Abstract
Selenium is a trace element essential to humans and forms complexes with proteins, which exert physiological functions in the body. In vitro studies suggested that selenium possesses anticancer effects and may be effective against osteosarcoma. This review aims to summarise current evidence on the anticancer activity of inorganic and organic selenium on osteosarcoma. Cellular studies revealed that inorganic and organic selenium shows cytotoxicity, anti-proliferative and pro-apoptotic effects on various osteosarcoma cell lines. These actions may be mediated by oxidative stress induced by selenium compounds, leading to the activation of p53, proapoptotic proteins and caspases. Inorganic selenium is selective towards cancer cells, but can cause non-selective cell death at a high dose. This condition challenges the controlled release of selenium from biomaterials. Selenium treatment in animals inoculated with osteosarcoma reduced the tumour size, but did not eliminate the incidence of osteosarcoma. Only one study investigated the relationship between selenium and osteosarcoma in humans, but the results were inconclusive. In summary, although selenium may exert anticancer properties on osteosarcoma in experimental model systems, its effects in humans require further investigation.
1. Introduction
Selenium is an essential trace element in humans and is a non-metal from Group 16 of the periodic table that shares some similar physicochemical properties with sulphur. This element is rarely found in its elementary state but rather in its inorganic or organic forms in natural compounds [1,2]. The examples of inorganic selenium are selenium dioxide (SeO2), selenite (SeO32−) and selenate (SeO42−), whereas those for organic selenium are selenide, diselenides, selenol or selenothiol and seleninic acid (selenium-based acid; RSeOH) [2,3,4,5].
Selenium can be obtained mainly from food, such as Brazil nuts, garlic, onion, mushroom, broccoli, meat, egg, seafood and internal organs and in negligible quantity from drinking water [6,7,8]. The main selenium species present in food are selenomethionine (SeMet), selenocysteine (SeC), selenium-methylselenocysteine (Se-MSC), SeO32 and SeO42− [9]. The organic forms of selenium are present in greater amount in food, and their bioavailability is higher because they are absorbed easily compared with inorganic selenium [7,8,10]. The US-recommended dietary allowance and UK-recommended reference nutrient intake of selenium for an adult are 55 and 60 µg/day, respectively [11,12]. The average dietary intake of selenium varies across countries [10,13,14,15,16]. People from several countries, such as the UK, China, New Zealand and Finland, are traditionally deficient in selenium dietary intake [7,8,10,15,16].
Selenium is vital in various physiological processes and can incorporate into a protein as selenoproteins (SeC-containing protein), selenium-binding proteins (without SeC) and certain proteins rich in cysteine or methionine (such as SeMet), where sulphur is replaced by selenium in a nonspecific manner [1,17]. This element is required as a cofactor in more than 25 selenoproteins including glutathione peroxidase, thioredoxin reductase and iodothyronine deiodinase [1,18]. Selenoproteins are essential in oxidative defence, detoxification, immune response and thyroid hormone regulation [8,18,19]. Minor selenoproteins, such as selenoprotein-P, -W and -R, have antioxidant functions [8,20,21]. Selenoprotein-S is involved in inflammatory response and protein quality control [1,8]. Inadequate selenium uptake is related to cardiovascular diseases (Keshan disease), muscular diseases and bone diseases (osteopenia and Kashin-Beck disease) [2,18,22].
Selenium also exhibits potential in vivo and in vitro anticancer activities. Inorganic and organic selenium induce cell cycle arrest, cytotoxicity and apoptosis induction on cancerous cell lines from colon, breast, lung or prostate origins [2,5,23,24,25,26,27]. High levels of inorganic and organic selenium induce oxidative stress in cancer cells by generating reactive oxygen species (ROS) [5,28]. Selenium also prompts oxidative damage on DNA and mitochondria, leading to mitochondrial dysfunction in caspase-dependent or -independent apoptosis [2]. Organic selenium, such as methyseleninic acid (MSeA) inhibits angiogenesis by downregulating integrin β3 signalling [29]. Inorganic and organic selenium activate calcium influx, endoplasmic reticulum stress, 5’ adenosine monophosphate-activated protein kinase (AMPK), AKT, tumour suppressor protein phosphatase and tensin homolog (pTEN) and p53 signalling upstream of apoptosis events [24,27,30,31,32]. The potency of selenium in cytotoxicity depends greatly on chemical forms, cancer cell types and bioavailability [5]. With the use of human findings, the Nutritional Prevention of Cancer (NPC) trial showed that selenium intake decreases the risk of lung, colorectal and prostate cancer [33,34,35]. The Linxian Nutritional Intervention Trials also reported that a high level of basal serum selenium is associated with a marked reduction in mortality among patients with oesophageal and gastric cancer [33,36].
Bone cancer is one of the most prevalent cancers with the lowest long-term survival rate among children and adolescents [37]. Osteosarcoma is the most common malignant primary bone tumour in children and teenagers with 400 new cases annually in the US [38]. Its annual incidence rate is relatively low, with approximately 2–3.4 per million people worldwide [39,40], as compared with the most common childhood malignancy, childhood leukaemia (46.7 per million people) [41]. Osteosarcoma can be classified histopathologically into low-, intermediate- and high-grade, and more than 90% of osteosarcomas are high-grade malignancies [42]. Osteosarcoma is commonly found at the metaphyseal of long bones and its aetiology remains unknown [43]. Patients with non-metastatic osteosarcoma have a 65–70% five-year survival rate, whereas those with metastatic osteosarcoma have a poor survival rate of 19–30% [38,42]. The relapse rate for osteosarcoma is high, whereby nearly 40% of patients initially diagnosed with non-metastatic disease develop recurring osteosarcoma [44]. The current treatment options for osteosarcoma include surgery, radiotherapy and chemotherapy [38,40,45]. Adjuvant chemotherapy can greatly improve the quality of life and 5-year survival rate of these patients, but 30–50% of them still die because of lung metastasis [43,46]. Selenium is crucial in bone metabolism and health [47,48]. The therapeutic effect of selenium against osteosarcoma has been widely explored. Given its anticancer potential, selenium may serve as a prophylaxis or therapeutic option for osteosarcoma. Therefore, this review aims to summarise the anticancer effects of selenium in primary osteosarcoma from pre-clinical and clinical studies.
2. Literature Search
The literature search was performed between 1st–25th March 2019 on PubMed and Scopus using the keywords ‘Selenium’ OR ‘Selenite’ OR ‘Selenate’ OR ‘Selenide’ OR ‘Selenol’ OR ‘Organoselenium’ OR ‘Selenocysteine’ OR ‘Selenomethionine’ OR ‘Selenoprotein’ AND ‘Osteosarcoma’. We also examined the reference lists of the retrieved articles. Original research articles regarding the anticancer effects of selenium in osteosarcoma published in English were included. A total of 20 relevant studies were included in this review.
