Progress in Tissue Culture and Genetic Transformation of Oil Palm: An Overview
Abstract
:1. Introduction
2. Oil palm Propagation Methods
In Vitro Propagation of Oil Palm
3. Genetic Diversity Conservation of Oil Palm
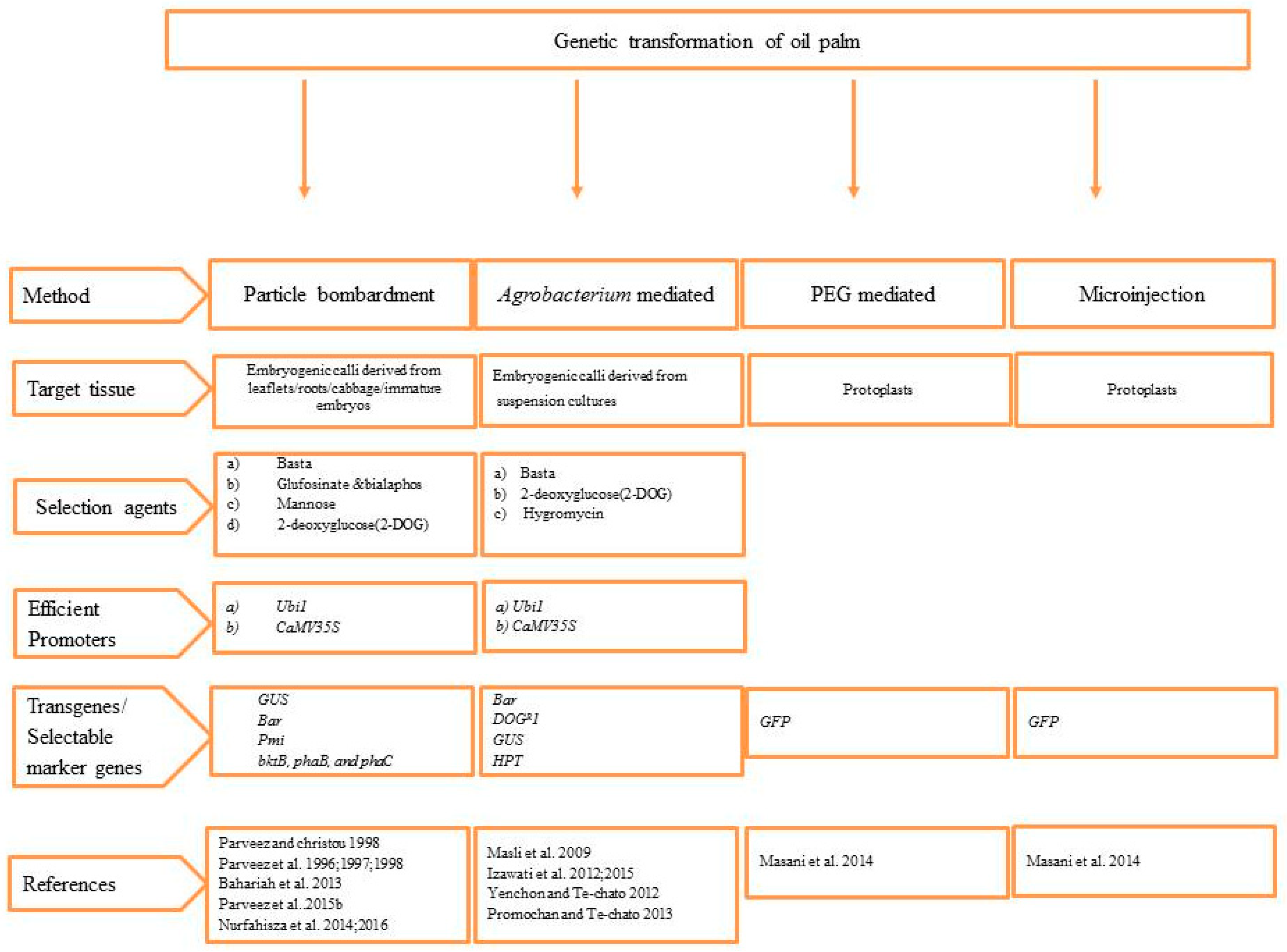
4. Genetic Transformation of Oil Palm
4.1. Particle Bombardment Mediated Genetic Transformation of Oil Palm
4.1.1. Factors Influencing Particle-Bombardment-Mediated Transformation
4.1.2. Selection of Promoters
4.1.3. Physical Parameters
4.1.4. Biological Parameters
4.1.5. Adequate Selection Conditions
4.1.6. Production of Oil Palm Transgenics Via Particle-Bombardment-Mediated Transformation
4.2. Agrobacterium-Mediated Genetic Transformation of Oil Palm
4.3. Protoplast Transformation of Oil Palm by PEG-Mediated and Microinjection Techniques
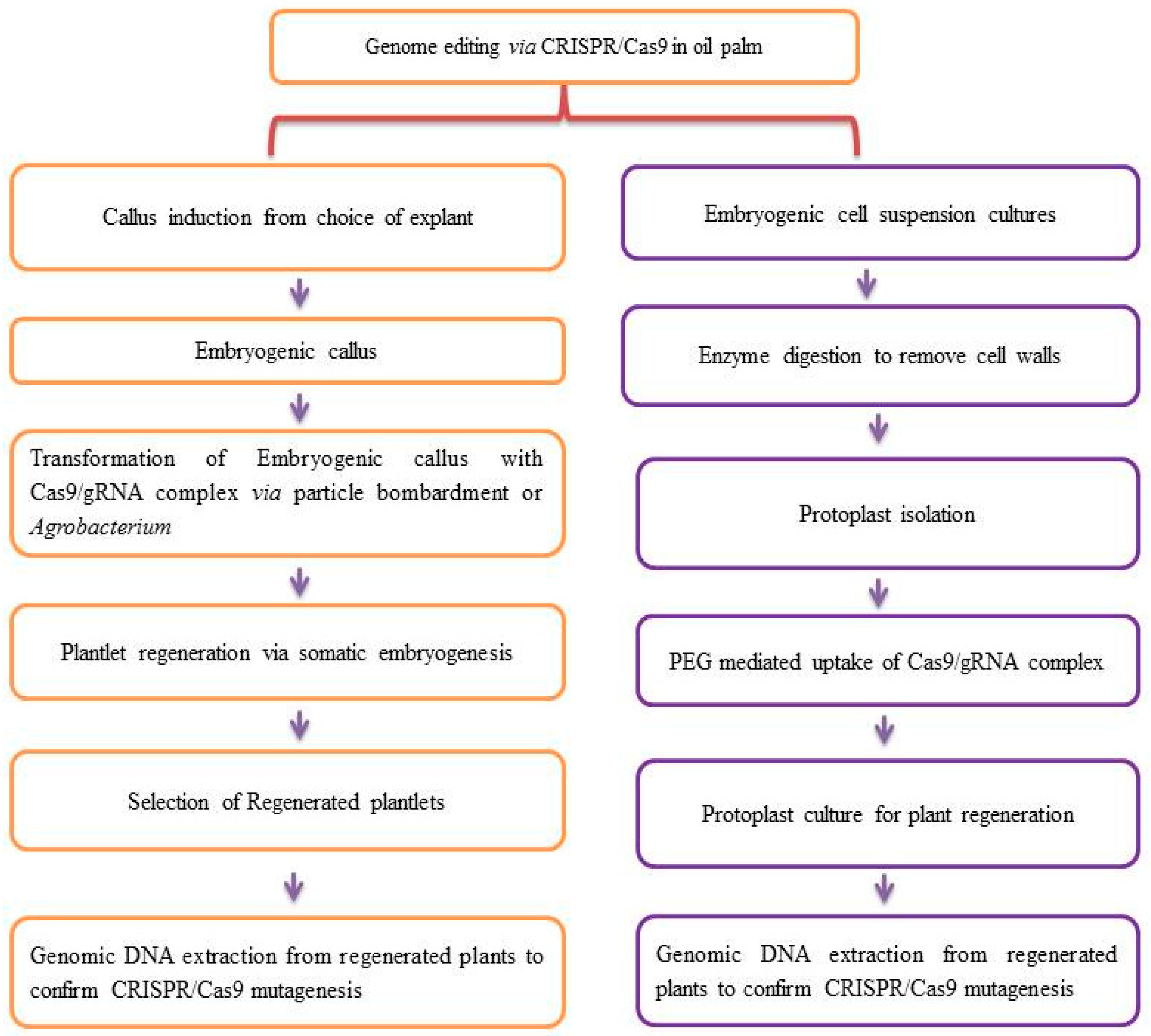
5. Oil Palm Genome Editing Through CRISPR/Cas9 Technology
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hormaza, P.; Fuquen, E.M.; Romero, H.M. Phenology of the oil palm interspecific hybrid Elaeis oleifera × Elaeis guineensis. Sci. Agric. 2012, 69, 275–280. [Google Scholar] [CrossRef]
- Sumaryono, R.I.; Saptari, R.T.; Rahmadi, H.Y.; Ernayunita. Embryogenic callus initiation from leaf explants of Elaeis oleifera x Elaeis guineensis (OxG) hybrids. IOP Conf. Series 2018, 183. [Google Scholar] [CrossRef]
- Weckx, S.; Inzé, D.; Maene, L. Tissue Culture of Oil Palm: Finding the Balance between Mass Propagation and Somaclonal Variation. Front. Plant Sci. 2019, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- Huth, N.I.; Banabas, M.; Nelson, P.N.; Webb, M. Development of an oil palm cropping systems model: Lessons learned and future directions. Environ. Modell. Softw. 2014, 62, 411–419. [Google Scholar] [CrossRef]
- FAOSTAT. Database Food and Agriculture Organization of the United Nations; FAOSTAT: Rome, Italy, 2013; Available online: www.faostat.fao.org (accessed on 16 May 2013).
