Present Scenario of Bioconjugates in Cancer Therapy: A Review
Abstract
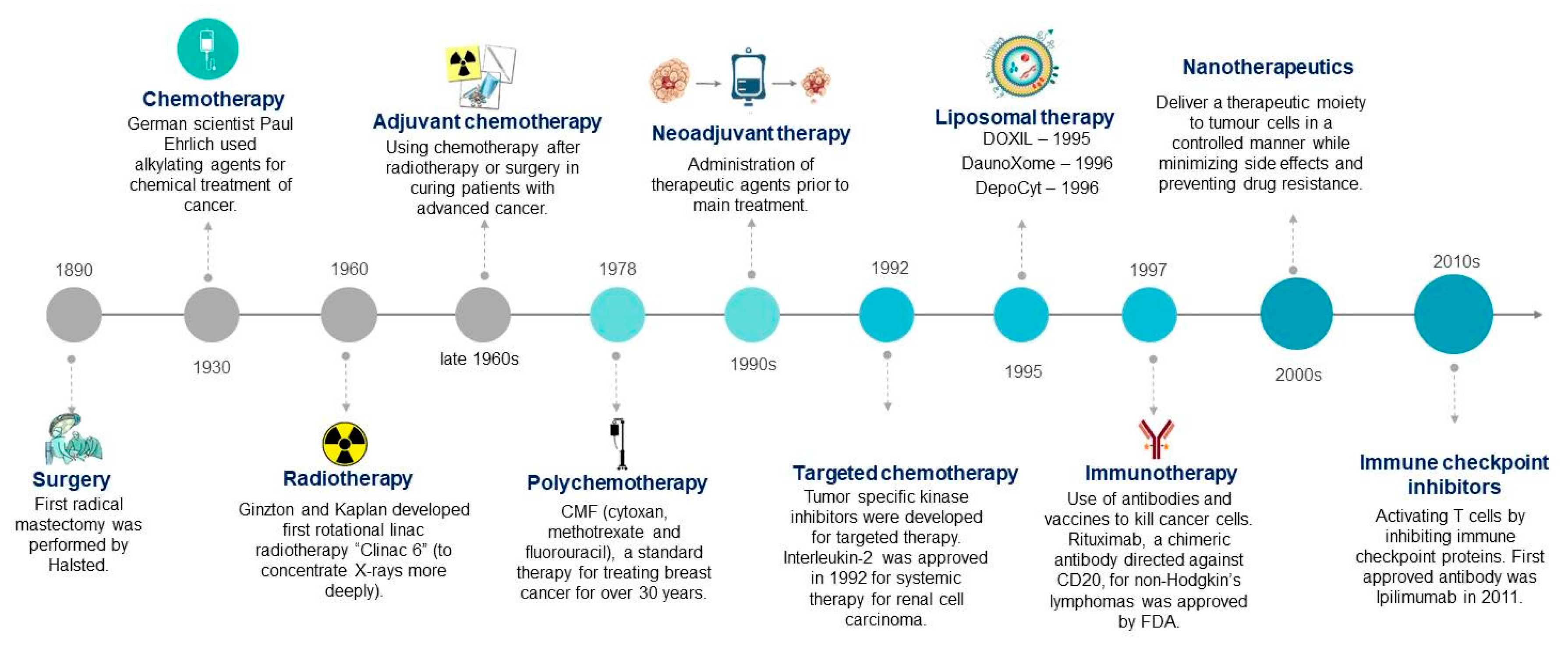
1. Introduction
- Ligand receptors (targeted anticancers)—antibodies, aptamers, and peptides
- Anticancer agents—peptides, glycoproteins, interferons, and biosurfactants
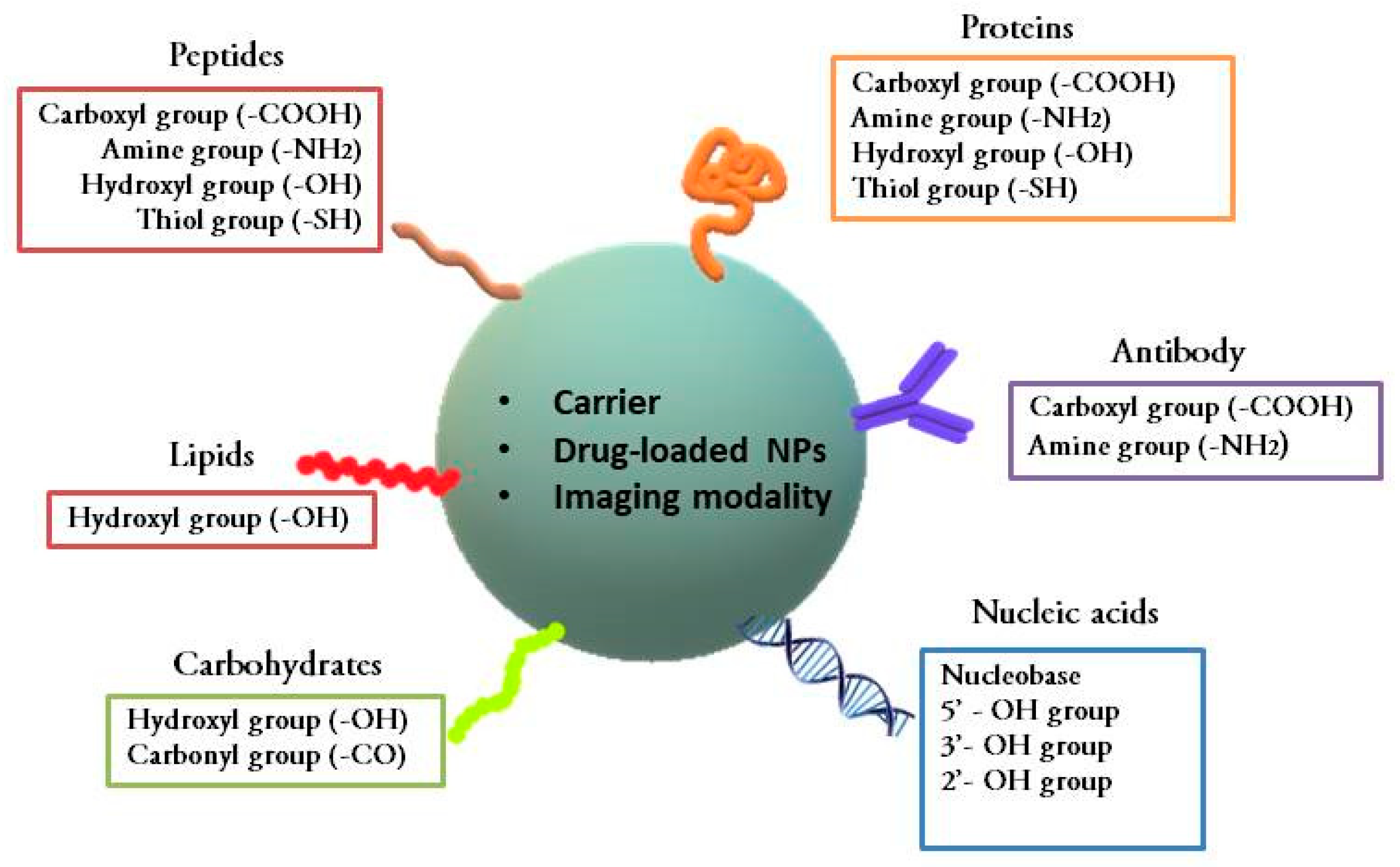
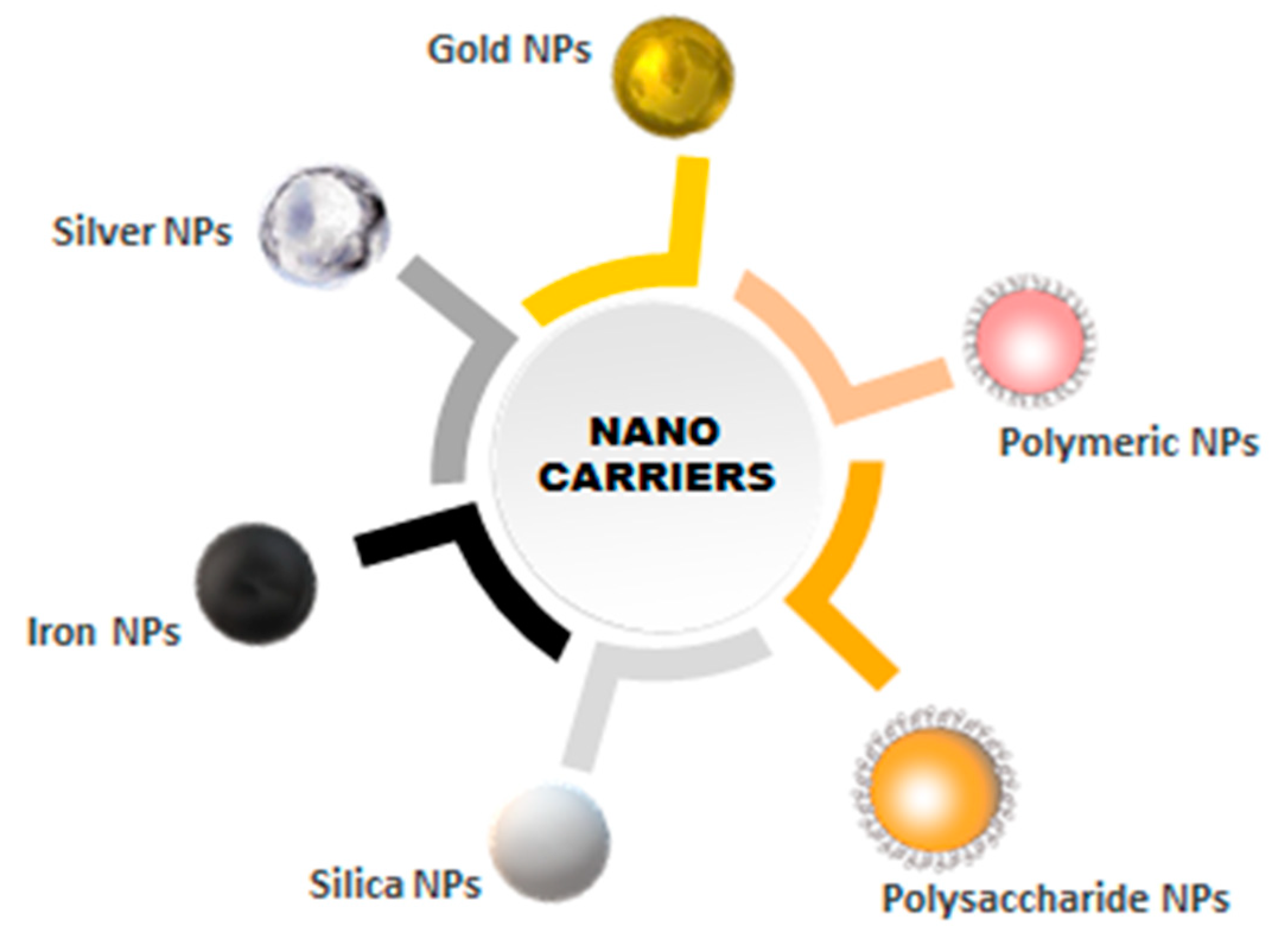
2. Nanomaterials as Carriers of Biomolecules in Conjugates
2.1. Inorganic Nanocarriers
2.1.1. Metallic Nanoparticles
Gold Nanoparticles (GNPs)
Silver Nanoparticles (AgNPs)
Magnetic Nanoparticles
2.1.2. Silica Nanoparticles
2.2. Organic Nanocarriers
2.2.1. Polymeric Nanoparticles
2.2.2. Polysaccharides
3. Combination Therapy Via Bioconjugates
Nucleic Acid or Aptamer-Based Therapeutic Agents
4. Nanotoxicity of Nanocarriers Used in Bioconjugates
5. Fate of Newly Developed Bioconjugates
6. Conclusion and Future Perspectives
Funding
Conflicts of Interest
References
- All Cancers Fact Sheet, GLOBOCAN 2018. The Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 31 March 2019).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- Cameron, F.; Whiteside, G.; Perry, C. Ipilimumab: First global approval. Drugs 2011, 71, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.M.; Butow, P.N.; Coates, A.S.; Childs, A.M.; Ellis, P.M.; Dunn, S.M.; Tattersall, M.H.N. On the receiving end V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann. Oncol. 1996, 7, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Remesh, A. Toxicities of anticancer drugs and its management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef]
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of Cancer Therapy: Oncogene and Non-oncogene Addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef]
- Sinha, R. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef]
- Salmaso, S.; Caliceti, P. Chapter 11—Peptide and Protein Bioconjugation: A Useful Tool to Improve the Biological Performance of Biotech Drugs. Peptide and Protein Delivery; Academic Press: Cambridge, MA, USA, 2011; pp. 247–290. ISBN 9780123849359. [Google Scholar]
- Werengowska-CieTwierz, K.; Wisniewski, M.; Terzyk, A.P.; Furmaniak, S. The Chemistry of Bioconjugation in Nanoparticles-Based Drug Delivery System. Adv. Condens. Matter Phys. 2015, 27, 198175. [Google Scholar]
- Du, W.; Yuan, Y.; Wang, L.; Cui, Y.; Wang, H.; Xu, H.; Liang, G. Multifunctional Bioconjugate for Cancer Cell-Targeted Theranostics. Bioconjugate Chem. 2015, 26, 2571–2578. [Google Scholar] [CrossRef]
- Hermanson, G.T. Chapter 1—Introduction to Bioconjugation. In Bioconjugate Tech., 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1–25. ISBN 9780123822390. [Google Scholar]
- Fernandes, C.S.M.; Teixeira, G.D.G.; Iranzo, O.; Roque, A.C.A. Chapter 5—Engineered Protein Variants for Bioconjugation. In Biomedical Applications of Functionalized Nanomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 105–138. ISBN 9780323508780. [Google Scholar]
- She, X.; Chen, L.; Velleman, L.; Li, C.; Zhu, H.; He, C.; Wang, T.; Shigdar, S.; Duan, W.; Kong, L. Fabrication of high specificity hollow mesoporous silica nanoparticles assisted by Eudragit for targeted drug delivery. J. Colloid Interface Sci. 2015, 445, 151–160. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hassanzadeh, F.; Aliabadi, H.S.; Khoraskani, F.R.; Mirian, M.; Behdadfar, B. Targeted delivery of doxorubicin to breast cancer cells by magnetic LHRH chitosan bioconjugated nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gupta, D.; Singh, G.; Sharma, S.; Bhat, M.; Prashant, C.K.; Dinda, A.K.; Kharbanda, S.; Kufe, D.; Singh, H. Novel polymeric nanoparticles for intracellular delivery of peptide cargos: Antitumor efficacy of the BCL-2 conversion peptide NuBCP-9. Cancer Res. 2014, 74, 3271–3281. [Google Scholar] [CrossRef] [PubMed]