3. Evidence from In Vitro Study
3.1. Selenium Exerts Cytotoxicity, Anti-Proliferation and Pro-Apoptotic Activities on Osteosarcoma Cells
Inorganic and organic forms of selenium including SeO2 [49], SeO32− [50], selenium-polysaccharide (Se-Poly) [51], MSeA [52,53], Se-MSC [54] and SeC [55] induce cytotoxicity, anti-proliferation and apoptosis on osteosarcoma cells. These anticancer properties of inorganic and organic selenium were studied using mouse osteosarcoma K7M2 cells [56,57] and several human osteosarcoma cell lines, such as MG-63 [49,54,55,58,59,60], U-2 OS [49,50,51,52,53,61], Saos-2 [49,62], drug-resistant Saos-2/MTX300 [54], 143B [63,64,65] and MNNG/HOS cells [66]. SeO32− concentration (as low as 10 µM) inhibits the 12−day colony formation activities of U-2 OS cells [50]. Organic selenium, such as SeC and Se-MSC, also induces cell cycle arrest and sub-G1 accumulation in MG-63 cells [54,55]. SeC-induced S-phase arrest is associated with the downregulation of cyclinA and cyclin-dependent kinase-2 (CDK-2) proteins [55]. Most of the cytotoxicity and anti-proliferation effects of selenium species are time-, concentration- or cell type-dependent [49,50,51,54,55]. For example, MSeA significantly increases the level of necrosis in U-2 OS cells within a longer treatment time period (48 h) [52]. Se-MSC inhibits the growth of MG-63 cells, but enhances that of U-2 OS cells in a concentration-independent manner [54]. This finding indicates that each selenium species may exert different effects on osteosarcoma cell models. At present, the most effective selenium species against osteosarcoma is difficult to determine on the basis of cellular findings. Direct comparison is impossible due to differences in treatment time, concentration and osteosarcoma cell models used. To date, only two studies reported the IC50 value of selenium (SeO32− and MSeA) in osteosarcoma cells [50,52]. Other selenium species such as SeO42−, selenide, selenol and selenoproteins have not been tested on osteosarcoma.
3.2. Molecular Mechanism of Selenium-Induced Osteosarcoma Cell Death
Various forms of selenium species, including SeO2 [49], SeO32− [50], Se-MSC [54] and SeC [55] induce apoptosis of human osteosarcoma cells by showing typical apoptotic morphological and ultrastructural changes. However, the molecular mechanism of selenium-induced cell death is poorly characterised. Selenium-induced osteosarcoma cell death may be modulated via ROS production. SeO32− [50] and SeO32−-substituted hydroxyapatite (HA) nanoparticles (SeHAN) [60,66] induce early ROS production in U-2 OS, MG-63 and MNNG/HOS cells. SeC also induces ROS production as shown by dichlorodihydrofluorescein diacetate and Mito-SOX staining [55]. ROS is an important modulator in p53 activation [67,68]. SeC also induces early p53 activation by phosphorylating the Ser 15, Ser 20 and Ser 392 sites of p53 protein [55]. Furthermore, ROS upregulation is crucial because glutathione pretreatment almost completely abrogates SeC-induced apoptosis and partially reduces p53 phosphorylation [55]. The crosstalks among p53, ataxia-telangiectasia mutated kinase (ATM) and forkhead box O3a (FOXO3a) have been identified [69]. However, MSeA induces U-2 OS cell death via ATM (Ser1981) and FOXO3a activation that is independent of ROS induction and phosphorylated H2A histone family member X (γH2AX) activation [52,53]. MSeA, not SeO32−, induces the nuclear translocation of FOXO3a protein [53]. The knockdown of ATM (by KU55933 inhibitor) and FOXO3a (by hairpin RNA vector transfection) further suppressed MSeA-induced cytotoxicity [53]. Additionally, Werner Syndrome protein (WRN) serves as a potential combinational target, and its downregulation further increased the MSeA-induced U-2 OS cell death [52]. A comprehensive molecular study is required to elucidate the upstream molecular mechanisms of different selenium species in p53/ATM/FOXO3a/WRN signalling pathway axis.
SeC also induces early mitochondrial dysfunction via mitochondrial fragmentation (from protonema to punctiform phenotype) and mitochondrial membrane potential loss, a possible result of p53 activation [55]. Additionally, proapoptotic proteins Bax, Bad and pTEN are transcriptionally upregulated by p53 activation [70,71]. In line with this finding, SeO32− [50], Se-MSC [54] and SeC [55] also downregulate the antiapoptotic Bcl-2 and Bcl-XL protein and upregulate the Bax and Bad protein in osteosarcoma cells. SeO32− treatment significantly increases the P53 and PTEN mRNA levels [50]. Caspase-9 is activated upon the assembly of the apoptosome after increase in the level of Bax or Bad protein, mitochondrial dysfunction and the release of mitochondrial apoptotic proteins, such as cytochrome c [70]. The activated caspase-9 subsequently activates the downstream executioner caspases (caspase-3, -6 and -7) and cleaves cellular proteins including poly(ADP-ribose) polymerase (PARP) [72,73]. SeC activates caspase-9 and caspase-3/7 and induces PARP cleavage [55]. SeO32− also increases CASP9 and CASP3 mRNA levels and caspase-3 protein level [50]. The mRNA levels for CASP6 and CASP8 are unaffected by SeO32− treatment [50].
The current understanding of the molecular mechanisms of selenium-induced osteosarcoma apoptosis is summarised in Figure 1.
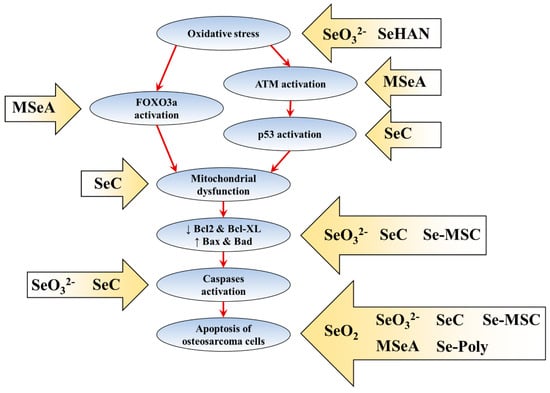
Figure 1.
The molecular mechanisms of selenium-induced osteosarcoma cell death. The arrow boxes with selenium species showed the reported effects of selenium species on the apoptosis pathway. The red arrows indicate the sequent of events in apoptosis pathway.
