Evaluation of novel urinary renal biomarkers: Bio- logical variation and reference change values. Toxicol. Pathol. 2012, 40, 541–549. [Google Scholar] [CrossRef]
- Tsuji, S.; Sugiura, M.; Tsutsumi, S.; Yamada, H. Sex differences in the excretion levels of traditional and novel urinary biomarkers of nephrotoxicity in rats. J. Toxicol. Sci. 2017, 42, 615–627. [Google Scholar] [CrossRef]
- Krawczeski, C.D.; Goldstein, S.L.; Woo, J.G.; Wang, Y.; Piyaphanee, N.; Ma, Q.; Bennett, M.; Devarajan, P. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J. Am. Coll. Cardiol. 2011, 58, 2301–2309. [Google Scholar] [CrossRef]
- Sun, I.O.; Shin, S.H.; Cho, A.Y.; Yoon, H.J.; Chang, M.Y.; Lee, K.Y. Clinical significance of NGAL and KIM-1 for acute kidney injury in patients with scrub typhus. PLoS ONE 2017, 12, e0175890. [Google Scholar] [CrossRef]
- Ornellas, F.M.; Ornellas, D.S.; Martini, S.V. Bone Marrow–Derived Mononuclear Cell Therapy Accelerates Renal Ischemia- Reperfusion Injury Recovery by Apoptotic Related Molecules. Cell. Physiol. Biochem. 2017, 41, 1736–1752. [Google Scholar] [CrossRef]
- Szeto, C.C.; Kwan, B.C.; Lai, K.B.; Lai, F.M.; Chow, K.M.; Wang, G.; Luk, C.C.; Li, P.K. Urinary expression of kidney injury markers in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2010, 5, 2329–2337. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Schjoedt, K.J.; Astrup, A.S.; Tarnow, L.; Lajer, M.; Hansen, P.R.; Parving, H.H.; Rossing, P. Neutrophil gelatinase-associated lipocalin (ngal) and kidney injury molecule 1 (kim1) in patients with diabetic nephropathy: A cross-sectional study and the effects of lisinopril. Diabet. Med. 2010, 27, 1144–1150. [Google Scholar] [CrossRef]
- Zhang, P.L.; Mashni, J.W.; Sabbisetti, V.S.; Schworer, C.M.; Wilson, G.D.; Wolforth, S.C.; Kernen, K.M.; Seifman, D.B.; Amin, B.M.; Geddes, T.J.; et al. Urine kidney injury molecule-1: A potential non-invasive biomarker for patients with renal cell carcinoma. Int. Urol. Nephrol. 2014, 46, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.C.; Hewitt, P. Biomarkers for drug-induced renal damage and nephrotoxicity-an overview for applied toxicology. AAPS J. 2011, 13, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, L.; Moerland, M.; Cohen, A.F.; Burggraaf, J. Urinary kidney biomarkers for early detection of nephrotoxicity in clinical drug development. Br. J. Clin. Pharmacol. 2014, 77, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Vokes, E.E. Induction chemotherapy for head and neck cancer: Recent data. Oncologist 2010, 15, 3–7. [Google Scholar] [CrossRef]
- Ismaili, N.; Amzerin, M.; Flechon, A. Chemotherapy in advanced bladder cancer: Current status and future. J. Hematol. Oncol. 2011, 4, 35. [Google Scholar] [CrossRef]
- Moxley, K.M.; McMeekin, D.S. Endometrial carcinoma: A review of chemotherapy, drug resistance, and the search for new agents. Oncologist 2010, 15, 1026–1033. [Google Scholar] [CrossRef]
- Gronwald, J.; Byrski, T.; Lubinski, J.; Narod, S.A. Cisplatin in breast cancer treatment in BRCA1 carriers. Hered Cancer Clin. Pract. 2012, 10 (Suppl. 4), A17. [Google Scholar] [CrossRef]
- Zarogoulidis, K.; Zarogoulidis, P.; Darwiche, K.; Boutsikou, E.; Machairiotis, N.; Tsakiridis, K.; Katsikogiannis, N.; Kougioumtzi, I.; Karapantzos, I.; Huang, H.; et al. Treatment of non-small cell lung cancer (NSCLC). J. Thorac. Dis. 2013, 5, S389–S396. [Google Scholar] [CrossRef]
- Chan, B.A.; Coward, J.I.G. Chemotherapy advances in small-cell lung cancer. J. Thorac. Dis. 2013, 5, S565–S578. [Google Scholar] [CrossRef]
- Tucker, B.M.; Perazella, M.A. Medications. In Nephrology Secrets, 4th ed.; Lerma, E.V., Sparks, M.A., Topf, J., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 78–83. [Google Scholar]
- Perazella, M.A. Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin. J. Am. Soc. Nephrol. 2012, 7, 1713–1721. [Google Scholar] [CrossRef]
- Dos Santos, N.A.; Carvalho Rodrigues, M.A.; Martins, N.M.; dos Santos, A.C. Cisplatininduced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Traylor, A.; Joseph, R.; Zarjou, A.; Agarwal, A. Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2016, 310, F385–F394. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.J. Pathophysiologic Mechanisms of Selected Types of Nephrotoxicity. 2017. Available online: https://emedicine.medscape.com/ article/1925868 (accessed on 13 September 2018).