- Szweda, R.; Trzebicka, B.; Dworak, A.; Otulakowski, L.; Kosowski, D.; Hertlein, J.; Haladjova, E.; Rangelov, S.; Szweda, D. Smart Polymeric Nanocarriers of Met-enkephalin. Biomacromolecules 2016, 17, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.G.C.; Robles Botero, V.; Martínez, E.S.M.; Gómez García, C.; Hinestroza, J.P. Soybean agglutinin-conjugated silver nanoparticles nanocarriers in the treatment of breast cancer cells. J. Biomater. Sci. Polym. Ed. 2016, 27, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Perepelyuk, M.; Maher, C.; Lakshmikuttyamma, A.; Shoyele, S.A. Aptamer-hybrid nanoparticle bioconjugate efficiently delivers miRNA-29b to non-small-cell lung cancer cells and inhibits growth by downregulating essential oncoproteins. Int. J. Nanomed. 2016, 11, 3533–3544. [Google Scholar]
- Tao, W.; Zeng, X.; Wu, J.; Zhu, X.; Yu, X.; Zhang, X.; Zhang, J.; Liu, G.; Mei, L. Polydopamine-based surface modification of novel nanoparticle-aptamer bioconjugates for in vivo breast cancer targeting and enhanced therapeutic effects. Theranostics 2016, 6, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Montagner, I.M.; Merlo, A.; Carpanese, D.; Dalla Pietà, A.; Mero, A.; Grigoletto, A.; Loregian, A.; Renier, D.; Campisi, M.; Zanovello, P.; et al. A site-selective hyaluronan-interferonα2a conjugate for the treatment of ovarian cancer. J. Control. Release 2016, 236, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, G.; Patel, D.; Stephens, D.; Gobin, A.M. Targeted cancer therapy by immunoconjugated gold-gold sulfide nanoparticles using protein g as a cofactor. Ann. Biomed. Eng. 2012, 40, 2131–2139. [Google Scholar] [CrossRef]
- Kaluzova, M.; Bouras, A.; Machaidze, R.; Hadjipanayis, C.G. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget 2015, 6, 8788–8806. [Google Scholar] [CrossRef]
- Wang, A.Z.; Bagalkot, V.; Vasilliou, C.C.; Gu, F.; Alexis, F.; Zhang, L.; Shaikh, M.; Yuet, K.; Cima, M.J.; Langer, R.; et al. Superparamagnetic Iron Oxide Nanoparticle–Aptamer Bioconjugates for Combined Prostate Cancer Imaging and Therapy. ChemMedChem 2008, 3, 1311–1315. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jeong, Y.Y.; Moon, W.K.; Jon, S. Integrin-targeting thermally cross-linked superparamagnetic iron oxide nanoparticles for combined cancer imaging and drug delivery. Nanotechnology 2010, 21, 415102. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Bioconjugated gold nanoparticles enhance cellular uptake: A proof of concept study for siRNA delivery in prostate cancer cells. Int. J. Pharm. 2016, 509, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, J.; Liu, T.; Yang, X. Anti-c-met antibody bioconjugated with hollow gold nanospheres as a novel nanomaterial for targeted radiation ablation of human cervical cancer cell. Oncol. Lett. 2017, 14, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and Pgp siRNA to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. pH-Sensitive Delivery Vehicle Based on Folic Acid-Conjugated Polydopamine-Modified Mesoporous Silica Nanoparticles for Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar] [CrossRef] [PubMed]