3.3. Role of Selenium in the Tumour Microenvironment
The bone tissue is made up of several cells, including osteoblasts, osteoclasts, chondrocytes, mesenchymal stem cells, blood cells and endothelial cells. The osteoclasts play an important role in the pathogenesis of osteosarcoma, especially in the progression and metastasis of the cancer [74]. Osteoclasts formation and activation are tightly regulated through both receptor-activation of nuclear factor κB (RANK) ligand (RANKL) and macrophage-colony stimulating factor [75]. The naturally-occurring inhibitor of RANKL, osteoprotegerin (OPG) synthesised by osteoblasts, protects against bone loss by acting as a negative regulator for RANK/RANKL pathway [75]. The RANKL is secreted by osteocytes, osteoblasts and mesenchymal stem cells, as well as osteosarcoma cells [76,77]. RANKL activates its receptor, RANK, and then triggers the bone resorption by osteoclasts, which in turn promotes invasion and metastasis of osteosarcoma [74]. RANKL inhibitors such as denosumab (currently in phase II clinical trial) may be effective against osteosarcoma [78,79]. Thus far, the effects of selenium on the RANK/RANKL/OPG pathway remain inconclusive. SeO32− suppresses RANKL-induced osteoclastogenesis through inhibition of ROS-induced signalling pathways in mouse bone marrow-derived monocytes and RAW 264.7 cell line [48,80]. Additionally, selenium (unknown species) was reported to inhibit the transcription activity of RANKL and downregulate the mRNA levels of OPG in human osteosarcoma Saos-2 cells [81] More research is required to confirm the effects of selenium in the RANK/RANKL/OPG pathway.
Additionally, mesenchymal stem cells serve as an important modulator in the pathogenesis of osteosarcoma, wherein it supports the osteosarcoma progression and metastasis via the secretion of cytokines/growth factors [74,82]. Mesenchymal stem cells can be targeted in osteosarcoma treatment via the restoration of cytokines/growth factors signalling, or transforming the stem cells into mature osteoblasts [82]. The effects of selenium on mesenchymal stem cells are not conclusive. SeO32− was reported to protect bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation through inhibiting oxidative stress and ERK activation [83]. A ruthenium (II) functional selenium nanoparticles and citrate functionalised selenium nanoparticles were reported to promote the proliferation and osteogenic differentiation of human umbilical cord mesenchymal stem cells [84]. Recently, Ahmed et al. demonstrated that 48 h of SeO2 nanoparticles treatment (2−25 μg/mL) significantly increases the proliferation of rat adipose stem cells and bone marrow stem cells [85]. The induction of proliferation and osteogenic differentiation of mesenchymal stem cells may interfere with metastasis of osteosarcoma. However, further study is required to confirm the role of mesenchymal stem cells and their therapeutic potentials in osteosarcoma.
Regarding anticancer selectivity, selenium selectively exerts anticancer actions on osteosarcoma cells. Both inorganic and organic selenium exhibit no or marginal toxicity on several primary and non-tumourigenic cells including primary rat growth plate chondrocytes [86], primary mouse lung fibroblasts [57], primary human calvarial osteoblasts [87], human bone marrow stem cells (BMSCs) [60,88], human umbilical cord stem cells [84], human lymphocytes [61], mouse preosteoblast MC3T3-E1 cells [57,58,86], mouse 3T3-L1 preadipocytes [89], rat skeletal muscle L6 cells [50], human embryonic kidney 293 cells [50] and human osteoblast hFOB1.19 cells [56,59]. Additionally a low concentration (0.01–1 µM) and 1 h transient treatment of SeO32− exert radioprotective effects on chondrocytes and osteoblasts by protecting them from 20-Gy irradiation-induced cytotoxicity [86]. SeO32− (0.01–1 µM) is also not genotoxic to human blood lymphocytes and does not cause mitotic index change, chromosomal aberration or chromatid break [61]. However, SeO32− (1 µM for 1 h) substantially increases the frequency of dicentric chromosomes (but not deletion and chromatid break) in γ rays-irradiated human blood lymphocytes via an unknown mechanism [61]. Additionally, selenium nanoparticles (500–6000 nm size, 0.005 mg/mL for 1 week) also induce significant cytotoxic effects and apoptosis events on mesenchymal stem cells via morphological observation [90]. These findings require further investigation to ensure the safety of selenium in daily consumption.
The molecular mechanisms on the selectivity of selenium have not been studied in detail in osteosarcoma cell models. Generally, cancerous cells are more sensitive to oxidative stress compared with normal cells [91,92]. This characteristic has been exploited by current chemotherapeutic agent, such as doxorubicin, to selectively induce osteosarcoma cell death via oxidative stress [93,94]. Therefore, the selective cytotoxicity of selenium toward osteosarcoma cells may be due to oxidative stress. A high concentration of inorganic or organic selenium induced non-selective cell death on non-tumourigenic cells and osteosarcoma cells [50,52,57,62], probably due to the overwhelming ROS production.
3.4. Selenium as a Bone Implant Material
Selenium serves as a potential candidate for bone implant material, especially for patients with osteosarcoma. In vitro osteogenic studies were conducted on selenium-doped titanium substrates [56,87], calcium phosphate [58], poly-L-lactic acid (PLLA) nanocomposites [59] or HA [57,60,62,66]. These selenium (SeO32−)-doped substrates suppress the growth of mouse and human osteosarcoma cells including K7M2 [56,57], MG-63 [58,59,60], Saos-2 [62] and MNNG/HOS cells [66]. In parallel with the in vitro studies, selenium-substituted HA substrate and nanoparticles induce apoptosis of osteosarcoma cells with early ROS generation, mitochondria dysfunction, cytochrome c release, tBid upregulation and caspase-8,-9,-3 activation [60,66]. The addition of manganese (III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP; an oxidative stress inhibitor) inhibits selenium-substituted HA nanoparticles-induced ROS generation and osteosarcoma apoptosis [66], thus, further emphasising the importance of ROS in anticancer activities of selenium. The addition of SeO32− into bone implant substrates does not affect their basic reaction chemistry [56]. Physicochemical analysis revealed that SeO32− is dose-dependently released from the substrate surface, thereby explaining its anticancer effects [56]. Similarly, acellular media collected from SeO32−-doped substrate are proven effective against mouse osteosarcoma cells [56]. Wang et al. reported that SeO32−-doped HA nanoparticles are directly internalised into the osteosarcoma cells via nonspecific endocytosis [66]. SeO32− in the vesicles is then released into the cytosol upon the degradation of HA nanoparticles during the merger of endosome and acidic lysosome in a pH-dependent manner [66]. The release of SeO32− increases ROS generation, leading to osteosarcoma cell apoptosis in 6% and 10% SeHANs and 2µM SeO32−, but not in HA nanoparticles control and 3% SeHAN groups [66].
SeO32−-doped substrates also showed osteoinductive activities by promoting the growth of non-cancerous human osteoblast hFOB 1.19 cells [56], non-cancerous human preosteoblast MC3T3-E1 cells [58] and primary human calvarial osteoblasts [87]. SeO32−-doped substrates increased the growth of mouse primary lung fibroblasts [57] and BMSCs [60] under a similar treatment. SeO32−-doped titanium substrate [56], selenium (SeO32−)-doped calcium phosphate coating [58] and SeO32−coated-PLLA nanocomposites (SeNP-PLLA) [59] promote bone-forming activities, as evidenced by the increase in alkaline phosphatase activities and extracellular calcium deposition. Furthermore, SeO32−-substituted HA exhibits osteoinductive activity on partially differentiated MC3T3-E1 cells by increasing the mRNA expression of bone γ-carboxyglutamate protein 3 (BGLAP3; osteocalcin-related protein) [57].