- Atilano-Roque, A.; Wen, X.; Aleksunes, L.M.; Joy, M.S. Nrf2 activators as potential modulators of injury in human kidney cell. Toxicol. Rep. 2016, 3, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fliedl, L.; Wieser, M.; Manhart, G.; Gerstl, M.P.; Khan, A.; Grillari, J.; Grillari-Voglauer, R. Controversial role of gamma-glutamyl transferase activity in cisplatin nephrotoxicity. ALTEX 2014, 31, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.D.; La, D.; McDuffie, J.E. Renal transporters and biomarkers in safety assessment. In New Insights into Toxicity and Drug Testing; Gowder, S., Ed.; InTech: Vienna, Austria, 2013. [Google Scholar]
- Ciarimboli, G. Membrane transporters as mediators of cisplatin side effects. Anticancer Res. 2014, 34, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Okamoto, K.; Kobayashi, M.; Narumi, K.; Furugen, A.; Yamada, T.; Iseki, K. Magnesium co-administration decreases cisplatin-induced nephrotoxicity in the multiple cisplatin administration. Life Sci. 2017, 189, 18–22. [Google Scholar] [CrossRef]
- Ciarimboli, G. Membrane Transporters as Mediators of Cisplatin Effects and Side Effects. Scientifica 2012, 2012, 473829. [Google Scholar] [CrossRef]
- Estrela, G.R.; Wasinski, F.; Felizardo, R.J.F.; Souza, L.L.; Câmara, N.O.S.; Bader, M.; Araujo, R.C. MATE-1 modulation by kinin B1 receptor enhances cisplatin efflux from renal cells. Mol. Cell. Biochem. 2017, 428, 101. [Google Scholar] [CrossRef]
- Harrach, S.; Ciarimboli, G. Role of transporters in the distribution of platinum-based drugs. Front. Pharmacol. 2015, 6, 85. [Google Scholar] [CrossRef]
- Zhu, N.; Pabla, C.; Tang, C.; He, L.; Dong, Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch. Toxicol. 2015, 89, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, H.; Shao, J.; Wu, J.; Zhou, L.; Zhang, Z.; Wang, Y.; Huang, Z.; Ren, J.; Liu, S.; et al. A role for Tubular Necroptosis in Cisplatin-Induced AKI. JASN 2015, 26, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Krishnamurthy, S. Cellular responses to Cisplatin-induced DNA damage. J. Nucl. Acids 2010, 2010, 201367. [Google Scholar] [CrossRef]
- Sharp, C.N.; Doll, M.A.; Dupre, T.V.; Shah, P.P.; Subathra, M.; Siow, D.; Arteel, G.E.; Megyesi, J.; Beverly, L.J.; Siskind, L.J. Repeated administration of low-dose cisplatin in mice induces fibrosis. Am. J. Physiol. Renal. Physiol. 2016, 310, F560–F568. [Google Scholar] [CrossRef]
- Vaseva, A.V.; Moll, U.M. The mitochondrial p53 pathway. Biochim. Biophys. Acta 2009, 1787, 414–420. [Google Scholar] [CrossRef]
- Siskind, L.J. Repeated administration of low-dose cisplatin in mice induces fibrosis. Am. J. Physiol. Ren. Physiol. 2016, 310, F560–F568. [Google Scholar] [CrossRef]
- Bhatt, K.; Zhou, L.; Mi, Q.S.; Huang, S.; She, J.X.; Dong, Z. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol. Med. 2010, 16, 409–416. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kakizoe, Y.; Iwata, Y.; Miyasato, Y.; Mizumoto, T.; Adachi, M.; Izumi, Y.; Kuwabara, T.; Suenaga, N.; Narita, Y.; et al. Doxycycline attenuates cisplatin-induced acute kidney injury through pleiotropic e_ects. Am. J. Physiol. Ren. Physiol. 2018, 315, F1347–F1357. [Google Scholar] [CrossRef]
- Watanabe, M.; Oe, Y.; Sato, E.; Sekimoto, A.; Sato, H.; Ito, S.; Takahashi, N. Protease-activated receptor 2 exacerbates cisplatin-induced nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2019, 316, F654–F659. [Google Scholar] [CrossRef]