- Masani, M.Y.A.; Izawati, A.M.D.; Rasid, O.A.; Parveez, G.K.A. Biotechnology of oil palm: Current status of oil palm genetic transformation. Biocat. Agric. Biotechnol. 2018, 15, 335–347. [Google Scholar] [CrossRef]
- Corley, R.H.V. How much palm oil do we need? Environ. Sci. Policy 2009, 12, 134–139. [Google Scholar] [CrossRef]
- Zulkifli, Y.; Norziha, A.; Naqiuddin, M.H.; Fadila, A.M.; Nor Azwani, A.B.; Suzana, M.; Samsul, K.R.; Ong-Abdullah, M.; Singh, R.; Parveez, G.K.A.; et al. Designing the oil palm of the future. J. Oil Palm Res. 2017, 29, 440–455. [Google Scholar]
- Mielke, T. Palm Oil the Leader in Global Oils & Fats Supply. 2013. Available online: http://www.mpoc.org.my/upload/Plenary_Paper-Thomas-Mielke (accessed on 16 May 2013).
- Jayanthi, M.; Susanthi, B.; Murali Mohan, N.; Mandal, P.K. In vitro somatic embryogenesis and plantlet regeneration from immature male inflorescence of adult dura and tenera palms of Elaeis guineensis (Jacq.). Springer Plus 2015, 4, 256. [Google Scholar] [CrossRef]
- Basiron, Y. The Palm-Oil Advantage in Biofuel. New Straits Times. 24 February 2007. Available online: http:// www.mpoc.org.my (accessed on 24 February 2007).
- Green, M.; Lima, W.A.A.; de Figueiredo, A.F.; Atroch, A.L.; Lopes, R.; da Cunha, R.N.V.; Teixeira, P.C. Heat-treatment and germination of oil palm seeds (Elaeis guineensis Jacq.). J. Seed Sci. 2013, 35, 296–301. [Google Scholar] [CrossRef]
- Kushairi, A.; Tarmizi, A.H.; Zamzuri, I.; Ong-Abdullah, M.; Samsul Kamal, R.; Ooi, S.E.; Rajanaidu, N. Production, performance and advances in oil palm tissue culture. In Proceedings of the International Society of Oil Palm Breeders (ISOPB) Seminar on Advances in Oil Palm Tissue Culture, Yogyakarta, Indonesia, 29 May 2010. [Google Scholar]
- Lee, F.C.; Ong-Abdullah, M.; Ooi, S.E.; Ho, C.L.; Namasivayam, P. Cloning and characterization of Somatic Embryogenesis Receptor Kinase I (EgSERK I) and its association with callus initiation in oil palm. In Vitro Cell Dev. Biol. Plant 2019, 55, 153. [Google Scholar] [CrossRef]
- Muniran, F.; Bhore, S.J.; Shah, F.H. Micropropagation of Elaies guineensis Jacq. ‘Dura’: comparison of three basal media for efficient regeneration. Indian J. Exp. Biol. 2008, 46, 79–82. [Google Scholar] [PubMed]
- Tan, C.C.; Wong, G.; Soh, A.C.; Hor, T.Y.; Chong, S.P.; Gopal, K. Experiences and lessons from oil palm clonal evaluation trials and commercial test plantings. In Proceedings of the 2003 PIPOC International Palm Oil Congress, Malaysian Palm Oil Board, Kuala Lumpur, Malaysia, 24–28 August 2003; pp. 1093–1119. [Google Scholar]
- Kushairi, A.; Singh, R.; Ong-Abdullah, M. The oil palm industry in Malaysia: Thriving with transformative technologies. J. Oil Palm Res. 2017, 29, 431–439. [Google Scholar]
- Kushairi, A.; Mohd Din, A.; Rajanaidu, N. Oil palm breeding and seed production. In Further Advances in Oil Palm Research (2000–2010) 1; Mohd Basri, W., Choo, Y.M., Chan, K.W., Eds.; Malaysian Palm Oil Board: Kuala Lumpur, Malaysia, 2011; pp. 47–93. [Google Scholar]
- Rajanaidu, N.; Kushairi, A.; Rafii, M.; Mohd Din, A.; Maizura, I.; Jalani, B.S. Oil palm breeding and genetic resources. In Advances in Oil Palm Research; Basiron, Y., Jalani, B.S., Chan, K.W., Eds.; Malaysian Palm Oil Board: Kuala Lumpur, Malaysia, 2000; pp. 171–237. [Google Scholar]
- Zou, J.; Zhang, Q.; Zhu, Z.; Gao, L.; Zheng, Y.; Li, D. Embryogenic callus induction and fatty acid composition analysis of oil palm (Elaeis guineensis cv. Tenera). J. Sci. 2019. [Google Scholar] [CrossRef]
- Padua, M.S.S.; Santos, R.S.; Paiva, L.V.; Steins, V.C.; Silva, L.C. In vitro rooting of tenera hybrid oil palm (Elaeis guineensis Jacq.) plants. Rev. Arvore. 2017, 41. [Google Scholar] [CrossRef]
- Jones, L.H. Propagation of clonal oil palms by tissue culture. Planter 1974, 50, 374–381. [Google Scholar]
- Rabéchault, H.; Martin, J.P. Multiplication végétative du palmier à huile (Elaeis guineensis Jacq.) à l’aide de cultures de tissus foliaires. C. R. Acad. Sci. Paris Ser. D 1976, 283, 1735–1737. [Google Scholar]