The cytotoxicity of SeO32−-doped substrates and nanoparticles relies on its pH-dependent release into the medium and a high concentration of selenium is suggested to induce non-selective cytotoxicity [57,59,62]. A high SeO32− content (3.0 wt%) induces non-cancerous MC3T3-E1 cell death with abnormal morphological changes as early as 24 h of treatment [57]. SeO32−-containing HA (SeHA) 3.0 wt% treatment also decreases BGLAP3 expression due to its cytotoxic effect [57]. SeNP-PLLA reduces the viability of human osteosarcoma MG-63 cells and non-cancerous hFOB cells, though the effects are more selective on former than on the latter [59]. Additionally, the media after overnight incubation with selenium-containing hydroxyapatite/alginate (SeHA/ALG) composite microgranules are cytotoxic to human osteosarcoma Saos-2 cells and non-cancerous hFOB 1.19 cells with almost 90% reduction on viability [62]. According to the authors, this non-selective cytotoxicity of SeHA/ALG microgranules may be due to the rapid release and accumulation of selenium in the culture media [62]. As previously discussed, excessive ROS production is one of the mechanisms of selenium in inducing unspecific cell death [95,96].
The in vitro studies of selenium and its derivatives on osteosarcoma cells are summarised in Table 1.

Table 1.
The anticancer effects of selenium from in vitro studies.
4. Evidence from Animal and Human Studies
Several animal studies were conducted to determine the effects of selenium on osteosarcoma [49,51,55,66,97,98]. Bierke and Svedenstal initiated the study on the effects of inorganic selenium in radioactive strontium (90Sr)-induced osteosarcoma mice [98]. Vitamin E (α-tocopherol acetate) with or without SeO32− (10 µg) was administrated intraperitoneally to the mice with osteosarcoma every 2 weeks from day 105 after the 90Sr exposure until 14 months [98]. The same injection was continued after 14 months but was changed to 30-day intervals for the rest of the life span [98]. Oestrogen (polyestradiol phosphate) was administered in certain groups during 30, 60 and 90 days after the 90Sr exposure. The results showed no significant difference in osteosarcoma tumour incidence after treatment with vitamin E with or without SeO32−. Post-exposure of antioxidants, including selenium and vitamin E, is not beneficial to prevent the development of 90Sr-induced osteosarcoma. The combined treatment somehow hastened the onset of osteosarcoma regardless of oestrogen induction [98].
Several studies of inorganic and organic selenium were conducted using osteosarcoma xenograft animal models [49,51,55,66,97]. Some in vitro human osteosarcoma cell lines were used, including human osteosarcoma KOS [49], U-2 OS [51], MG-63 [55] and SOSP-9607 cells [97]. Hiraoka et al. investigated the effect of SeO2 in BALB/c nude mice implanted with osteosarcoma xenograft [49]. The back of nude mice was subcutaneously inoculated with KOS cells, and the mice were fed with SeO2−containing drinking water (0.2 and 2 µg/mL) until day 44 after inoculation [49]. SeO2 dose-dependently decreased the tumour volume to 2.5-fold lower compared with that of control [49]. SeO2 also induces apoptosis in xenograft tumour tissues without affecting visceral organs. However, this compound does not prevent tumour incidence [49].
For the organic selenium, Wang et al. reported that daily oral administration of Se-Poly isolated from Ziyang green tea (200 and 400 mg/kg) for 28 days significantly reduces tumour volume and weight of U-2 OS xenograft in BALB/c nude mice [51]. Similar to that used by Hiraoka et al., the Se-Poly is non-toxic to nude mice where it does not affect the body weight or cause any lethal incidence [51]. Wang et al. showed that the intravenous injection of SeC (5 and 10 mg/kg; every other day for 2 weeks) significantly and dose-dependently reduces the osteosarcoma MG-63 tumour xenograft volume and weight in nude mice [55]. Mechanistically, SeC induces p53 phosphorylation (Ser 15) and caspase-3 activation in tumour xenograft in a dose-dependent manner [55]. SeC also significantly suppresses cell proliferation and angiogenesis of tumour xenografts as evidenced by the downregulation of Ki-67 and CD-34 biomarkers [55]. Similar to other selenium species, SeC does not affect the body weight, suggesting the lack of systemic toxicity in nude mice [55].
The anticancer effect of SeO32−-doped substrates on osteosarcoma xenograft animal models has also been reported [66,97]. Wang et al. revealed that intratumoural injection of SeHAN for 30 days significantly reduces the tumour volume of intrafemoral human osteosarcoma SOSP-9607 xenograft in nude mice [97]. SeHAN also inhibits osteosarcoma tumour metastasis into the lung and protects other vital organs, such as liver, kidney and cardiac muscles from osteosarcoma-mediated damages [97]. The anticancer effect of SeHAN is mediated by the suppression of tumour invasion but not proliferation, as indicated by the reduction of matrix metallopeptidase-9 (MMP-9; invasion marker) and the lack of change in Ki-67 level (mitotic marker) [97]. Intratumour 10% SeHAN injection (every 3 days for 30 days) significantly reduces the tumour size, weight and volume of osteosarcoma MNNG/HOS tumour xenograft in BALB/c nude mice [66]. SeHAN induces oxidative DNA damage, which will hypothetically trigger the subsequent activation of caspases and the apoptosis in tumour tissues [66]. In parallel with in vitro studies, the anticancer effect of SeHAN is related to the release of SeO32− ions into aqueous solution [97]. SeHAN is completely degraded within tumour tissues with less calcium aggregation and blood vessel vascularization upon histological analysis [66]. Similar to findings in other selenium studies, SeHAN does not cause any significant systemic toxicity in nude mice and has no effect on body weight, lethality, haematological indices and serum biochemical profile, including aspartate aminotransferase, blood urea nitrogen, creatinine and lactate dehydrogenase levels [66,97]. Furthermore, no pathological change has been detected in the liver of nude mice that received SeHAN treatment [66].
One human study was conducted by Huang et al. to identify the relationship between selenium level and osteosarcoma disease [54]. No significant difference was found in the plasma selenium levels between patients with and without osteosarcoma [54]. Selenium levels were significantly higher in osteosarcoma tissues compared with those in normal bone tissues among patients with osteosarcoma [54]. However, further investigation is needed to identify the role of high selenium levels in osteosarcoma tissues. To date, no human study has revealed the beneficial effect of selenium supplementation in preventing osteosarcoma and no clinical trial is being conducted to evaluate the therapeutic effect of selenium in patients with osteosarcoma.