- Soni, H.; Matthews, A.T.; Pallikkuth, S.; Gangaraju, R.; Adebiyi, A. Gamma-secretase inhibitor DAPT mitigates cisplatin-induced acute kidney injury by suppressing Notch1 signaling. J. Cell. Mol. Med. 2019, 23, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hou, J.; Yan, X.; Leng, J.; Li, R.; Zhang, J.; Xing, J.; Chen, C.; Wang, Z.; Li, W. Platycodon grandiflorum Saponins Ameliorate Cisplatin-Induced Acute Nephrotoxicity through the NF-kappaB-Mediated Inflammation and PI3K/Akt/Apoptosis Signaling Pathways. Nutrients 2018, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Sen, Z.; Jie, M.; Jingzhi, Y.; Dongjie, W.; Dongming, Z.; Xiaoguang, C. Total Coumarins from Hydrangea paniculata Protect against Cisplatin-Induced Acute Kidney Damage in Mice by Suppressing Renal Inflammation and Apoptosis. Evid. Based Complement. Altern. Med. 2017, 2017, 5350161. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Dupre, T.V.; Doll, M.A.; Shah, P.P.; Sharp, C.N.; Kiefer, A.; Scherzer, M.T.; Saurabh, K.; Saforo, D.; Siow, D.; Casson, L.; et al. Suramin protects from cisplatin-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2016, 310, F248–F258. [Google Scholar] [CrossRef]
- Dupre, T.V.; Doll, M.A.; Shah, P.P.; Sharp, C.N.; Siow, D.; Megyesi, J.; Shayman, J.; Bielawska, A.; Bielawski, J.; Beverly, L.J.; et al. Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury. J. Lipid Res. 2017, 58, 1439–1452. [Google Scholar] [CrossRef]
- Kong, D.; Zhuo, L.; Gao, C.; Shi, S.; Wang, N.; Huang, Z.; Li, W.; Hao, L. Erythropoieti protects against cisplatin-induced nephrotoxicity by attenuating endoplasmic reticulum stress-induced apoptosis. J. Nephrol. 2013, 26, 219–227. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Wen, H.; Holanda, D.G.; Rastogi, P.; Steinbach, E.; Han, R.; Coleman, M.C.; Attanasio, M.; Riley, D.P.; Spitz, D.R.; et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 2019, 20, 98–106. [Google Scholar] [CrossRef]
- Tang, J.; Shi, Y.; Liu, N.; Xu, L.; Zang, X.; Li, P.; Zhang, J.; Zheng, X.; Qiu, A.; Zhuang, S. Blockade of histone deacetylase 6 protects against cisplatin-induced acute kidney injury. Clin. Sci. 2018, 132, 339–359. [Google Scholar] [CrossRef]
- Perico, L.; Morigi, M.; Rota, C.; Breno, M.; Mele, C.; Noris, M.; Introna, M.; Capelli, C.; Longaretti, L.; Rottoli, D.; et al. Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat. Commun. 2017, 8, 983. [Google Scholar] [CrossRef]
- Volarevic, V.; Djokovic, B.; Gazdic, M.J.; Harrell, R.; Fellabaum, C.; Djono, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 25. [Google Scholar] [CrossRef]
- Hosohata, K. Role of oxidative stress in drug-induced kidney injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.A.; da Cunha, A.D., Jr. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef]
- Ibrahim, A.; Al-Hizab, F.A.; Abushouk, A.I.; Abdel-Daim, M.M. Nephroprotective E_ects of Benzyl Isothiocyanate and Resveratrol Against Cisplatin-Induced Oxidative Stress and Inflammation. Front. Pharmacol. 2018, 9, 1268. [Google Scholar] [CrossRef]
- Qi, Z.; Li, Z.; Li, W.; Liu, Y.; Wang, C.; Lin, H.; Liu, J.; Li, P. Pseudoginsengenin DQ Exhibits Therapeutic E_ects in Cisplatin-Induced Acute Kidney Injury via Sirt1/NF-kappaB and Caspase Signaling Pathway without Compromising Its Antitumor Activity in Mice. Molecules 2018, 23, 3038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.F.; Han, X.Y.; Sun, Y.S.; Zhang, L.X.; Liu, W.; Liu, X.X.; Li, W.; Liu, Y.Y. Kidney Protection E_ect of Ginsenoside Re and Its Underlying Mechanisms on Cisplatin-Induced Kidney Injury. Cell. Physiol. Biochem. 2018, 48, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Yang, S.H.; Shim, H.; Young Cho, E.; Beom Kwon, K.; Hwan Kwak, T.; So, H.S. New Therapeutic Concept of NAD Redox Balance for Cisplatin Nephrotoxicity. BioMed Res. Int. 2016, 2016, 4048390. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, D.; Saha, P.P.; Marthala, H.; D’Silva, P. Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages. Cell Death Dis. 2014, 5, e1394. [Google Scholar] [CrossRef]