- Le Trinh, T.; Zhu, G.; Xiao, X.; Puszyk, W.; Sefah, K.; Wu, Q.; Tan, W.; Liu, C. A Synthetic Aptamer-Drug Adduct for Targeted Liver Cancer Therapy. PLoS ONE 2015, 10, e0136673. [Google Scholar] [CrossRef]
- Jalalian, S.H.; Taghdisi, S.M.; Hamedani, N.S.; Kalat, S.A.M.; Lavaee, P.; ZandKarimi, M.; Ghows, N.; Jaafari, M.R.; Naghibi, S.; Danesh, N.M.; et al. Epirubicin loaded super paramagnetic iron oxide nanoparticle-aptamer bioconjugate for combined colon cancer therapy and imaging in vivo. Eur. J. Pharm. Sci. 2013, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Cultrara, C.N.; Kozuch, S.D.; Patel, M.R.; Ramos, J.A.; Samuni, U.; Zilberberg, J.; Sabatino, D. Direct Transfection of Fatty Acid Conjugated siRNAs and Knockdown of the Glucose-Regulated Chaperones in Prostate Cancer Cells. Bioconjug. Chem. 2018, 29, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, X.; Su, L.; Xia, H.; Gu, G.; Pang, Z.; Jiang, X.; Yao, L.; Chen, J.; Chen, H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef]
- Dhankar, R.; Jain, A.K.; Arora, S.; Rath, G.; Goyal, A.K.; Rathee, P.; Kumar, M.S.; Saxena, A.K.; Sharma, P.R.; Kumar, S.; et al. HER-2 targeted immunonanoparticles for breast cancer chemotherapy. J. Appl. Pharm. Sci. 2011, 1, 132–139. [Google Scholar]
- Bonzi, G.; Salmaso, S.; Scomparin, A.; Eldar-Boock, A.; Satchi-Fainaro, R.; Caliceti, P. Novel Pullulan Bioconjugate for Selective Breast Cancer Bone Metastases Treatment. Bioconjug. Chem. 2015, 26, 489–501. [Google Scholar] [CrossRef]
- Li, M.; Tang, Z.; Zhang, Y.; Lv, S.; Li, Q.; Chen, X. Targeted delivery of cisplatin by LHRH-peptide conjugated dextran nanoparticles suppresses breast cancer growth and metastasis. Acta Biomater. 2015, 18, 132–143. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Carvalho, S.M.; Lobato, Z.I.P.; Leite, M.D.F.; Armando da Silva Cunha, J.; Mansur, H.S. Design and Development of Polysaccharide-Doxorubicin-Peptide Bioconjugates for Dual Synergistic E ff ects of Integrin-Targeted and Cell-Penetrating Peptides for Cancer Chemotherapy. Bioconjug. Chem. 2018, 29, 1973–2000. [Google Scholar] [CrossRef] [PubMed]
- Biabanikhankahdani, R.; Ho, K.; Alitheen, N.; Tan, W. A Dual Bioconjugated Virus-Like Nanoparticle as a Drug Delivery System and Comparison with a pH-Responsive Delivery System. Nanomaterials 2018, 8, 236. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, D.; He, W.; Zhang, H.; Li, Z.; Luan, Y. Nanoassemblies from amphiphilic cytarabine prodrug for leukemia targeted therapy. J. Colloid Interface Sci. 2017, 487, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Q.; Jiang, J.; Luo, Y.; Sun, Z. A conjugate of methotrexate and an analog of luteinizing hormone releasing hormone shows increased efficacy against prostate cancer. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sangtani, A.; Petryayeva, E.; Susumu, K.; Oh, E.; Huston, A.L.; Lasarte-aragones, G.; Medintz, I.L.; Algar, W.R.; Delehanty, J.B. Nanoparticle-Peptide-Drug Bioconjugates for Unassisted Defeat of Multidrug Resistance in a Model Cancer Cell Line. Bioconjug. Chem. 2019, 30, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Singh, J.; Kumari, U.; Kaur, I.P.; Barnwal, R.P.; Kumar, R.; Singh, S.; Singh, G.; Chatterjee, M. Development of biosurfactant-based graphene quantum dot conjugate as a novel and fluorescent theranostic tool for cancer. Int. J. Nanomed. 2019, 14, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.Y.; Liu, S.Z. A simple and general method for preparing antibody-PEG-PLGA sub-micron particles using electrospray technique: An in vitro study of targeted delivery of cisplatin to ovarian cancer cells. Colloids Surf. B Biointerfaces 2014, 117, 346–353. [Google Scholar] [CrossRef]