Several epidemiological studies and clinical trials were conducted to determine the relationship between selenium intake and the risk of other solid cancers; however, the findings are heterogeneous [5,34,99,100,101,102,103,104,105]. The NPC and Linxian Nutritional Intervention Trials reported that selenium intake reduces the risk of lung, colorectal and prostate cancer and mortality related to oesophageal and gastric cancer [33,34,35,36]. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) and Selenium and Celecoxib (Sel/Cel) Trial showed that selenium does not reduce the risk of prostate [33,101] and colorectal cancer [106]. Additionally, a recent systematic review and meta-analysis by Vinceti et al. concluded that selenium supplementation does not reduce the overall cancer incidence or mortality [103,107]. The contradicting findings may be confounded by experimental biases, chemical forms of selenium, basal selenium status, nutritional status and lifestyle factors of the subjects [35,107]. Vinceti et al. emphasised on randomised controlled clinical trials of selenium on various cancers [103]. The osteosarcoma is not involved as currently the relevant clinical trial is not available. Further studies are required to confirm the relationship of selenium and cancer risk, especially on osteosarcoma. Table 2 summarises the effects of selenium in osteosarcoma in vivo.

Table 2.
The effects of selenium in osteosarcoma animal and human studies.
5. Conclusions
Selenium and selenium-containing proteins possess potential anticancer activity, as evidenced from cellular and animal studies. Evidence was provided that the underlying mechanism for their anticancer effects involves increased intracellular ROS generation, leading to activation of the p53/ATM/FOXO3a pathway, proapoptotic proteins and caspases. This results in cytotoxicity, antiproliferative and proapoptotic effects of selenium compounds on osteosarcoma. The action of selenium can be selective on osteosarcoma cells without affecting adjacent normal cells, such as chondrocytes and fibroblasts. Moreover, this element is osteogenic for normal bone tissues. Developing SeO32−-doped bone biomaterials, which release this essential element in a controlled manner for therapeutic purposes, remains a challenge because SeO32− at a high concentration exerts non-specific cell death via oxidative stress. Another challenge is identifying the most active selenium form and dose that exert the best anti-osteosarcoma effects in the various cellular models used in previous experiments. In vivo studies showed that selenium can reduce the tumour size in animals with osteosarcoma, but does not diminish the incidence of the tumour. However, the effects of selenium on osteosarcoma have not been validated in a clinical trial. A paucity of epidemiological data revealed a relationship between selenium intake and osteosarcoma. Thus, the role of inorganic and/or organic selenium on osteosarcoma tumour formation remains unknown. These gaps in our knowledge should be filled by researchers to determine the role of selenium in preventing or treating osteosarcoma.
Author Contributions
Conceptualization, K.-L.P. and K.-Y.C.; writing—original draft preparation, K.-L.P.; writing—review and editing, K.-Y.C.; funding acquisition, K.-Y.C.
Funding
The authors are funded by research grants FRGS/1/2018/SKK10/UKM/03/1 provided by Ministry of Education, Malaysia and GUP-2017-060 provided by Universiti Kebangsaan Malaysia.
Acknowledgments
The authors expressed gratitude to Ministry of Education, Malaysia and Universiti Kebangsaan Malaysia for their generous support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMPK | 5’ adenosine monophosphate-activated protein kinase |
| ATM | Ataxia-telangiectasia mutated kinase |
| Bad | Bcl-2−associated death promoter protein |
| BAX | Bcl-2−associated x gene |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-XL | B-cell lymphoma-extra large |
| BGLAP3 | Bone γ-carboxyglutamate protein 3 |
| BMSCs | Bone marrow stem cells |
| BRCA1 | Breast cancer type 1 susceptibility gene |
| CASP3 | Caspase-3 gene |
| CASP9 | Caspase-9 gene |
| CDK-2 | Cyclin-dependent kinase-2 |
| FOXO3a | Forkhead Box O3a protein |
| HA | Hydroxyapatite |
| MMP-9 | Matrix metallopeptidase-9 |
| MnTMPyP | Manganese(iii) tetrakis(1-methyl-4-pyridyl)porphyrin |
| MSeA | Methyseleninic acid |
| NO | Nitric oxide |
| NPC | Nutritional Prevention of Cancer trial |
| Nrf2 | Nuclear factor erythroid 2−related factor 2 |
| O2−• | Superoxide anion |
| PARP | Poly(ADP-ribose) polymerase |
| PLLA | Poly-l-lactic acid |
| pTEN | Tumour suppressor protein phosphatase and tensin homolog |
| ROS | Reactive oxygen species |
| RSeOH | Seleninic acid |
| SeC | Selenocysteine |
| SeHA | Seo32−-containing HA |
| SeHA/ALG | Selenium-containing Hydroxyapatite/Alginate |
| SeHA/ALG RIS I | Seha/AIG granules with risedronate sodium during formation |
| SeHA/ALG RIS II | Seha/AIG granules with risedronate sodium and potassium chloride during formation |
| SeHAN | Selenite-substituted HA nanoparticles |
| SeMet | Selenomethionine |
| Se-MSC | Selenium-methylselenocysteine |
| SeNP-PLLA | Selenium-coated poly-l-lactic acid nanocomposites |
| SeO2 | Selenium dioxide |
| SeO32− | Selenite |
| SeO42− | Selenate |
| Se-Poly | Selenium-polysaccharide |
| Ser | Serine |
| tBid | Truncated BH3 interacting-domain death agonist protein |
| WRN | Werner syndrome protein |
| γH2Ax | Phosphorylated H2A histone family member x |
References
- Cheng, W.-H.; Lei, X.G. Selenium: Basic nutritional aspects. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Collins, J.F., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 449–461. [Google Scholar]
- Tan, H.W.; Mo, H.Y.; Lau, A.T.Y.; Xu, Y.M. Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2018, 20. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Perez, M.; Ali, W.; Marc, M.A.; Handzlik, J.; Dominguez-Alvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Sancineto, L.; de Bem, A.F.; Tew, K.D.; Santi, C.; Radi, R.; Toquato, P.; Galli, F. Selenocompounds in Cancer Therapy: An Overview. Adv. Cancer Res. 2017, 136, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B.; Blazejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6. [Google Scholar] [CrossRef]