- Tang, C.; Dong, Z. Mitochondria in Kidney Injury: When the Power Plant Fails. J. Am. Soc. Nephrol. 2016, 27, 1869–1872. [Google Scholar] [CrossRef]
- Hall, A.M.; Schuh, C.D. Mitochondria as therapeutic targets in acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2016, 25, 355–362. [Google Scholar] [CrossRef]
- Oh, G.S.; Kim, H.J.; Choi, J.H.; Shen, A.; Choe, S.K.; Karna, A.; Lee, S.H.; Jo, H.J.; Yang, S.H.; Kwak, T.H.; et al. Pharmacological activation of NQO1 increases NAD(+) levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014, 85, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Perse, M.; Veceric-Haler, Z. Cisplatin-Induced Rodent Model of Kidney Injury: Characteristics and Challenges. BioMed Res. Int. 2018, 2018, 1462802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ramesh, G.; Norbury, C.C.; Reeves, W.B. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007, 72, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Horváth, B.; Kechrid, M.; Tanchian, G.; Rajesh, M.; Naura, A.S.; Boulares, A.H.; Pacher, P. Poly (ADP-ribose) polymerase-1 is a key mediator of cisplatin-induced kidney inflammation and injury. Free Radic. Biol. Med. 2011, 51, 1774–1788. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T. M1 macrophage triggered by Mincle leads to a deterioration of acute kidney injury. Kidney Int. 2017, 91, 526–529. [Google Scholar] [CrossRef]
- Summers, S.A.; Chan, J.; Gan, P.Y.; Dewage, L.; Nozaki, Y.; Steinmetz, O.M.; Nikolic-Paterson, D.J.; Kitching, A.R.; Holdsworth, S.R. Mast cells mediate acute kidney injury through the production of TNF. J. Am. Soc. Nephrol. 2011, 22, 2226–2236. [Google Scholar] [CrossRef]
- Soni, H.; Kaminski, D.; Gangaraju, R.; Adebiyi, A. Cisplatin-induced oxidative stress stimulates renal Fas ligand shedding. Ren. Fail. 2018, 40, 314–322. [Google Scholar] [CrossRef]
- Yang, L.; Brooks, C.R.; Xiao, S.; Sabbisetti, V.; Yeung, M.Y.; Hsiao, L.L.; Ichimura, T.; Kuchroo, V.; Bonventre, J.V. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J. Clin. Investig. 2015, 125, 1620–1636. [Google Scholar] [CrossRef]
- Endre, Z.H. Recovery from Acute Kidney Injury: The Role of Biomarkers. Nephron Clin. Pract. 2014, 127, 101–105. [Google Scholar] [CrossRef]
- Liu, X.; Guan, Y.; Xu, S.; Li, Q.; Sun, Y.; Han, R.; Jiang, C. Early Predictors of Acute Kidney Injury: A Narrative Review. Kidney Blood Press. Res. 2016, 41, 680–700. [Google Scholar] [CrossRef]
- Rizo-Topete, L.M.; Rosner, M.H.; Ronco, C. Acute Kidney Injury Risk Assessment and the Nephrology Rapid Response Team, Role of the Nephrologist in Multidisciplinary Management of AKI. Blood Purif. 2017, 43, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Kinoshita, K.; Hino, S.; Yano, T.; Niki, K.; Hirooka, Y.; Kishimoto, K.; Funauchi, M.; Matsumura, I. Signaling Rho-kinase mediates inflammation and apoptosis in T cells and renal tubules in cisplatin nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2015, 308, F899–F909. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.; Vence, L.M.; Salahudeen, A.K. Urinary tubular proteinbased biomarkers in the rodent model of cisplatin nephrotoxicity: A comparative analysis of serum creatinine, renal histology, and urinary KIM-1, NGAL, and NAG in the initiation, maintenance, and recovery phases of acute kidney injury. J. Investig. Med. 2013, 61, 564–568. [Google Scholar] [PubMed]