- Schuster, S.; Biri-Kovács, B.; Szeder, B.; Buday, L.; Gardi, J.; Szabó, Z.; Halmos, G.; Mezo, G. Enhanced In Vitro Antitumor Activity of GnRH-III-Daunorubicin Bioconjugates Influenced by Sequence Modification. Pharmaceutics 2018, 10, 223. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of Atp-Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Coley, H.M. Overcoming Multidrug Resistance in Cancer: An Update on the Clinical Strategy of Inhibiting P-Glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lelle, M.; Freidel, C.; Kaloyanova, S.; Tabujew, I.; Schramm, A.; Musheev, M.; Niehrs, C.; Müllen, K.; Peneva, K. Overcoming drug resistance by cell-penetrating peptide-mediated delivery of a doxorubicin dimer with high DNA-binding affinity. Eur. J. Med. Chem. 2017, 130, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yu, D.; Jia, B.; Jin, C.; Liu, X.; Zhao, X.; Zhang, G. Targeting CD133 + laryngeal carcinoma cells with chemotherapeutic drugs and siRNA against ABCG2 mediated by thermo/pH-sensitive mesoporous silica nanoparticles. Tumor Biol. 2016, 37, 2209–2217. [Google Scholar] [CrossRef]
- Sangtani, A.; Petryayeva, E.; Wu, M.; Susumu, K.; Oh, E.; Huston, A.L.; Lasarte-aragones, G.; Medintz, I.L.; Algar, W.R.; Delehanty, J.B. Intracellularly Actuated Quantum Dot-Peptide-Doxorubicin Nanobioconjugates for Controlled Drug Delivery via the Endocytic Pathway. Bioconjug. Chem. 2018, 29, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2016, 12, 252–264. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Previously Untreated Melanoma. N. Engl. J. Med. 2017, 373, 23–34. [Google Scholar] [CrossRef]
- Ishihara, J.; Fukunaga, K.; Ishihara, A.; Larsson, H.M.; Potin, L.; Hosseinchi, P.; Galliverti, G.; Swartz, M.A.; Hubbell, J.A. Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events. Sci. Transl. Med. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Sasaki, K.; Lee, S.S.-Y.; Williford, J.M.; Yasui, M.; Abe, H.; Potin, L.; Hosseinchi, P.; Fukunaga, K.; et al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci. Transl. Med. 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Olivo, M.; Dinish, U.S.; Goh, D.; Kong, K.V.; Yong, K.T. Engineering bioconjugated gold nanospheres and gold nanorods as label-free plasmon scattering probes for ultrasensitive multiplex dark-field imaging of cancer cells. J. Biomed. Nanotechnol. 2013, 9, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chandrasekaran, N.; Mukherjee, A.; Kumar, M.; Kumaraguru, A.K. Cancerous cell targeting and destruction using pH stabilized amperometric bioconjugated gold nanoparticles from marine macroalgae, Padina gymnospora. Bioprocess Biosyst. Eng. 2014, 37, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yong, K.T.; Roy, I.; Hu, R.; Ding, H.; Zhao, L.; Swihart, M.T.; He, G.S.; Cui, Y.; Prasad, P.N. Additive controlled synthesis of gold nanorods (GNRs) for two-photon luminescence imaging of cancer cells. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Fixler, D.; Ankri, R.; Kaplan, I.; Novikov, I.; Hirshberg, A. Diffusion reflection: A novel method for detection of oral cancer. J. Dent. Res. 2014, 93, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Bickford, L.R.; Agollah, G.; Drezek, R.; Yu, T.K. Silica-gold nanoshells as potential intraoperative molecular probes for HER2-overexpression in ex vivo breast tissue using near-infrared reflectance confocal microscopy. Breast Cancer Res. Treat. 2010, 120, 547–555. [Google Scholar] [CrossRef]