- Staritsky, G. Tissue culture of the oil palm (Elaeis guineensis Jacq.) as a tool for vegetative propagation. Euphytica 1970, 19, 5. [Google Scholar] [CrossRef]
- Monteiro, T.R.; Freitas, E.O.; Nogueira, G.F.; Scherwinski-Pereira, J.E. Assessing the influence of subcultures and liquidmedium during somatic embryogenesis and plant regeneration in oilpalm (Elaeis guineensis Jacq.). J. Hortic. Sci. Biotechnol. 2017, 93, 196–203. [Google Scholar] [CrossRef]
- Feher, A.; Pasternak, T.P.; Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell. Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Hashim, A.T.; Ishak, Z.; Rosli, S.K.; Ong-Abdullah, M.; Ooi, S.-E.; Husri, M.N.; Bakar, D.A. Oil palm (Elaeis guineensis Jacq.) somatic embryogenesis. In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Jain, S.M., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; pp. 209–229. [Google Scholar] [CrossRef]
- Constantin, M.; Nchu, W.A.; Godswill, N.; Wiendi, N.M.A.; Wachjar, A.; Frank, N.E.G. Induction of oil palm (Elaeis guineensis Jacq. var. Tenera) callogenesis and somatic embryogenesis from young leaf explants. J. Appl. Biol. Biotechnol. 2015, 3, 4–10. [Google Scholar] [CrossRef]
- Eeuwens, C.J.; Lord, S.; Donough, C.R.; Rao, V.; Vallejo, G.; Nelson, S. Effects of tissue culture conditions during embryoid multiplication on the incidence of “mantled” flowering in clonally propagated oil palm. Plant Cell. Tiss Org Cult. 2002, 70, 311–323. [Google Scholar] [CrossRef]
- Rajesh, M.K.; Radha, E.; Karun, A.; Parthasarathy, V.A. Plant regeneration from embryo-derived callus of oil palm—The effect of exogenous polyamines. Plant Cell. Tiss Org Cult. 2003, 75, 41–47. [Google Scholar] [CrossRef]
- Corrêa, T.R.; Motoike, S.Y.; de Souza Andrade, A.P.; Coser, S.M.; Queiroz, V.; Granja, M.M.C.; Caetano, D.D.N.; Pena, C.N.M.; Picoli, E.A.d.T. Accelerated in vitro propagation of elite oil palm genotypes (Elaeis guineensis Jacq.) by substituting cytokinin with putrescine. Afr. J. Biotechnol. 2016, 15, 2767–2775. [Google Scholar] [CrossRef]
- Ong-Abdullah, M.; Ordway, J.M.; Jiang, N.; Ooi, S.E.; Kok, S.Y.; Sarpan, N.; Azimi, N.; Hashim, A.T.; Ishak, Z.; Rosli, S.K.; et al. Loss of Karma transposon methylation underlies the mantled somaclonal variant of oil palm. Nature 2015, 525, 533–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwee, S.S.; Linder, V.; Schoot, J.V.T.; Folter, S.; Angent, G.C.; Cheach, S.C.; Smulders, M.J.M. Characterization of oil palm MADS box genes in relation to the mantled flower abnormality. Plant Cell. Tissue Org. Cult. 2006, 85, 331–344. [Google Scholar] [CrossRef]
- Gomes, H.T.; Bartos, P.M.C.; Balzon, T.A.; Scherwinski-Pereira, J.E. Regeneration of somatic embryos of oil palm (Elaeis guineensis) using temporary immersion bioreactors. Ind. Crops Prod. 2016, 89, 244–249. [Google Scholar] [CrossRef]
- Gomes, H.T.; Bartos, P.M.C.; Scherwinski-Pereira, J.E. Optimizing rooting and survival of oil palm (Elaeis guineensis) plantlets derived from somatic embryos. In Vitro Cell. Dev. Biol. 2015, 51, 111–117. [Google Scholar] [CrossRef]
- De Carvalho Silva, R.; Luis, Z.G.; Scherwinski-Pereira, J.E. The histo differentiation events involved during the acquisition and development of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.). Plant Growth Regul. 2014, 72, 67–80. [Google Scholar] [CrossRef]
- Balzon, T.A.; Luis, Z.G.; Scherwinski-Pereira, J.E. New approaches to improve the efficiency of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.) from mature zygotic embryos. In Vitro Cell. Dev. Biol. 2013, 49, 41–50. [Google Scholar] [CrossRef]
- Wan Nur Syuhada, W.S.; Rasid, O.A.; Parveez, G.K.A. Evaluation on the effects of culture medium on regeneration of oil palm plantlets from immature embryos (IE). J. Oil Palm Res. 2016, 28, 234–239. [Google Scholar] [CrossRef]
- Thuzar, M.; Vanavichit, A.; Tragoonrung, S.; Jantasuriyarat, C. Efficient and rapid plant regeneration of oil palm zygotic embryos cv. “Tenera” through somatic embryogenesis. Acta Physiol. Plant. 2011, 33, 123–128. [Google Scholar] [CrossRef]