- Shinke, H.; Masuda, S.; Togashi, Y.; Ikemi, Y.; Ozawa, A.; Sato, T.; Kim, Y.H.; Mishima, M.; Ichimura, T.; Bonventre, J.V.; et al. Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother. Pharmacol. 2015, 76, 989–996. [Google Scholar] [CrossRef]
- Faig, J.; Haughton, M.; Taylor, R.C.; D’Agostino, R.B., Jr.; Whelen, M.J.; Rodriguez, K.A.; Bonomi, M.; Murea, M.; Porosnicu, M. Retrospective analysis of cisplatin nephrotoxicity in patients with head and neck Cancer receiving outpatient treatment with concurrent high-dose cisplatin and radiotherapy. Am. J. Clin. Oncol. 2018, 41, 432–440. [Google Scholar] [CrossRef]
- Prasaja, Y.; Sutandyo, N.; Andrajati, R. Incidence of cisplatin-induced nephrotoxicity and associated factors among cancer patients in Indonesia. Asian Pac. J. Cancer Prev. 2015, 16, 1117–1122. [Google Scholar] [CrossRef]
- Tekce, B.K.; Uyeturk, U.; Tekce, H.; Uyeturk, U.; Aktas, G.; Akkaya, A. Does the kidney injury molecule-1 predict cisplatin-induced kidney injury in early stage? Ann. Clin. Biochem. 2015, 52, 88–94. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Elmorsy, E.; Abdelwahab, H. Urinary biomarkers for early detection of platinum based drugs induced nephrotoxicity. BMC Nephrol. 2018, 19, 219. [Google Scholar] [CrossRef]
- Oh, S.M.; Park, G.; Lee, S.H.; Seo, C.S.; Shin, H.K.; Oh, D.S. Assessing the recovery from prerenal and renal acute kidney injury after treatment with single herbal medicine via activity of the biomarkers HMGB1, NGAL and KIM-1 in kidney proximal tubular cells treated by cisplatin with different doses and exposure times. BMC Complement. Altern. Med. 2017, 17, 544. [Google Scholar] [CrossRef]
- McMahon, K.R.; Rod Rassekh, S.; Schultz, K.R. Design and Methods of the Pan-Canadian Applying Biomarkers to Minimize Long-Term Effects of Childhood/Adolescent Cancer Treatment (ABLE) Nephrotoxicity Study: A Prospective Observational Cohort Study. Can. J. Kidney Health Dis. 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Salem, N.; Helmi, N.; Assaf, N. Renoprotective Effect of Platelet-Rich Plasma on Cisplatin-Induced Nephrotoxicity in Rats. Oxid. Med. Cell. Longev. 2018, 2018, 9658230. [Google Scholar] [CrossRef] [PubMed]
- Jun, D.Y.; Kim, S.Y.; Na, J.C.; Lee, H.H.; Kim, J.; Yoon, Y.E.; Hong, S.J.; Han, W.K. Tubular organotypic culture model of human kidney. PLoS ONE 2018, 13, e0206447. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Kaeslin, G.; Ranall, M.V. Evaluation of biomarkers for in vitro prediction of druginduced nephrotoxicity: Comparison of HK-2, immortalized human proximal tubule epithelial, and primary cultures of human proximal tubular cells. Pharmacol. Res. Perspect. 2015, 3, e00148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Grunz-Borgmann, E.A.; Parrish, A.R. Loss of _(E)-Catenin Potentiates Cisplatin-Induced Nephrotoxicity via Increasing Apoptosis in Renal Tubular Epithelial Cells. Toxicol. Sci. 2014, 141, 254–262. [Google Scholar] [CrossRef]
- Gardiner, L.; Akintola, A.; Chen, G.; Catania, J.M.; Vaidya, V.; Burghardt, R.C.; Bonventre, J.V.; Trzeciakowski, J.; Parrish, A.R. Structural equation modeling highlights the potential of Kim-1 as a biomarker for chronic kidney disease. Am. J. Nephrol. 2012, 35, 152–163. [Google Scholar] [CrossRef]
- Timothy, J.; Piantaa, B.; John, W.; Succar, P.L.; Chin, M.; Davidson, T.; Buckley, N.A.; Mohamed, F.; Endre, Z.H. Dexamethasone Modifies Cystatin C-Based Diagnosis of Acute Kidney Injury During Cisplatin-Based Chemotherapy. Kidney Blood Press. Res. 2017, 42, 62–75. [Google Scholar] [CrossRef]