- Retnakumari, A.; Setua, S.; Menon, D.; Ravindran, P.; Muhammed, H.; Pradeep, T.; Nair, S.; Koyakutty, M. Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology 2010, 21, 055103. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. Poly(Ethylene Glycol)- Coated Gold Nanocages Bioconjugated with [Nle4,D-Phe7]-α-Melanotropin-Stimulating Hormone. 2011. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21755635 (accessed on 14 July 2011).
- Day, E.S.; Bickford, L.R.; Slater, J.H.; Riggall, N.S.; Drezek, R.A.; West, J.L. Antibody-conjugated gold-gold sulfide nanoparticles as multifunctional agents for imaging and therapy of breast cancer. Int. J. Nanomed. 2010, 5, 445–454. [Google Scholar] [CrossRef]
- Rana, S.; Yeh, Y.-C.; Rotello, V.M. Engineering the Nanoparticle-Protein Interface: Applications and Possibilities. Curr. Opin. Chem. Biol. 2010, 14, 828–834. [Google Scholar] [CrossRef]
- Mocan, L.; Matea, C.; Tabaran, F.A.; Mosteanu, O.; Pop, T.; Puia, C.; Agoston-Coldea, L.; Zaharie, G.; Mocan, T.; Buzoianu, A.D.; et al. Selective ex vivo photothermal nano-therapy of solid liver tumors mediated by albumin conjugated gold nanoparticles. Biomaterials 2017, 119, 33–42. [Google Scholar] [CrossRef]
- Diem, P.H.N.; Thao, D.T.T.; Van Phu, D.; Duy, N.N.; Quy, H.T.D.; Hoa, T.T.; Hien, N.Q. Synthesis of Gold Nanoparticles Stabilized in Dextran Solution by Gamma Co-60 Ray Irradiation and Preparation of Gold Nanoparticles/Dextran Powder. J. Chem. 2017, 2017, 6836375. [Google Scholar] [CrossRef]
- Spadavecchia, J.; Movia, D.; Moore, C.; Maguire, C.M.; Moustaoui, H.; Casale, S.; Volkov, Y.; Prina-mello, A. Targeted polyethylene glycol gold nanoparticles for the treatment of pancreatic cancer: From synthesis to proof-of-concept in vitro studies. Int. J. Nanomed. 2016, 11, 791–822. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sacco, P.; Marsich, E.; Furlani, F.; Arib, C.; Djaker, N.; de la Chapelle, M.L.; Donati, I.; Spadavecchia, J. Lactose-Modified Chitosan Gold(III)-PEGylated Complex-Bioconjugates: From Synthesis to Interaction with Targeted Galectin-1 Protein. Bioconjug. Chem. 2018, 29, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis Paradigm and Applications of Silver Nanoparticles (AgNPs), A Review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials 2018, 8, 319. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, K.L.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jun, Y.W.; Yeon, S.I.; Kim, H.C.; Shin, J.S.; Cheon, J. Biocompatible heterostructured nanoparticles for multimodal biological detection. J. Am. Chem. Soc. 2006, 128, 15982–15983. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J. Neurooncol. 2015, 124, 13–22. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef]
- Caltagirone, C.; Bettoschi, A.; Garau, A.; Montis, R. Silica-based nanoparticles: A versatile tool for the development of efficient imaging agents. Chem. Soc. Rev. 2015, 44, 4645–4671. [Google Scholar] [CrossRef]
- US Food and Drug Administration GRAS Substances (SCOGS) Database—Select Committee on GRAS Substances (SCOGS) Opinion: Silicates. Available online: http://wayback.archive-it.org/7993/20171031063508/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm260849.htm (accessed on 2 September 1979).