- Guedes, S.; Da, R.; da Silva, T.L.; Luis, Z.G.; Scherwinski-Pereira, J.E. Initial requirements for embryogenic calluses initiation in thin cell layers explants from immature female oil palm inflorescences. Afr. J. Biotechnol. 2011, 10, 10774–10780. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, J.B.; Söndahl, M.R.; Kirby, E.G. Somatic embryogenesis from immature inflorescences of oil palm. Plant Cell Rep. 1994, 13, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.T.; Bartos, P.M.C.; Scherwinski-Pereira, J.E. Dynamics of morphological and anatomical changes in leaf tissues of an interspecific hybrid of oil palm during acquisition and development of somatic embryogenesis. Plant Cell. Tissue Organ Cult. 2017, 131, 269–282. [Google Scholar] [CrossRef] [Green Version]
- De Touchet, B.; Duval, Y.; Pannetier, C. Plant regeneration from embryogenic suspension cultures of oil palm (Elaeis guineensis Jacq.). Plant Cell Rep. 1991, 10, 529–532. [Google Scholar] [CrossRef]
- Jayanthi, M.; Mohan, N.M.; Mandal, P.K. Direct somatic embryogenesis and plantlet regeneration in oil palm. J. Plant Biochem. Biotechnol. 2011, 20, 249–251. [Google Scholar] [CrossRef]
- Scherwinski-Pereira, J.E.; da Guedes, R.S.; Fermino, P.C.P.; Silva, T.L.; Costa, F.H.S. Somatic embryogenesis and plant regeneration in oil palm using the thin cell layer technique. In Vitro Cell. Dev. Biol. 2010, 46, 378–385. [Google Scholar] [CrossRef]
- Romyanon, K.; Mosaleeyanon, K.; Kirdmanee, C. Direct-shoot organogenesis as an alternative protocol for in vitro regeneration of oil palm (Elaeis guineensis Jacq.). Sci. Hortic-Amsterdam 2015, 195, 1–7. [Google Scholar] [CrossRef]
- Ho, W.K.; Ooi, S.E.; Mayes, S.; Namasivayam, P.; Ong-Abdullah, M.; Chin, C.F. Methylation levels of a novel genetic element, EgNB3 as a candidate biomarker associated with the embryogenic competency of oil palm. Tree Genet. Genomes. 2013, 9, 1099–1107. [Google Scholar] [CrossRef]
- Mariani, T.S.; Sasmitamiharja, D.; Mienanti, D.; Latif, S.; Ginting, G.; Miyake, H. Somatic Embryogenesis of Oil Palm (Elaeis guineensis Jacq.) for Synthetic Seed Production. As. J. Appl. Sci. 2014, 2, 3. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Eeuwens, C.J. Effects of organic nutrients and hormones on growth and development of tissue explants from coconut (Cocos nucifera) and Date (Phoenix dactylifera) palms cultured in vitro. Physiol. Plant. 1978, 42, 173–178. [Google Scholar] [CrossRef]
- Chu, C.C.; Wang, C.C.; Sun, C.S.; Hsu, C.; Yin, K.C.; Chu, C.Y.; Bi, F.Y. Establishment of an efficient medium for another culture of rice through comparative experiments on nitrogen sources. Sci. Sin. 1975, 18, 659–668. [Google Scholar]
- Bakoumé, C. Genetic Diversity, Erosion, and Conservation in Oil Palm (Elaeis guineensis Jacq.). In Genetic Diversity and Erosion in Plants; Sustainable Development and Biodiversity; Ahuja, M., Jain, S., Eds.; Springer: Cham, Switzerland, 2018; p. 8. [Google Scholar]
- Teixeira da Silva, J.A.; Engelmann, F. Cryopreservation of oil palm (Elaeis guineensis Jacq.). Cryobiology 2017, 77, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F.; Duval, J.Y. Dereuddre Survival and proliferation of oil palm (Elaeis guineensis Jacq.) somatic embryos after freezing in liquid nitrogen. C. R. Acad. Sci. Paris 1985, 301, 111–116. [Google Scholar]
- Parveez, G.K.A.; Rasid, O.A.; Masani, M.Y.A.; Sambanthamurthi, R. Biotechnology of oil palm: Strategies towards manipulation of lipid content and composition. Plant Cell Rep. 2015, 34, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Izawati, A.M.D.; Masani, M.Y.A.; Ismanizan, I.; Parveez, G.K.A. Evaluation on the effectiveness of 2-deoxyglucose-6-phosphate phosphatase (DOGR1) gene as a selectable marker for oil palm (Elaeis guineensis Jacq.) embryogenic calli transformation mediated by Agrobacterium tumefaciens. Front. Plant Sci. 2015, 6, 727. [Google Scholar] [CrossRef]