- Meng, H.; Fu, G.; Shen, J.; Shen, K.; Xu, Z.; Wang, Y.; Jin, B.; Pan, H. Ameliorative Effect of Daidzein on Cisplatin-Induced Nephrotoxicity in Mice via Modulation of Inflammation, Oxidative Stress, and Cell Death. Oxid. Med. Cell. Longev. 2017, 2017, 3140680. [Google Scholar] [CrossRef]
- McDuffie, J.E.; Chen, Y.; Ma, J.Y.; Lee, K.M.; Lynch, D.M.; Hamlin, L.; Nguyen, M.; Rizzolio, M.; Soneed, M.; Snooka, S. Cisplatin nephrotoxicity in male beagle dogs: Next-generation protein kidney safety biomarker tissue expression and related changes in urine. Toxicol. Res. 2016, 5, 1202. [Google Scholar] [CrossRef]
- Nishihara, K.; Masuda, S.; Shinke, H.; Ozawa, A.; Ichimura, T.; Yonezawa, A.; Nakagawa, S.; Inui, K.; Bonventre, J.V.; Matsubara, K. Urinary chemokine (C-C motif) ligand 2 (monocyte chemotactic protein-1) as a tubular injury marker for early detection of cisplatin-induced nephrotoxicity. Biochem. Pharmacol. 2013, 15, 570–582. [Google Scholar] [CrossRef]
- DesRochers, T.M.; Suter, L.; Roth, A.; Kaplan, D.L. Bioengineered 3D Human Kidney Tissue, a Platform for the Determination of Nephrotoxicity. PLoS ONE 2013, 8, e59219. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Franczyk, B.; Jabłonowski, Z.; Ciałkowska-Rysz, A. Novel Biomarkers in the Diagnosis of Chronic Kidney Disease and the Prediction of Its Outcome. Int. J. Mol. Sci. 2017, 18, 1702. [Google Scholar] [CrossRef] [PubMed]
- Vinken, P.; Starckx, S.; Barale-Thomas, E.; Looszova, A.; Sonee, M.; Goeminne, N.; Vermissen, L.; Buyens, K.; Lampo, A. Tissue Kim-1and urinary clusterin as early indicators of cisplatin-induced acute kidney injury in rats. Toxicol. Pathol. 2012, 40, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Hany, H.; Araba, B.; Samir, A.; Salamaa, C.; Maghrabi, I.A. Camel Milk Ameliorates 5-Fluorouracil- Induced Renal Injury in Rats: Targeting MAPKs, NF-κB and PI3K/Akt/eNOS Pathways. Cell. Physiol. Biochem. 2018, 46, 1628–1642. [Google Scholar] [CrossRef]
- Abdelrahman, A.M.; Al Suleimani, Y.; Shalaby, A.; Ashique, M.; Manoj, P.; Al-Saadi, H.; Ali, B.H. Effect of levosimendan, a calcium sensitizer, on cisplatin-induced nephrotoxicity in rats. Toxicol. Rep. 2019, 6, 232–238. [Google Scholar] [CrossRef]
- Sharp, C.N.; Siskind, L.J. Developing better mouse models to study cisplatin-induced kidney injury. Am. J. Physiol. Ren. Physiol. 2017, 313, F835–F841. [Google Scholar] [CrossRef]
- Ali, B.H.; Abdelrahman, A.M.; Al-Salam, S.; Sudhadevi, M.; AlMahruqi, A.S.; Al-Husseni, I.S.; Beegam, S.; Dhanasekaran, S.; Nemmar, A.; Al-Moundhri, M. The effect of sildenafil on cisplatin nephrotoxicity in rats. Basic Clin. Pharmacol. Toxicol. 2011, 109, 300–308. [Google Scholar] [CrossRef]
- Al Suleimani, Y.M.; Abdelrahman, A.M.; AlMahruqi, A.S.; Alhseini, I.S.; Tageldin, M.H.; Mansour, M.E.; Ali, B.H. Interaction of nimesulide, a cyclooxygenase- 2 inhibitor, with cisplatin in normotensive and spontaneously hypertensive rats. Food Chem. Toxicol. 2010, 48, 139–144. [Google Scholar] [CrossRef]
- Khames, A.; Khalaf, M.M.; Gad, A.M.; Abd El-Raouf, O.M.; Kandeil, M.A. Nicorandil combats doxorubicin-induced nephrotoxicity via amendment of TLR4/P38 MAPK/NFκ-B signaling pathway. Chem. Biol. Interact. 2019, 311, 108777. [Google Scholar] [CrossRef]