- Reilly, C. Selenium in Foods. In Selenium in Food and Health, 2nd ed.; Springer: Salmon Tower Building, NY, USA, 2006; pp. 158–172. [Google Scholar]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484–1491. [Google Scholar] [CrossRef]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef]
- Nakayama, S.F.; Iwai-Shimada, M.; Oguri, T.; Isobe, T.; Takeuchi, A.; Kobayashi, Y.; Michikawa, T.; Yamazaki, S.; Nitta, H.; Kawamoto, T.; et al. Blood mercury, lead, cadmium, manganese and selenium levels in pregnant women and their determinants: The Japan Environment and Children’s Study (JECS). J. Expo. Sci. Environ. Epidemiol. 2019, 29, 633–647. [Google Scholar] [CrossRef]
- Monsen, E.R. Dietary Reference Intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Department of Health. Dietary reference values for food energy and nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy, 8th ed.; Stationery Office: London, UK, 1991; Volume 41, pp. 1–210. [Google Scholar]
- Al-Ahmary, K.M. Selenium content in selected foods from the Saudi Arabia market and estimation of the daily intake. Arab. J. Chem. 2009, 2, 95–99. [Google Scholar] [CrossRef]
- Al-Othman, A.M.; Al-Othman, Z.A.; El-Desoky, G.E.; Aboul-Soud, M.A.; Habila, M.A.; Giesy, J.P. Daily intake of selenium and concentrations in blood of residents of Riyadh City, Saudi Arabia. Environ. Geochem Health 2012, 34, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Sunde, R.A.; Paterson, E.; Evenson, J.K.; Barnes, K.M.; Lovegrove, J.A.; Gordon, M.H. Longitudinal selenium status in healthy British adults: Assessment using biochemical and molecular biomarkers. Br. J. Nutr. 2008, 99 (Suppl.3), 37–47. [Google Scholar] [CrossRef] [PubMed]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Selenomethionine: A Review of Its Nutritional Significance, Metabolism and Toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Gladyshev, V.N.; Hatfield, D.L. Selenocysteine-containing proteins in mammals. J. Biomed. Sci. 1999, 6, 151–160. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Egrise, D.; Nève, J.; Pasteels, J.L.; Schoutens, A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Min. Res. 2001, 16, 1556–1563. [Google Scholar] [CrossRef]
- Badr, D.M.; Hafez, H.F.; Agha, A.M.; Shouman, S.A. The Combination of alpha-Tocopheryl Succinate and Sodium Selenite on Breast Cancer: A Merit or a Demerit? Oxidative Med. Cell Longev. 2016, 2016, 4741694. [Google Scholar] [CrossRef]
- Berggren, M.; Sittadjody, S.; Song, Z.; Samira, J.L.; Burd, R.; Meuillet, E.J. Sodium selenite increases the activity of the tumor suppressor protein, PTEN, in DU-145 prostate cancer cells. Nutr. Cancer 2009, 61, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Lee, H.J.; Wang, Z.; Hu, H.; Liao, J.D.; Watts, J.C.; Combs, G.F., Jr.; Lu, J. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis 2008, 29, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Schroterova, L.; Kralova, V.; Voracova, A.; Haskova, P.; Rudolf, E.; Cervinka, M. Antiproliferative effects of selenium compounds in colon cancer cells: Comparison of different cytotoxicity assays. Toxicol. in Vitro 2009, 23, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother. Pharmacol. 2010, 66, 475–484. [Google Scholar] [CrossRef]
- Qi, Y.; Schoene, N.W.; Lartey, F.M.; Cheng, W.H. Selenium compounds activate ATM-dependent DNA damage response via the mismatch repair protein hMLH1 in colorectal cancer cells. J. Biol. Chem. 2010, 285, 33010–33017. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Dong, L.; Song, C.; Zhang, Y.; Zhu, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Huang, Z.; et al. Methylseleninic Acid Provided at Nutritional Selenium Levels Inhibits Angiogenesis by Down-regulating Integrin beta3 Signaling. Sci. Rep. 2017, 7, 9445. [Google Scholar] [CrossRef]
- Kao, R.H.; Lai, G.M.; Chow, J.M.; Liao, C.H.; Zheng, Y.M.; Tsai, W.L.; Hsia, S.; Lai, I.C.; Lee, H.L.; Chuang, S.E.; et al. Opposite Regulation of CHOP and GRP78 and Synergistic Apoptosis Induction by Selenium Yeast and Fish Oil via AMPK Activation in Lung Adenocarcinoma Cells. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Ren, H.; Mu, J.; Ma, J.; Gong, J.; Li, J.; Wang, J.; Gao, T.; Zhu, P.; Zheng, S.; Xie, J.; et al. Selenium Inhibits Homocysteine-Induced Endothelial Dysfunction and Apoptosis via Activation of AKT. Cell. Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem. Pharmacol. 2016, 38, 871–882. [Google Scholar] [CrossRef]
- Sakalli Cetin, E.; Naziroglu, M.; Cig, B.; Ovey, I.S.; Aslan Kosar, P. Selenium potentiates the anticancer effect of cisplatin against oxidative stress and calcium ion signaling-induced intracellular toxicity in MCF-7 breast cancer cells: Involvement of the TRPV1 channel. J. Recept. Signal. Transduct. Res. 2017, 37, 84–93. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Dunn, B.K. Selenium and prostate cancer prevention: Insights from the selenium and vitamin E cancer prevention trial (SELECT). Nutrients 2013, 5, 1122–1148. [Google Scholar] [CrossRef]
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of Selenium Supplementation for Cancer Prevention in Patients With Carcinoma of the Skin. A Randomized Controlled Trial. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The Epidemiology of Selenium and Human Cancer. In Selenium and Selenoproteins in Cancer; Tew, K.D., Galli, F., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 136, pp. 1–48. [Google Scholar]
- Wei, W.-Q.; Abnet, C.C.; Qiao, Y.-L.; Dawsey, S.M.; Dong, Z.-W.; Sun, X.-D.; Fan, J.-H.; Gunter, E.W.; Taylor, P.R.; Mark, S.D. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am. J. Clin. Nutr. 2004, 79, 80–85. [Google Scholar] [CrossRef]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.W.; Janeway, K.A.; Gorlick, R. Future directions in the treatment of osteosarcoma. Curr. Opin. Pediatrics 2016, 28, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. Sicot-J. 2018, 4, 12. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 320–325. [Google Scholar] [CrossRef]
- Stiller, C. Incidence of Childhood Leukaemia. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/97016/4.1.-Incidence-of-childhood-leukaemia-EDITED_layouted.pdf (accessed on 3 October 2019).
