Reduction-Triggered Paclitaxel Release Nano-Hybrid System Based on Core-Crosslinked Polymer Dots with a pH-Responsive Shell-Cleavable Colorimetric Biosensor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of PTX Loaded L-PD
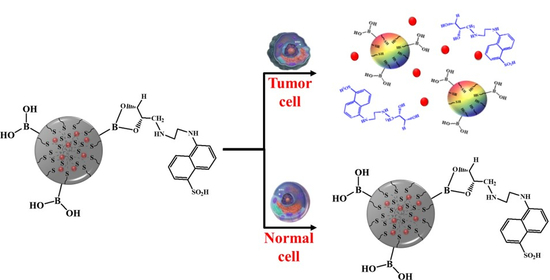
2.2. Stimuli-Responsive Drug Release and Anticancer Efficacy Evaluation of PTX Loaded L-PD
2.3. Fluorescence OFF/ON Behavior Based on Confocal Imaging
2.4. Apoptosis/Necrosis Analysis and Live/Dead Assay
3. Materials and Methods
3.1. Materials and Characterization
3.2. Synthesis of Carbonized Disulfide-Crosslinked Pluronic-Grafted Poly(DMA), Poly(HEMA) Quaternized Boronic Acid–Cysteamine (B-PD)
3.3. Synthesis of the Diol-Conjugated Fluorescent Probe- B-PD (L-PD)
3.4. Drug Loading into L-PD (PTX Loaded L-PD) and Release Profiles
3.5. Cytotoxicity Assay
3.6. Flow Cytometry
3.7. Confocal Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| B-PD | Disulfide-crosslinked Pluronic-grafted poly(DMA), poly(HEMA) quaternized boronic acid–cysteamine |
| B/S-Pluronic | Pluronic-grafted poly(DMA), poly(HEMA) quaternized boronic acid–cysteamine |
| BSA | Bovine serum albumin |
| DDS | Drug delivery system |
| DDW | Double distilled water |
| DMA | 2-(dimethylamino)ethyl methacrylate |
| FBS | Fetal bovine serum |
| GSH | Glutathione |
| HEMA | 2-hydroxyethyl methacrylate |
| L-PD | Diol-conjugated fluorescent probe- B-PD |
| MC | Methylene chloride |
| MDCK | Madin-Darby Canine Kidney |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NPC | Nitrophenyl chloroformate |
| PD | Polymer dots |
| PI | Propidium iodide |
| PTX | Paclitaxel |
| TEA | Triethylamine |
References
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C. 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnology 2018, 16, 74. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Li, M.; Tang, Z.; Sun, H.; Ding, J.; Song, W.; Chen, X. pH and reduction dual-responsive nanogel cross-linked by quaternization reaction for enhanced cellular internalization and intracellular drug delivery. Polym. Chem. 2013, 4, 1199–1207. [Google Scholar] [CrossRef]
- Miao, K.; Liu, H.; Zhao, Y. Thermo, pH and reduction responsive coaggregates comprising AB 2 C 2 star terpolymers for multi-triggered release of doxorubicin. Polym. Chem. 2014, 5, 3335–3345. [Google Scholar] [CrossRef]
- Su, Z.; Xu, Y.; Wang, Y.; Shi, W.; Han, S.; Shuai, X. A pH and reduction dual-sensitive polymeric nanomicelle for tumor microenvironment triggered cellular uptake and controlled intracellular drug release. Biomater. Sci. 2019, 7, 3821–3831. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Liu, Z.; Wang, L.; Wang, X.; Su, L.; Chang, J. Smart pH- and reduction-dual-responsive folate–PEG-coated polymeric lipid vesicles for tumor-triggered targeted drug delivery. Nanoscale 2014, 6, 7635–7642. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine: NBM. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.-F.; Zhuang, W.-R.; Hu, X.; Xing, L.; Yu, R.-Y.; Qiao, J.-B.; He, Y.-J.; Li, F.; Ling, D.; Jiang, H.-L. A new strategy for hydrophobic drug delivery using a hydrophilic polymer equipped with stacking units. Chem. Commun. 2018, 54, 8218–8221. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lammers, T.; Storm, G.; Hennink, W.E. Physico-Chemical Strategies to Enhance Stability and Drug Retention of Polymeric Micelles for Tumor-Targeted Drug Delivery. Macromol. Biosci. 2017, 17, 1600160. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, K.; Cao, W.; Sun, Y.; Sheng, W.; Li, F.; Wu, Y.; Liang, X.-J. pH-responsive biocompatible fluorescent polymer nanoparticles based on phenylboronic acid for intracellular imaging and drug delivery. Nanoscale 2014, 6, 13701–13709. [Google Scholar] [CrossRef] [PubMed]
- Mazrad, Z.A.I.; Phuong, P.T.M.; Choi, C.A.; In, I.; Lee, K.D.; Park, S.Y. pH/Redox-Triggered Photothermal Treatment for Cancer Therapy Based on a Dual-Responsive Cationic Polymer Dot. ChemMedChem 2018, 13, 2437–2447. [Google Scholar] [CrossRef]
- Ryplida, B.; Lee, K.D.; In, I.; Park, S.Y. Light-Induced Swelling-Responsive Conductive, Adhesive, and Stretchable Wireless Film Hydrogel as Electronic Artificial Skin. Adv. Funct. Mater. 2019, 1903209. [Google Scholar] [CrossRef]
- Du, J.; Choi, B.; Liu, Y.; Feng, A.; Thang, S.H. Degradable pH and redox dual responsive nanoparticles for efficient covalent drug delivery. Polym. Chem. 2019, 10, 1291–1298. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-Cleavage-Triggered Chemosensors and Their Biological Applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Xia, Y. One-step synthesis of boronic acid functionalized gold nanoclusters for photoluminescence sensing of dopamine. Methods Appl. Fluoresc. 2017, 5, 014006. [Google Scholar] [CrossRef] [PubMed]