- Hashim, A.T.; Ishak, Z.; Ooi, S.E.; Rosli, S.K.; Chan, P.L.; Rohani, O.; Ong-Abdullah, M. Forging ahead with clones. In Further Advances in Oil Palm Research; Wahid, M.B., Choo, Y.M., Chan, K.W., Eds.; Malaysian Palm Oil Board: Kuala Lumpur, Malaysia, 2011; pp. 102–140. [Google Scholar]
- Parveez, G.K.A. Production of transgenic oil palm (Elaeis guineensis Jacq.) using biolistic techniques. In Molecular Biology of Woody Plants 2; Jain, S.N., Minocha, S.C., Eds.; Kluwer Academic Publishers: Tranbjerg, Denmark, 2000; pp. 327–350. [Google Scholar]
- Sanford, J.C.; Klein, T.M.; Wolf, E.D.; Allen, N. Delivery of substances into cells and tissues using a particle bombardment process. J. Part. Sci. Tech. 1987, 6, 559–563. [Google Scholar] [CrossRef]
- Christou, P.; McCabe, D.E.; Swain, W.F. Stable transformation of soybean callus by DNA coated particles. Plant Physiol. 1988, X7, 671–674. [Google Scholar] [CrossRef]
- Klein, T.M.; Harper, E.C.; Svab, Z.; Sanford, L.C.; Fromm, M.E.; Maliga, P. Stable genetic transformation of intact Nicotiana cells by the particle bombardment process. Proc. Natl. Acad. Sci. USA 1988, 85, 8502–8505. [Google Scholar] [CrossRef]
- Finer, J.J.; Finer, K.R.; Ponappa, T. Particle Bombardment Mediated Transformation. In Current Topics Microbiology and Immunology; Plant Biotechnology; Hammond, J., McGarvey, P., Yusibov, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; p. 240. [Google Scholar]
- Parveez, G.K.A.; Chowdhury, M.K.U.; Saleh, N.M. Biological parameters affecting transient GUS gene expression in oil palm (Elaeis guineensis Jacq.) embryogenic calli via microprojectile bombardment. Ind. Crops Prod. 1998, 8, 17–27. [Google Scholar] [CrossRef]
- Parveez, G.K.A.; Chowdhury, M.K.U.; Saleh, N.M. Physical parameters affecting transient GUS gene expression in oil palm (Elaeis guineensis Jacq.) using the biolistic device. Ind. Crops Prod. 1997, 6, 41–50. [Google Scholar] [CrossRef]
- Chowdhury, M.K.U.; Parveez, G.K.A.; Saleh, N.M. Evaluation of five promoters for use in transformation of oil palm (Elaeis guineensis Jacq.). Plant Cell Rep. 1997, 16, 277–281. [Google Scholar] [PubMed]
- Parveez, G.K.A.; Chowdhury, M.K.U.; Saleh, N.M. Determination of minimal inhibitory concentration of selection agents for oil palm (Elaies guineesis Jacq.) transformation. As. Pac. J. Mol. Biol. Biotechnol. 1996, 4, 219–228. [Google Scholar]
- Parveez, G.K.A.; Christou, P. Biolistic-mediated DNA delivery and isolation of transgenic oil palm (Elaeis guineensis Jacq.) embryogenic callus cultures. J. Oil Palm Res. 1998, 10, 29–38. [Google Scholar]
- Nurfahisza, A.R.; Rafiqah, M.A.; Masani, M.Y.A.; Hanin, A.N.; Rasid, O.A.; Parveez, G.K.A.; Ismail, I. Molecular analysis of transgenic oil palm to detect the presence of transgenes. J. Oil Palm Res. 2014, 26, 73–80. [Google Scholar]
- Nurfahisza, A.R.; Rafiqah, M.A.; Parveez, G.K.A.; Rasid, O.A. Comparison of the effectiveness of Basta, bialaphos and glufosinate ammonium for selecting transformed oil palm tissues. J. Oil Palm Res. 2016, 28, 247–255. [Google Scholar] [CrossRef]
- Bahariah, B.; Parveez, G.K.A.; Khalid, N. Determination of optimal concentration of mannose as a selection agent for selecting transformed oil palm cells using the phosphomannose isomerase (pmi) gene as the positive selectable marker. J. Oil Palm Res. 2012, 24, 1250–1259. [Google Scholar]
- Bahariah, B.; Parveez, G.K.A.; Masani, M.Y.A.; Masura, S.S.; Khalid, N.; Othman, R.Y. Biolistic transformation of oil palm using the phosphomannose isomerase (pmi) gene as a positive selectable marker. Biocatal. Agric. Biotechnol. 2013, 2, 295–304. [Google Scholar] [CrossRef]
- Masli, D.I.A.; Parveez, G.K.A.; Ismail, I. Optimisation of 2-deoxyglucose concentration for identifying the sensitivity level for oil palm embryogenic calli. J. Oil Palm Res. 2012, 24, 1296–1302. [Google Scholar]
- Parveez, G.K.A.; Bahariah, B.; Ayub, N.H.; Masani, M.Y.A.; Rasid, O.A.; Tarmizi, A.H.; Ishak, Z. Production of polyhydroxybutyrate in oil palm (Elaeis guineensis Jacq.) mediated by microprojectile bombardment of PHB biosynthesis genes into embryogenic calli. Front. Plant Sci. 2015, 6, 598. [Google Scholar] [CrossRef] [PubMed]