- Kager, L.; Tamamyan, G.; Bielack, S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017, 13, 357–368. [Google Scholar] [CrossRef]
- Moore, D.D.; Luu, H.H. Osteosarcoma. Cancer Treat. Res. 2014, 162, 65–92. [Google Scholar] [CrossRef]
- Daw, N.C.; Chou, A.J.; Jaffe, N.; Rao, B.N.; Billups, C.A.; Rodriguez-Galindo, C.; Meyers, P.A.; Huh, W.W. Recurrent osteosarcoma with a single pulmonary metastasis: A multi-institutional review. Br. J. Cancer 2015, 112, 278–282. [Google Scholar] [CrossRef]
- Anderson, M.E. Update on Survival in Osteosarcoma. Orthop. Clin. North. Am. 2016, 47, 283–292. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Hou, C.-H.; Hou, S.-M.; Yang, R.-S. The Metastasectomy and Timing of Pulmonary Metastases on the Outcome of Osteosarcoma Patients. Clin. Med. Oncol. 2009, 3, 99–105. [Google Scholar] [CrossRef]
- Beukhof, C.M.; Medici, M.; van den Beld, A.W.; Hollenbach, B.; Hoeg, A.; Visser, W.E.; de Herder, W.W.; Visser, T.J.; Schomburg, L.; Peeters, R.P. Selenium Status Is Positively Associated with Bone Mineral Density in Healthy Aging European Men. PLoS ONE 2016, 11, e0152748. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Cao, J.J.; Combs, G.F., Jr. Selenium in bone health: Roles in antioxidant protection and cell proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Komiya, S.; Hamada, T.; Zenmyo, M.; Inoue, A. Osteosarcoma cell apoptosis induced by selenium. J. Orthop Res. 2001, 19, 809–814. [Google Scholar] [CrossRef]
- Chen, X.J.; Duan, F.D.; Zhang, H.H.; Xiong, Y.; Wang, J. Sodium selenite-induced apoptosis mediated by ROS attack in human osteosarcoma U2OS cells. Biol. Trace Elem. Res. 2012, 145, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Zhang, D.; Zhang, Y.; Wen, Y.; Li, L.; Zheng, L. Tumoricidal effects of a selenium (Se)-polysaccharide from Ziyang green tea on human osteosarcoma U-2 OS cells. Carbohydr Polym 2013, 98, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Wu, R.T.; Wu, M.; Rocourt, C.R.; Carrillo, J.A.; Song, J.; Bohr, C.T.; Tzeng, T.J. Targeting Werner syndrome protein sensitizes U-2 OS osteosarcoma cells to selenium-induced DNA damage response and necrotic death. Biochem Biophys Res. Commun. 2012, 420, 24–28. [Google Scholar] [CrossRef]
- Tarrado-Castellarnau, M.; Cortes, R.; Zanuy, M.; Tarrago-Celada, J.; Polat, I.H.; Hill, R.; Fan, T.W.; Link, W.; Cascante, M. Methylseleninic acid promotes antitumour effects via nuclear FOXO3a translocation through Akt inhibition. Pharmacol. Res. 2015, 102, 218–234. [Google Scholar] [CrossRef]
- Huang, G.; Yong, B.C.; Xu, M.H.; Li, J.C.; Guo, H.H.; Shen, J.N. Analysis of Selenium Levels in Osteosarcoma Patients and the Effects of Se-Methylselenocysteine on Osteosarcoma Cells In Vitro. Nutr. Cancer 2015, 67, 847–856. [Google Scholar] [CrossRef]
- Wang, W.; Meng, F.B.; Wang, Z.X.; Li, X.; Zhou, D.S. Selenocysteine inhibits human osteosarcoma cells growth through triggering mitochondrial dysfunction and ROS-mediated p53 phosphorylation. Cell Biol. Int. 2018, 42, 580–588. [Google Scholar] [CrossRef]
- Tran, P.A.; Sarin, L.; Hurt, R.H.; Webster, T.J. Titanium surfaces with adherent selenium nanoclusters as a novel anticancer orthopedic material. J. Biomed. Mater. Res. A 2010, 93, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, V.; Iyer, M.A.; Wu, V.M. One Ion to Rule Them All: Combined Antibacterial, Osteoinductive and Anticancer Properties of Selenite-Incorporated Hydroxyapatite. J. Mater. Chem. B 2017, 5, 1430–1445. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Valencia, C.; Freixeiro, P.; Serra, J.; Ferreiros, C.M.; Gonzalez, P.; Lopez-Alvarez, M. In vitro evaluation of the antibacterial and osteogenic activity promoted by selenium-doped calcium phosphate coatings. Biomed. Mater. 2017, 12, 015028. [Google Scholar] [CrossRef] [PubMed]
- Stolzoff, M.; Webster, T.J. Reducing bone cancer cell functions using selenium nanocomposites. J. Biomed. Mater. Res. A 2016, 104, 476–482. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Zhou, L.; Chen, J.; Liu, Y.; Qiu, Z.; Zhang, S. Dual functional selenium-substituted hydroxyapatite. Interface Focus 2012, 2, 378–386. [Google Scholar] [CrossRef]
- Abul-Hassan, K.S.; Lehnert, B.E.; Guant, L.; Walmsley, R. Abnormal DNA repair in selenium-treated human cells. Mutat. Res. 2004, 565, 45–51. [Google Scholar] [CrossRef]
- Kolmas, J.; Pajor, K.; Pajchel, L.; Przekora, A.; Ginalska, G.; Oledzka, E.; Sobczak, M. Fabrication and physicochemical characterization of porous composite microgranules with selenium oxyanions and risedronate sodium for potential applications in bone tumors. Int. J. Nanomed. 2017, 12, 5633–5642. [Google Scholar] [CrossRef]
- Wojewoda, M.; Duszynski, J.; Szczepanowska, J. Antioxidant defence systems and generation of reactive oxygen species in osteosarcoma cells with defective mitochondria: Effect of selenium. Biochim Biophys Acta. 2010, 1797, 890–896. [Google Scholar] [CrossRef]
- Wojewoda, M.; Duszynski, J.; Wieckowski, M.; Szczepanowska, J. Effect of selenite on basic mitochondrial function in human osteosarcoma cells with chronic mitochondrial stress. Mitochondrion 2012, 12, 149–155. [Google Scholar] [CrossRef]
- Wojewoda, M.; Walczak, J.; Duszynski, J.; Szczepanowska, J. Selenite activates the ATM kinase-dependent DNA repair pathway in human osteosarcoma cells with mitochondrial dysfunction. Biochem. Pharmacol. 2015, 95, 170–176. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In Vitro and in Vivo Mechanism of Bone Tumor Inhibition by Selenium-Doped Bone Mineral Nanoparticles. ACS Nano 2016, 10, 9927–9937. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Simon, H.-U. A novel link between p53 and ROS. Cell Cycle 2013, 12, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Y.; St Clair, D.K. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Renault, V.M.; Thekkat, P.U.; Hoang, K.L.; White, J.L.; Brady, C.A.; Kenzelmann Broz, D.; Venturelli, O.S.; Johnson, T.M.; Oskoui, P.R.; Xuan, Z.; et al. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene 2011, 30, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Du, W.; Heese, K.; Wu, M. The Bad guy cooperates with good cop p53: Bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol. Cell Biol 2006, 26, 9071–9082. [Google Scholar] [CrossRef]