- Reckmeier, C.J.; Schneider, J.; Xiong, Y.; Häusler, J.; Kasák, P.; Schnick, W.; Rogach, A.L. Aggregated Molecular Fluorophores in the Ammonothermal Synthesis of Carbon Dots. Chem. Mater. 2017, 29, 10352–10361. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.A.; Mazrad, Z.A.I.; Lee, G.; In, I.; Lee, K.D.; Park, S.Y. Boronate-based fluorescent carbon dot for rapid and selectively bacterial sensing by luminescence off/on system. J. Pharm. Biomed. Anal. 2018, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.A.; Ryplida, B.; In, I.; Park, S.Y. Selective redox-responsive theragnosis nanocarrier for breast tumor cells mediated by MnO2/fluorescent carbon nanogel. Eur. J. Pharm. Sci. 2019, 134, 256–265. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, L.; Miao, Y.; Liu, C.; Zhang, C. Construction of a Turn Off-On-Off Fluorescent System Based on Competitive Coordination of Cu2+ between 6,7-Dihydroxycoumarin and Pyrophosphate Ion for Sensitive Assay of Pyrophosphatase Activity. J. Anal. Methods Chem. 2016, 2016, 1–10. [Google Scholar]
- Mazrad, Z.A.I.; Lee, K.; Chae, A.; In, I.; Lee, H.; Park, S.Y. Progress in internal/external stimuli responsive fluorescent carbon nanoparticles for theranostic and sensing applications. J. Mater. Chem. B 2018, 6, 1149–1178. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, W.; Xiao, K.; Berti, L.; Luo, J.; Tseng, H.P.; Fung, G.; Lam, K.S. Well-Defined, Reversible Boronate Crosslinked Nanocarriers for Targeted Drug Delivery in Response to Acidic pH Values and cis -Diols. Angew. Chemie Int. Ed. 2012, 51, 2864–2869. [Google Scholar] [CrossRef]
- Choi, C.A.; Lee, J.E.; Mazrad, Z.A.I.; In, I.; Jeong, J.H.; Park, S.Y. Redox- and pH-responsive fluorescent carbon nanoparticles-MnO2-based FRET system for tumor-targeted drug delivery in vivo and in vitro. J. Ind. Eng. Chem. 2018, 63, 208–219. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-J.; Hsu, Z.-R.; Wang, L.-F. Synthesis and characterization of pluronic-block-poly(N,N-dimethylamino-2-ethyl methacrylate) pentablock copolymers for drug/gene co-delivery systems. RSC Adv. 2014, 4, 31552–31563. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; An, J.; Su, C.; Guo, Q.; Li, C. Boronate ester bond-based core–shell nanocarriers with pH response for anticancer drug delivery. RSC Adv. 2014, 4, 20208–20215. [Google Scholar] [CrossRef]
- Wang, X.; Xia, N.; Liu, L. Boronic Acid-Based Approach for Separation and Immobilization of Glycoproteins and Its Application in Sensing. Int. J. Mol. Sci. 2013, 14, 20890–20912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Meng, Q.; Hu, H.; Xu, T.; Shen, Y.; Cong, H. Construction of Dimeric Drug-Loaded Polymeric Micelles with High Loading Efficiency for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 1961. [Google Scholar] [CrossRef] [PubMed]
- Basiruddin, S.; Swain, S.K. Phenylboronic acid functionalized reduced graphene oxide based fluorescence nano sensor for glucose sensing. Mater. Sci. Eng. C 2016, 58, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Phuong, P.T.M.; Won, H.J.; Oh, Y.J.; Lee, H.S.; Lee, K.D.; Park, S.Y. The chemistry and engineering of mussel-inspired glue matrix for tissue adhesive and hemostatic. J. Ind. Eng. Chem. 2019. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.G.; Ryplida, B.; Phuong, P.T.M.; Won, H.J.; Lee, G.; Bhang, S.H.; Park, S.Y. Reduction-Triggered Paclitaxel Release Nano-Hybrid System Based on Core-Crosslinked Polymer Dots with a pH-Responsive Shell-Cleavable Colorimetric Biosensor. Int. J. Mol. Sci. 2019, 20, 5368. https://doi.org/10.3390/ijms20215368
Kim SG, Ryplida B, Phuong PTM, Won HJ, Lee G, Bhang SH, Park SY. Reduction-Triggered Paclitaxel Release Nano-Hybrid System Based on Core-Crosslinked Polymer Dots with a pH-Responsive Shell-Cleavable Colorimetric Biosensor. International Journal of Molecular Sciences. 2019; 20(21):5368. https://doi.org/10.3390/ijms20215368
Chicago/Turabian StyleKim, Seul Gi, Benny Ryplida, Pham Thi My Phuong, Hyun Jeong Won, Gibaek Lee, Suk Ho Bhang, and Sung Young Park. 2019. "Reduction-Triggered Paclitaxel Release Nano-Hybrid System Based on Core-Crosslinked Polymer Dots with a pH-Responsive Shell-Cleavable Colorimetric Biosensor" International Journal of Molecular Sciences 20, no. 21: 5368. https://doi.org/10.3390/ijms20215368
APA StyleKim, S. G., Ryplida, B., Phuong, P. T. M., Won, H. J., Lee, G., Bhang, S. H., & Park, S. Y. (2019). Reduction-Triggered Paclitaxel Release Nano-Hybrid System Based on Core-Crosslinked Polymer Dots with a pH-Responsive Shell-Cleavable Colorimetric Biosensor. International Journal of Molecular Sciences, 20(21), 5368. https://doi.org/10.3390/ijms20215368