- Khan, T.H.; Ganaie, M.A.; Alharthy, K.M.; Madkhali, H.; Jan, B.L.; Sheikh, I.A. Naringenin prevents doxorubicin-induced toxicity in kidney tissues by regulating the oxidative and inflammatory insult in Wistar rats. Arch. Physiol. Biochem. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Rashid, S.; Ali, N.; Nafees, S.; Hasan, S.K.; Sultana, S. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem. Toxicol. 2014, 66, 185–193. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ford, G.M.; Waikar, S.S.; Wang, Y.; Clement, M.B.; Ramirez, V.; Glaab, W.E.; Troth, S.P.; Bonventre, J.V. A rapid urine test for early detection of kidney injury. Kidney Int. 2009, 76, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sabbisetti, V.S.; Ito, K.; Wang, C.; Mefferd, S.C.; Bonventre, J.V. Novel Assays for Detection of Urinary KIM-1 in Mouse Models of Kidney Injury. Toxicol. Sci. 2013, 131, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Abdallah, D.M.; El-Abhar, H.S. Cilostazol Renoprotective Effect: Modulation of PPAR-c, NGAL, KIM-1 and IL-18 Underlies Its Novel Effect in a Model of Ischemia-Reperfusion. PLoS ONE 2014, 9, e95313. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, L.; Sun, W.; Tian, J.; Guo, H. Targeted Silencing of Kim-1 Inhibits the Growth of Clear Cell Renal Cell Carcinoma Cell Line 786-0 In Vitro and in Vivo. Oncol. Res. 2018, 26, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Hayati, F.; Hossainzadeh, M.; Shayanpour, S.; Abedi-Gheshlaghi, Z.; Beladi Mousavi, S.S. Prevention of cisplatin nephrotoxicity. J. Nephropharmacol. 2016, 5, 57–60. [Google Scholar] [PubMed]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Baradaran, A.; Rafieian, M. Plants antioxidants: From laboratory to clinic. J. Nephropathol. 2013, 2, 152–153. [Google Scholar] [CrossRef]
- Hemati, S.; Arbab Jolfaie, N.; Rafienia, M.; Ghavamnasiri, M. The effects of vitamin E and selenium on cisplatininduced nephrotoxicity in cancer patients treated with cisplatin-based chemotherapy: A randomized, placebo-controlled study. J. Res. Med. Sci. 2012, 17, S49–S58. [Google Scholar]
- Abdel-Daim, M.M.; Abushouk, A.I.; Donia, T.; Alarifi, S.; Alkahtani, S.; Aleya, L.; Bungau, S.G. The nephroprotective effects of allicin and ascorbic acid against cisplatin-induced toxicity in rats. Environ. Sci. Pollut. Res. 2019, 26, 13502. [Google Scholar] [CrossRef]
- Ridzuan, N.R.; Rashid, N.A.; Othman, F.; Budin, S.B.; Hussan, F.; Teoh, S.L. Protective Role of Natural Products in Cisplatin-Induced Nephrotoxicity. Mini Rev. Med. Chem. 2019, 19, 1134. [Google Scholar] [CrossRef]
- Cascella, M.; Palma, G.; Barbieri, A.; Bimonte, S.; Amruthraj, N.J.; Muzio, M.R.; Del Vecchio, V.; Rea, D.; Falco, M.; Luciano, A.; et al. Role of Nigella sativa and Its Constituent Thymoquinone on Chemotherapy-Induced Nephrotoxicity: Evidences from Experimental Animal Studies. Nutrients 2017, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, S.; Khajavi Rad, A.; Hadjzadeh, M.A.R.; Mohamadian Roshan, N.; Havakhah, S.; Shafiee, S. The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats. Avicenna J. Phytomed. 2016, 6, 44–54. [Google Scholar]
- Santos, N.A.; Bezerra, C.S.; Martins, N.M.; Curti, C.; Bianchi, M.L.; Santos, A.C. Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemother. Pharmacol. 2008, 61, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.D.; Gu, R.; Pierce, C.; Kil, J. Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear. Res. 2005, 201, 81–89. [Google Scholar] [CrossRef]