- Stambolic, V.; MacPherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T.W. Regulation of PTEN transcription by p53. Mol. Cell 2001, 8, 317–325. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of Poly(ADP-ribose) Polymerase (PARP) Cleavage in Apoptosis. Caspase 3-Resistant PARP Mutant Increases Rates of Apoptosis in Transfected Cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. CCS 2010, 8, 31. [Google Scholar] [CrossRef]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; de Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. CMLS 2015, 72, 3097–3113. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem Biophys 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Branstetter, D.; Rohrbach, K.; Huang, L.Y.; Soriano, R.; Tometsko, M.; Blake, M.; Jacob, A.P.; Dougall, W.C. RANK and RANK ligand expression in primary human osteosarcoma. J. Bone Oncol. 2015, 4, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Navet, B.; Ando, K.; Vargas-Franco, J.W.; Brion, R.; Amiaud, J.; Mori, K.; Yagita, H.; Mueller, C.G.; Verrecchia, F.; Dumars, C.; et al. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, E.M.; Gonzalez-Suarez, E. RANKL inhibitors for osteosarcoma treatment: Hope and caution. Ann. Transl. Med. 2016, 4, 534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Di Grappa, M.A.; Molyneux, S.D.; McKee, T.D.; Waterhouse, P.; Penninger, J.M.; Khokha, R. RANKL blockage prevents and treats aggressive osteosarcomas. Sci. Transl. Med. 2015, 7, 317ra197. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Ko, W.K.; Han, S.W.; Kim, D.S.; Hwang, Y.S.; Park, H.K.; Kwon, I.K. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem Biophys Res. Commun. 2012, 418, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Nadiminty, N.; Liao, H.; Cao, A.C. Selenium inhibits the expression of osteogenic genes associated with bone remodeling. In Proceedings of the AACR Annual Meeting, Los Angeles, CA, USA, 14–18 April 2007; American Association for Cancer Research: Philadephia, PA, USA, 2007; p. 2192. [Google Scholar]
- Zheng, Y.; Wang, G.; Chen, R.; Hua, Y.; Cai, Z. Mesenchymal stem cells in the osteosarcoma microenvironment: Their biological properties, influence on tumor growth, and therapeutic implications. Stem Cell Res. Ther. 2018, 9, 22. [Google Scholar] [CrossRef]
- Liu, H.; Bian, W.; Liu, S.; Huang, K. Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway. Biol. Trace Elem. Res. 2012, 150, 441–450. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, J.; Liu, Y.; Yu, Q.; Liu, Y.; Deng, N.; Liu, J. Functional Selenium Nanoparticles Enhanced Stem Cell Osteoblastic Differentiation through BMP Signaling Pathways. Adv. Funct. Mater. 2014, 24, 6872–6883. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Aglan, H.A.; Mabrouk, M.; Abd-Rabou, A.A.; Beherei, H.H. Enhanced mesenchymal stem cell proliferation through complexation of selenium/titanium nanocomposites. J. Mater. Sci. Mater. Med. 2019, 30, 24. [Google Scholar] [CrossRef]
- Margulies, B.S.; Damron, T.A.; Allen, M.J. The differential effects of the radioprotectant drugs amifostine and sodium selenite treatment in combination with radiation therapy on constituent bone cells, Ewing’s sarcoma of bone tumor cells, and rhabdomyosarcoma tumor cells in vitro. J. Orthop Res. 2008, 26, 1512–1519. [Google Scholar] [CrossRef]
- Tran, P.A.; Sarin, L.; Hurt, R.H.; Webster, T.J. Differential effects of nanoselenium doping on healthy and cancerous osteoblasts in coculture on titanium. Int. J. Nanomed. 2010, 5, 351–358. [Google Scholar]
- Ebert, R.; Ulmer, M.; Zeck, S.; Meissner-Weigl, J.; Schneider, D.; Stopper, H.; Schupp, N.; Kassem, M.; Jakob, F. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells 2006, 24, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.H.; Nam, S.W.; Kim, B.W.; Kim, G.Y.; Kim, W.J.; Choi, Y.H. Selenium improves stem cell potency by stimulating the proliferation and active migration of 3T3-L1 preadipocytes. Int. J. Oncol. 2014, 44, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, V. Influence of silver and selenium nanoparticles on mesenchymal stromal cells. Environ. Res. Eng. Manag. 2019, 74. [Google Scholar] [CrossRef]
- Burgess, D.J. Anticancer Drugs: Selective oxycution? Nat. Rev. Drug Discov. 2011, 10, 658. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z. Increased Oxidative Stress as a Selective Anticancer Therapy. Oxidative Med. Cell. Longev. 2015, 2015, 294303. [Google Scholar] [CrossRef]
- Carrle, D.; Bielack, S.S. Current strategies of chemotherapy in osteosarcoma. Int. Orthop. (SICOT) 2006, 30, 445–451. [Google Scholar] [CrossRef]
- Spina, A.; Sorvillo, L.; Di Maiolo, F.; Esposito, A.; D’Auria, R.; Di Gesto, D.; Chiosi, E.; Naviglio, S. Inorganic phosphate enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin via a p53-dependent pathway. J. Cell. Physiol. 2013, 228, 198–206. [Google Scholar] [CrossRef]
- Clement, M.V.; Pervaiz, S. Intracellular superoxide and hydrogen peroxide concentrations: A critical balance that determines survival or death. Redox Rep. Commun. Free Radic. Res. 2001, 6, 211–214. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2014, 7, 1875–1884. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, H.; Liu, H.; Wang, Y.; Li, Y.; Yang, G.; Ma, J.; Mao, C.; Zhang, S. Selenite-Releasing Bone Mineral Nanoparticles Retard Bone Tumor Growth and Improve Healthy Tissue Functions In Vivo. Adv. Healthc. Mater. 2015, 4, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Bierke, P.; Svedenstal, B.M. The influence of selenium, vitamin E, and oestrogen on the development of tumours in mice exposed to 90Sr. Acta Oncol. (Stockh. Swed.) 1994, 33, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Combs, G.F., Jr. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.F.; Cantor, K.P.; Silverman, D.T.; Malats, N. Selenium and bladder cancer risk: A meta-analysis. Cancer Epidemiol Biomark. Prev. 2010, 19, 2407–2415. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; Karp, D.D.; et al. Vitamin E and the Risk of Prostate Cancer. The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- WHO. Diet. Nutrition and the Prevention of Chronic Diseases; World Health Organization: Geneva, Switzerland, 2003; pp. 1–149. [Google Scholar]
- Vinceti, M.; Filippini, T.; del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018, 1. [Google Scholar] [CrossRef]
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J. Natl Cancer Inst. 2014, 106, djt456. [Google Scholar] [CrossRef]
- Lener, M.R.; Gupta, S.; Scott, R.J.; Tootsi, M.; Kulp, M.; Tammesoo, M.-L.; Viitak, A.; Metspalu, A.; Serrano-Fernández, P.; Kładny, J.; et al. Can selenium levels act as a marker of colorectal cancer risk? BMC Cancer 2013, 13, 214. [Google Scholar] [CrossRef]
- Thompson, P.A.; Ashbeck, E.L.; Roe, D.J.; Fales, L.; Buckmeier, J.; Wang, F.; Bhattacharyya, A.; Hsu, C.H.; Chow, H.H.; Ahnen, D.J.; et al. Selenium Supplementation for Prevention of Colorectal Adenomas and Risk of Associated Type 2 Diabetes. J. Natl Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Vinceti, M.; Dennert, G.; Crespi, C.M.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.A.; Horneber, M.; D’Amico, R.; del Giovance, C. Selenium for preventing cancer. Cochrane Database Syst Rev. 2014. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).