- Tzfira, T.; Citovsky, V. Agrobacterium-mediated genetic transformation of plants: Biology and biotechnology. Curr. Opin. Biotech. 2006, 17, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Raineri, D.M.; Bottino, P.; Gordon, M.P.; Nester, E.W. Agrobacterium tumefaciens mediated transformation of rice (Oryza sativa L.). Bio/Technology 1990, 8, 33–38. [Google Scholar]
- Ahmed, R.I.; Ding, A.; Xie, M.; Kong, Y. Progress in Optimization of Agrobacterium-Mediated Transformation in Sorghum (Sorghum bicolor). Int. J. Mol. Sci. 2018, 29, 19. [Google Scholar] [CrossRef]
- Koetle, M.J.; Finnie, J.F.; Balázs, E.; Van Staden, J. A review on factors affecting the Agrobacterium-mediated genetic transformation in ornamental monocotyledonous geophytes. S. Afr. J. Bot. 2015, 98, 37–44. [Google Scholar] [CrossRef]
- Hiei, Y.; Ishida, Y.; Komari, T. Progress of cereal transformation technology mediated by Agrobacterium tumefaciens. Front. Plant Sci. 2014, 5, 628. [Google Scholar] [CrossRef]
- Masli, D.I.A.; Parveez, G.K.A.; Yunus, A.M.M. Transformation of oil palm using Agrobacterium tumefaciens. J. Oil Palm Res. 2009, 21, 643–652. [Google Scholar]
- Izawati, A.M.D.; Parveez, G.K.A.; Masani, M.Y.A. Transformation of oil palm using Agrobacterium tumefaciens. Methods Mol. Biol. 2012, 847, 177–188. [Google Scholar]
- Yenchon, S.; Te-chato, S. Effect of bacteria density, inoculation and cocultivation period on Agrobacterium-mediated transformation of oil palm embryogenic callus. J. Agric. Technol. 2012, 8, 1485–1496. [Google Scholar]
- Promochan, T.; Te-Chato, S. Strains of Agrobacterium affecting gene transformation through embryogenic cell suspension of hybrid tenera oil palm. J. Agric. Technol. 2013, 9, 669–679. [Google Scholar]
- Parveez, G.K.A. Optimization of Parameters Involved in the Transformation of Oil Palm Using the Biolistics Method. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Malaysia, 1998. [Google Scholar]
- Masani, M.Y.A.; Noll, G.; Parveez, G.K.A.; Sambanthamurthi, R.; Prüfer, D. Regeneration of viable oil palm plants from protoplasts by optimizing media components, growth regulators and cultivation procedures. Plant Sci. 2013, 210, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Masani, M.Y.A.; Noll, G.; Parveez, G.K.A.; Sambanthamurthi, R.; Pruefer, D. Efficient transformation of oil palm protoplasts by PEG-mediated transfection and DNA microinjection. PLoS ONE 2014, 9, e96831. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Ann. Rev. Plant Bio. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Haque, E.; Taniguchi, H.; Hassan, M.M.; Bhowmik, P.; Karim, M.R.; Śmiech, M.; Zhao, K.; Rahman, M.; Islam, T. Application of CRISPR/Cas9 Genome Editing Technology for the Improvement of Crops Cultivated in Tropical Climates: Recent Progress, Prospects, and Challenges. Front. Plant Sci. 2018, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.N.; Iqbal, Z.; Tahir, M.N.; Shahid, M.S.; Khurshid, M.; Al-Khateeb, A.A.; Al-Khateeb, S.A. CRISPR/Cas9: A Practical Approach in Date Palm Genome Editing. Front. Plant Sci. 2017, 8, 1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, S.N.A.; Azzeme, A.M.; Ebrahimi, M.; Ariff, E.A.K.E.; Hanifiah, F.H.A. Transcription Factors Associated with Abiotic Stress and Fruit Development in Oil Palm. In Crop Improvement; Abdullah, S., Chai-Ling, H., Wagstaff, C., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Arshad, C.M.; Armanto, M.E.; Zain, A.M. Evaluation of climate suitability for oil palm (Elaeis guineensis Jacq.) cultivation. J. Environ. Sci. Eng. 2012, B1, 272–276. [Google Scholar]
- Singh, R.; Ong-Abdullah, M.; Low, E.T.; Manaf, M.A.; Rosli, R.; Nookiah, R.; Ooi, L.C.; Ooi, S.E.; Chan, K.L.; Halim, M.A.; et al. Oil palm genome sequence reveals divergence of interfertile species in Old and New worlds. Nature 2013. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, L.; Lei, X.; Cao, H.; Wang, Y.; Dou, Y.; Tang, W.; Xia, W. Genome-wide identification of WRKY genes and their expression profiles under different abiotic stresses in Elaeis guineensis. PLoS ONE 2017, 12, e0189224. [Google Scholar] [CrossRef]