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Ji, X.; He, X.; Yin, Q.; Zhang, Z.; Shi, J.; Li, Y. Controlled Intracellular Release of Doxorubicin in Multidrug-Resistant Cancer Cells by Tuning the Shell-Pore Sizes of Mesoporous Silica Nanoparticles. Am. Chem. Soc. 2011, 5, 9788–9798. [Google Scholar] [CrossRef]
- Duncan, R.; Vicent, M.J. Polymer therapeutics-prospects for 21st century: The end of the beginning. Adv. Drug Deliv. Rev. 2013, 65, 60–70. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef]
- Quesnel, R.; Hildgen, P. Synthesis of PLA-b-PEG multiblock copolymers for stealth drug carrier preparation. Molecules 2005, 10, 98–104. [Google Scholar] [CrossRef]
- Lupold, S.E.; Hicke, B.J.; Lin, Y.; Coffey, D.S. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002, 62, 4029–4033. [Google Scholar]
- Farokhzad, O.; Jon, S.; Khadelmhosseini, A.; Tran, T.; LaVan, D.; Langer, R. Nanopartideaptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef]
- Farokhzad, O.; Cheng, J.; Teply, B.; Sherifi, I.; Jon, S.; Kantoff, P.; Richie, J.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbauma, E.; Moreno, A.F.R.; Langer, R.; Farokhzad, O.C. Formulation of Functionalized PLGA-PEG Nanoparticles for In Vivo Targeted Drug Delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lipparda, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef]
- Dhar, S.; Kolishetti, N.; Lippard, S.J.; Farokhzad, O.C. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 1850–1855. [Google Scholar] [CrossRef]
- Hami, Z.; Amini, M.; Ghazi-Khansari, M.; Rezayat, S.M.; Gilani, K. Doxorubicin-conjugated PLA-PEG-Folate based polymeric micelle for tumor-targeted delivery: Synthesis and in vitro evaluation. DARU J. Pharm. Sci. 2014, 22, 1–7. [Google Scholar] [CrossRef]
- Quester, K.; Juarez-Moreno, K.; Secundino, I.; Roseinstein, Y.; Alejo, K.P.; Huerta-Saquero, A.; Vazquez-Duhalt, R. Cytochrome P450 Bioconjugate as a Nanovehicle for Improved Chemotherapy Treatment. Macromol. Biosci. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Alyafeeu, Y.A.; Alaamery, M.; Bawazeer, S.; Almutairi, M.S.; Alghamdi, B.; Alomran, N.; Sheereen, A.; Daghestani, M.; Massadeh, S. Preparation of anastrozole loaded PEG-PLA nanoparticles: Evaluation of apoptotic response of breast cancer cell lines. Int. J. Nanomed. 2018, 13, 199–208. [Google Scholar] [CrossRef]
- Lossignol, D. A little help from steroids in oncology. J. Transl. Intern. Med. 2016, 4, 52–54. [Google Scholar] [CrossRef]
- Andima, M.; Costabile, G.; Isert, L.; Ndakala, A.; Derese, S.; Merkel, O.; Andima, M.; Costabile, G.; Isert, L.; Ndakala, A.J.; et al. Evaluation of β-Sitosterol Loaded PLGA and PEG-PLA Nanoparticles for Effective Treatment of Breast Cancer: Preparation, Physicochemical Characterization, and Antitumor Activity. Pharmaceutics 2018, 10, 232. [Google Scholar] [CrossRef]
- Goodarzi, N.; Varshochian, R.; Kamalinia, G.; Atyabi, F.; Dinarvand, R. A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydr. Polym. 2013, 92, 1280–1293. [Google Scholar] [CrossRef]
- Basu, A.; Kunduru, K.R.; Abtew, E.; Domb, A.J. Polysaccharide-Based Conjugates for Biomedical Applications. Bioconjug. Chem. 2015, 26, 1396–1412. [Google Scholar] [CrossRef]
- Lopalco, A.; Cutrignelli, A.; Denora, N.; Perrone, M.; Iacobazzi, R.M.; Fanizza, E.; Lopedota, A.; Depalo, N.; De Candia, M.; Franco, M.; et al. Delivery of proapoptotic agents in glioma cell lines by TSPO ligand–dextran nanogels. Int. J. Mol. Sci. 2018, 19, 1155. [Google Scholar] [CrossRef]
- Pednekar, P.P.; Jadhav, K.R.; Kadam, V.J. Aptamer-dendrimer bioconjugate: A nanotool for therapeutics, diagnosis, and imaging. Expert Opin. Drug Deliv. 2012, 9, 1273–1288. [Google Scholar] [CrossRef]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef]
- Lee, J.H.; Yigit, M.V.; Mazumdar, D.; Lu, Y. Molecular Diagnostic and Drug Delivery Agents based on Aptamer-Nanomaterial Conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605. [Google Scholar] [CrossRef]
- Hwang, D.W.; Ko, H.Y.; Lee, J.H.; Kang, H.; Ryu, S.H.; Song, I.C.; Lee, D.S.; Kim, S. A Nucleolin-Targeted Multimodal Nanoparticle Imaging Probe for Tracking Cancer Cells Using an Aptamer. J. Nucl. Med. 2010, 51, 98–105. [Google Scholar] [CrossRef]
- Li, Y.; Duo, Y.; Bao, S.; He, L.; Ling, K.; Luo, J.; Zhang, Y.; Huang, H.; Zhang, H.; Yu, X. EpCAM aptamer-functionalized polydopamine- coated mesoporous silica nanoparticles loaded with DM1 for targeted therapy in colorectal cancer. Int. J. Nanomed. 2017, 12, 6239–6257. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Benvidi, A.; Banaei, M.; Nikukar, H.; Tezerjani, M.D.; Azimzadeh, M. Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly (glutamic acid)/MWNT nanocomposite. Microchim. Acta 2018, 185, 1–10. [Google Scholar] [CrossRef]