- Hensley, M.L.; Hagerty, K.L.; Kewalramani, T.; Green, D.M.; Meropol, N.J.; Wasserman, T.H.; Cohen, G.I.; Emami, B.; Gradishar, W.J.; Mitchell, R.B.; et al. American Society of Clinical Oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2009, 27, 127–145. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Zadeh, M.H.; Shahbazian, H.; Khanzadeh, A.; Hayati, F.; Ghorbani, A.; Golzari, K.; Valavi, E.; Motemednia, F.; Mousavi, M.B. The protective effect of theophylline in cisplatin nephrotoxicity. Saudi J. Kidney Dis. Transpl. 2014, 25, 333–337. [Google Scholar] [CrossRef]
- Katsuda, H.; Yamashita, M.; Katsura, H.; Yu, J.; Waki, Y.; Nagata, N.; Miyamoto, K. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumoractivity. Biol. Pharm. Bull. 2010, 33, 1867–1871. [Google Scholar] [CrossRef]
- Mercantepe, F.; Mercantepe, T.; Topcu, A.; Ylmaz, A.; Tumkaya, L. Protective effects of amifostine, curcumin, and melatonin against cisplatin- induced acute kidney injury Naunyn-Schmiedeberg’s. Arch. Pharmacol. 2018, 391, 915. [Google Scholar] [CrossRef]
- Wei, L.; Chen, W.; Zou, Y.; Huang, H.; Pan, B.; Jin, S.; Huang, R.; Nie, S.; Kong, G. AMP-activated protein kinase regulates autophagic protection against cisplatin-induced tissue injury in the kidney. Genet. Mol. Res. 2015, 14, 12006–12015. [Google Scholar] [CrossRef] [PubMed]
- Gaião, S.M.; Paiva, J.A.O.D.C. Biomarkers of renal recovery after acute kidney injury. Rev. Bras. Ter. Intensiva 2017, 29, 373–381. [Google Scholar] [CrossRef]

| Chemotherapy Agents | Therapeutic Doses | Administration Time and Detection | Increased Detection of Serum/Urine/Immunostaining | References |
|---|---|---|---|---|
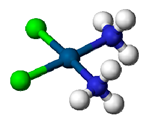
| Cisplatin (Cl2H6N2P) | Dose ranging from 0.01 mM to 100 mM | KIM-1 detection after 2 weeks | ↑ KIM-1, ↑NGAL | [137] |
 | 6 mg/kg | 3rd day | ↑ KIM-1, clusterin | [24] |
| 1 mg/kg/day | 3rd day | ↑ KIM-1, clusterin | [139] | |
| 6 mg/kg (1 mg/mL) | 3rd day | ↑ KIM-1, clusterin, plasma cystatin | [133] | |
| One dose: 6 mg/kg | 7th day and 10th day | ↑ NGAL ↑ urinary NAG, ↑ KIM-1 | [141] | |
| 50 mg/m2 | 2nd and 3rd day | ↑ KIM-1, NGAL and cystatin | [125] | |
| 100 µmol/L | 3rd day | ↑ KIM-1, ↑NGAL | [130] | |
| 5 μM | 24 h | ↑ KIM-1, ↑NGAL | [129] | |
| 5 mg/kg | 2nd day | ↑KIM-1 | [136] | |
| 400 μM during 6 h or 10 μM during 1 day | 6, 24, 48 h | ↑ NGAL, ↑KIM-1, ↑HMGB1 | [126] | |
| 80 mg/m2, 125 mg/body | 7th day | ↑ KIM-1, ↑ MCP-1 ↑ NGAL | [127] | |
| Doxorubicin (C27H29NO11) | 0.001 mM to 0.2 mM | 0, 3, 7, 10, 14 day | ↑KIM-1 ↑NGAL | [137] |
| 20 mg/kg | 20th day | ↑ KIM-1 | [145] | |
| Fluorouracil (5-FU) (C4H3FN2O2) | 50 mg/kg/day | 17th–21st day | ↑ KIM-1, ↑ NGAL | [140] |
| 150 mg/kg | 21st day | ↑ KIM-1 | [147] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. https://doi.org/10.3390/ijms20205238
Tanase DM, Gosav EM, Radu S, Costea CF, Ciocoiu M, Carauleanu A, Lacatusu CM, Maranduca MA, Floria M, Rezus C. The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity. International Journal of Molecular Sciences. 2019; 20(20):5238. https://doi.org/10.3390/ijms20205238
Chicago/Turabian StyleTanase, Daniela Maria, Evelina Maria Gosav, Smaranda Radu, Claudia Florida Costea, Manuela Ciocoiu, Alexandru Carauleanu, Cristina Mihaela Lacatusu, Minela Aida Maranduca, Mariana Floria, and Ciprian Rezus. 2019. "The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity" International Journal of Molecular Sciences 20, no. 20: 5238. https://doi.org/10.3390/ijms20205238
APA StyleTanase, D. M., Gosav, E. M., Radu, S., Costea, C. F., Ciocoiu, M., Carauleanu, A., Lacatusu, C. M., Maranduca, M. A., Floria, M., & Rezus, C. (2019). The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity. International Journal of Molecular Sciences, 20(20), 5238. https://doi.org/10.3390/ijms20205238