| References | Initial Ex-Plant | Growth Phase | Culture Media & Plant Growth Regulators |
|---|---|---|---|
| Hashim et al. [27] | Young leaf | Callus induction Somatic embryo initiation/proliferation/maturation Plantlet regeneration Rooting | MS + 27–54 µM NAA; 0–4.5 µM 2,4-D MS + Half of the above PGR’s conc. followed by a gradual decrease MS + 0.11 µM NAA MS + 1.1 µM NAA |
| Gomes et al. [42] | Young leaf | Callus induction Somatic embryogenesis Maturation of SE | MS + 450 µM Picloram + 2.5 g/L Activated Charcoal MS + 12.3 ip; 0.54 NAA MS + 2.5g/L Activated Charcoal |
| Corrêa et al. [31] | Young leaf | Callus induction Callus Proliferation Somatic embryo initiation/maturation Plantlet regeneration | Y3 + 800 µM 2,4-D + 3.0g/L Activated Charcoal Y3 + 9 µM 2,4-D; 1000 µM Putrescine Y3 + 0.1 µM 2,4-D; 1000 µM Putrescine Y3 + 0.54 µM NAA; 1000 µM Putrescine |
| Constantin et al. [28] | Young leaf | Callus induction Callus Proliferation Somatic embryo initiation/maturation | MS + 107.41 µM NAA MS + Gradual decrease in NAA |
| Wan Nur Syuhada et al. [38] | Immature zygotic Embryo | Callus induction Callus Proliferation Somatic embryo initiation/maturation Plantlet regeneration | MS + 9.95 µM 2,4-D MS + 25 µM 2,4-D MS + 0.1 µM NAA MS + 0.1 µM NAA |
| Thuzar et al. [39] | Immature zygotic Embryo | Callus induction Somatic embryo initiation/maturation Plantlet regeneration | N6 + 9.05 µM 2,4-D N6 + 0.45 µM 2,4-D N6 + 0.5 g/L Activated Charcoal |
| Monteiro et al. [25] | Mature zygotic Embryo | Callus induction Callus Proliferation Somatic embryo initiation/maturation Plantlet regeneration | MS + 450 µM Picloram + 2.5 g/L Activated Charcoal MS + 5 µM 2,4-D ½ MS + 2.5g/L Activated Charcoal MS |
| Balzon et al. [37] de Carvalho Silva et al. [36] Gomes et al. [35] Gomes et al. [34] | Mature zygotic Embryo | Callus induction Callus Proliferation Somatic embryo initiation/maturation Plantlet regeneration Rooting | MS + 450 µM Picloram + 2.5g/L Activated Charcoal MS + 40 µM Picloram; 10 µM 2-ip MS + 0.54 µM NAA; 12.3 µM 2-ip + 0–0.3g/L Activated Charcoal ½ MS + 0–2.5g/L Activated Charcoal MS + 53.7 µM IBA |
| Jayanthi et al. [10] | Immature male Inflorescence | Callus induction Somatic embryo initiation/maturation/plantlet regeneration Rooting | Y3 + 150 µM 2,4-D; 150 µM Picloram + 3.0 g/L Activated Charcoal Y3 + 18 µM BA; 3.78 µM ABA; 5.78 µM GA |
| Guedes et al.[40] | Immature female Inflorescence | Callus induction | Y3 + 23 µM IAA; 19.6 µM IBA + 0.5 g/L activated Charcoal |
| Teixeira et al. [41] | Immature female Inflorescence | Callus induction Somatic embryo initiation/maturation Plantlet regeneration | ½ MS + 225–450 µM 2,4-D + 3.0 g/L Activated Charcoal MS + 475 µM 2,4-D + 3.0 g/L Activated Charcoal Y3 + 15 µM NAA; 2 µM ABA ½ MS + 3.0 g/L Activated Charcoal |
| Jayanthi et al. [44] | Young plantlet | Somatic embryogenesisPlantlet regeneration | Y3 + 40 µM 2,4-D; 10 µM 2,4,5-T; 40 µM NAA; 10 µM TDZ; 10 µM BA + 3.0 g/L Activated Charcoal Y3 + 2 µM BA; 1 µM ABA |
| Scherwinski-Pereira et al. [45] | Young plantlet | Callus induction Somatic embryo initiation/maturation Plantlet regeneration | MS + 450 µM Picloram + 0.3 g/L Activated Charcoal MS + 0.6 µM NAA; 12.3 µM 2-ip + 0.3 g/L Activated Charcoal ½ MS + 1.0 g/L Activated Charcoal |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarra, R.; Jin, L.; Zhao, Z.; Cao, H. Progress in Tissue Culture and Genetic Transformation of Oil Palm: An Overview. Int. J. Mol. Sci. 2019, 20, 5353. https://doi.org/10.3390/ijms20215353
Yarra R, Jin L, Zhao Z, Cao H. Progress in Tissue Culture and Genetic Transformation of Oil Palm: An Overview. International Journal of Molecular Sciences. 2019; 20(21):5353. https://doi.org/10.3390/ijms20215353
Chicago/Turabian StyleYarra, Rajesh, Longfei Jin, Zhihao Zhao, and Hongxing Cao. 2019. "Progress in Tissue Culture and Genetic Transformation of Oil Palm: An Overview" International Journal of Molecular Sciences 20, no. 21: 5353. https://doi.org/10.3390/ijms20215353