- Tietze, S.; Schau, I.; Michen, S.; Ennen, F.; Janke, A.; Schackert, G.; Aigner, A.; Appelhans, D.; Temme, A. A Poly(Propyleneimine) Dendrimer-Based Polyplex-System for Single-Chain Antibody-Mediated Targeted Delivery and Cellular Uptake of SiRNA. Small 2017, 13, 1700072. [Google Scholar] [CrossRef]
- Misra, S.K.; Kampert, T.L.; Pan, D. Nano-Assembly of Pamitoyl-Bioconjugated Coenzyme-A for Combinatorial Chemo-Biologics in Transcriptional Therapy. Bioconjug. Chem. 2018, 29, 1419–1427. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Dong, Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, 1–25. [Google Scholar] [CrossRef]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef]
- Uchida, S.; Kinoh, H.; Ishii, T.; Matsui, A.; Tockary, T.A.; Takeda, K.M.; Uchida, H.; Osada, K.; Itaka, K.; Kataoka, K. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials 2016, 82, 221–228. [Google Scholar] [CrossRef]
- Yeom, J.H.; Ryou, S.M.; Won, M.; Park, M.; Bae, J.; Lee, K. Inhibition of Xenograft Tumor Growth by Gold Nanoparticle-DNA Oligonucleotide Conjugates-Assisted Delivery of BAX mRNA. PLoS ONE 2013, 8, e75369. [Google Scholar] [CrossRef]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Bagherifam, S.; Bekhradnia, S.; Nyström, B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wells, S. Surface-Modified Superparamagnetic Nanoparticles for Drug Delivery: Preparation, Characterization, and Cytotoxicity Studies. IEEE Trans. Nanobiosci. 2004, 3, 66–73. [Google Scholar] [CrossRef]
- Mioc, M.; Pavel, I.Z.; Ghiulai, R.; Coricovac, D.E.; Farcas, C.; Mihali, C.; Oprean, C.; Serafim, V.; Popovici, R.A.; Dehelean, C.A.; et al. The Cytotoxic Effects of Betulin-Conjugated Gold Nanoparticles as Stable Formulations in Normal and Melanoma Cells. Front. Pharmacol. 2018, 9, 429. [Google Scholar] [CrossRef]
- Curado, N.; Dewaele-Le Roi, G.; Poty, S.; Lewis, J.S.; Contel, M. Trastuzumab gold-conjugates: Synthetic approach and in vitro evaluation of anticancer activities in breast cancer cell lines. Chem. Commun. 2019, 55, 1394–1397. [Google Scholar] [CrossRef]
- Li, Y.; Duo, Y.; Zhai, P.; He, L.; Zhong, K.; Zhang, Y.; Huang, K.; Luo, J.; Zhang, H.; Yu, X. Dual targeting delivery of miR-328 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Nanomedicine 2018, 13, 1753–1772. [Google Scholar] [CrossRef]
- Zellmer, S.; Schmidt-Heck, W.; Godoy, P.; Weng, H.; Meyer, C.; Lehmann, T.; Sparna, T.; Schormann, W.; Hammad, S.; Kreutz, C.; et al. Transcription Factors ETF, E2F, and SP-1 Are Involved in Cytokine-Independent Proliferation of Murine Hepatocytes. Hepatology 2010, 52, 2127–2136. [Google Scholar] [CrossRef]
- Tice, R.R.; Austin, C.P.; Kavlock, R.J.; Bucher, J.R. Improving the Human Hazard Characterization of Chemicals: A Tox21 Update. Environ. Health Perspect. 2013, 121, 756–765. [Google Scholar] [CrossRef]
- Centre for Drug Evaluation and Research. New Drug Therapy Approvals. 2017. Available online: https://www.fda.gov/files/about%20fda/published/2017-New-Drug-Therapy-Approvals-Report.pdf (accessed on 1 January 2017).
- Yurkiewicz, I.R.; Muffly, L.; Liedtke, M. Inotuzumab ozogamicin: A CD22 mAb—Drug conjugate for adult relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Drug Des. Dev. Ther. 2018, 12, 2293–2300. [Google Scholar] [CrossRef]
- Norsworthy, K.J.; Ko, C.-W.; Lee, J.E.; Liu, J.; John, C.S.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. Oncologist 2018, 23, 1–6. [Google Scholar] [CrossRef]
- Amiri-kordestani, L.; Blumenthal, G.M.; Xu, Q.C.; Zhang, L.; Tang, S.W.; Ha, L.; Weinberg, W.C.; Chi, B.; Candau-chacon, R.; Hughes, P.; et al. FDA Approval: Ado-Trastuzumab Emtansine for the Treatment of Patients with HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2014, 20, 4436–4442. [Google Scholar] [CrossRef]

| S No. | Carrier | Therapeutic Agent | Ligand | Targeted Site | Mechanism of Action | Study Model | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | IONPs | Cituximab | Cituximab/EGFRvIIIAb | EGFR glioblastoma | Caspase-3 activation, apoptosis | In vitro and in vivo studies | [23] |
| 2 | SPIONS | Doxorubicin | A10 RNA Aptamer | Prostate-specific membrane antigen of prostate cancer cells | Caspase-3 activation, apoptosis | In vitro | [24] |
| 3 | SPIONS | Doxorubicin | cRGD peptide | Integrin positive U87MG cell lines | Caspase-3 activation, apoptosis | In vitro | [25] |
| 4 | Magnetic Fe-Zn NPs | Doxorubicin | LHRH | LHRH-expressing breast cancer cells | Caspase-3 activation, apoptosis | In vitro | [15] |
| 5 | Gold NPs | relAsiRNA | Transferrin targeting ligand (Tf)/folic acid (FA) | Transferrin-receptor- and folate-receptor- expressing prostate cancer | Downregulates relA gene (protooncogene) | In vitro | [26] |
| 6 | Gold nanospheres | Radiation therapy | Anti-c-Met antibody | Cervical cancer | Fas signaling pathway (apoptosis) | In vitro | [27] |
| 7 | Silver NPs | Soybean agglutinin | Soybean agglutinin | N-acetylgalactosamine and β-D galactose (breast cancer cells) | Autophagy, apoptosis, DNA damage | In vitro | [18] |
| 8 | Silica NPs | 5-Fluorouracil | Epidermal growth factor | Epidermal-growth-factor-receptor-expressing colorectal cancer cells | Inhibits thymidylate synthase, causes thymineless death | In vitro | [14] |
| 9 | Silica NPs | Doxorubicin | Pgp siRNA | Knockdown pgp gene, multidrug-resistant KBV1 cell line | Caspase-3 activation, apoptosis | In vitro | [28] |
| 10 | Silica NPs | Doxorubicin | PEG-folic acid | Folate-expressing cervical cancer cells | Caspase-3 activation, apoptosis | In vitro and in vivo | [29] |
| 11 | _ | IFNα2a | Hyaluranan acid | CD44+ ovarian cancer cells | JAK-STAT pathway, apoptosis | In vitro and in vivo | [21] |
| 12 | Aptamer | miRNA 29b | MUC1 aptamer | MUC1 transmembrane protein, lung cancer | Downregulates antiapoptotic proteins MCL1 and DNMT3B | In vitro | [19] |
| 13 | Aptamer | Doxorubicin | AS1411 | Nucleolin present on membrane of hepatocellular carcinoma | Apoptosis | In vitro and in vivo | [30] |
| 14 | SPIONs | Epirubicin | 5TR1 aptamer | Mucin1-glycoprotein-expressing colon cancer cell lines (C26) | Topoisomerase inhibitor | In vitro and in vivo | [31] |
| 15 | Fatty acid | siRNAs | siRNAs | Oncogenic glucose-regulated proteins (GRPs) in prostate cancer cells (PC-3) | siRNAs downregulates GRPs, apoptosis | In vitro | [32] |
| 16 | PLGA-TPGS NPs | Docitaxel | AS1411 aptamer | Nucleolin present on membrane of adenocarcinoma | Inhibition of mitotic cell division between metaphase and anaphase, blocks bcl2 oncoprotein, apoptosis | In vitro and in vivo | [20] |
| 17 | Thermoresponsive polymers | Met-enkaphalin peptide | RGD targeting ligand | RGD peptide | Halt growth of cells by immunological mechanisms | - | [17] |
| 18 | PLGA-PEG | Paclitaxel | AS1411 | Nucleolin expressed on C6 glioma cells | Inhibition of mitotic cell division between metaphase and anaphase, blocks bcl-2 oncoprotein, apoptosis | In vitro and in vivo | [33] |
| 19 | PLGA-PEG | Docetaxel | HER-2 Ab | HER-2-expressing breast cancer cells | Inhibition of mitotic cell division between metaphase and anaphase, prevents microtubule depolymerization, apoptosis | In vitro | [34] |
| 20 | PLA-PEG | NuBCP9 peptide | Bcl-2 | MCF-7 breast cancer and HepG2 hepatocellular carcinoma cells | Bcl-2-conversion-dependent apoptosis | In vitro and in vivo | [16] |
| 21 | Pullalan | Paclitaxel | Alendronate (ALN) | Hydroxyapatite, breast cancer bone metastasis | Inhibition of mitotic cell division between metaphase and anaphase, blocks bcl-2 oncoprotein, apoptosis | In vitro | [35] |
| 22 | Dextran | Cisplatin | LHRH-targeting ligand | LHRH receptors on breast cancer cells | Caspase-3- and caspase-7-activated apoptosis | In vitro and in vivo | [36] |
| 23 | Polysaccharide (carboxymethyl cellulose) | Doxorubicin | Integrin target receptor tripeptide (RGD), L-arginine | Integrin-expressing HEK293t cell lines | Apoptosis | In vitro and in vivo | [37] |
| 24 | Virus-like NPs (tHBcAg) | Doxorubicin | Folic acid | Folate-expressing HeLa cells | Caspase-3 activation, apoptosis | In vitro | [38] |
| 25 | _ | HA-cytarabine (Ara-C) | Folic acid | Folate-expressing leukemia cancer cells | Inhibition of DNA polymerase, apoptosis | In vitro | [39] |
| 26 | _ | Methotrexate | (D-Lys6)- LHRH | LHRH-expressing prostate cancer cells | Apoptosis | In vitro and in vivo | [40] |
| 27 | CdSe/ZnS core/shell QD | Doxorubicin | JB434 (cell uptake peptide) | H69AR (human small cell lung carcinoma) | JB434 allows QDs to penetrate MDR cancer cells, doxorubicin leads to apoptosis | In vitro | [41] |
| 28 | Graphene QD | Biosurfactant | Folic acid | Folate-expressing breast cancer cell lines (MCF-7) | Not determined | In vitro | [42] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadhawan, A.; Chatterjee, M.; Singh, G. Present Scenario of Bioconjugates in Cancer Therapy: A Review. Int. J. Mol. Sci. 2019, 20, 5243. https://doi.org/10.3390/ijms20215243
Wadhawan A, Chatterjee M, Singh G. Present Scenario of Bioconjugates in Cancer Therapy: A Review. International Journal of Molecular Sciences. 2019; 20(21):5243. https://doi.org/10.3390/ijms20215243
Chicago/Turabian StyleWadhawan, Aishani, Mary Chatterjee, and Gurpal Singh. 2019. "Present Scenario of Bioconjugates in Cancer Therapy: A Review" International Journal of Molecular Sciences 20, no. 21: 5243. https://doi.org/10.3390/ijms20215243
APA StyleWadhawan, A., Chatterjee, M., & Singh, G. (2019). Present Scenario of Bioconjugates in Cancer Therapy: A Review. International Journal of Molecular Sciences, 20(21), 5243. https://doi.org/10.3390/ijms20215243





