The Pathogenesis of Sepsis and Potential Therapeutic Targets
Abstract
:1. Definition
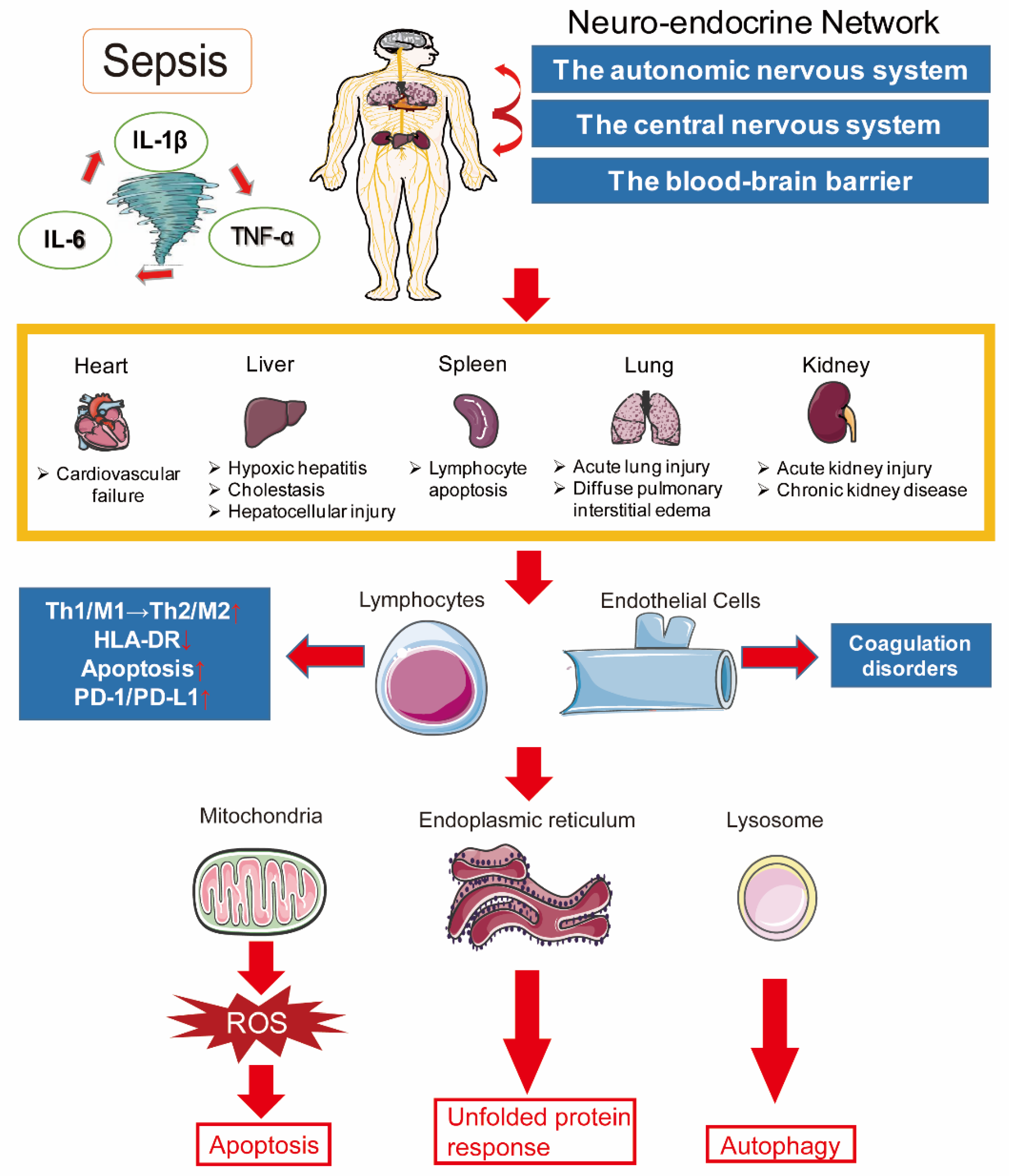
2. Pathogenesis of Sepsis
2.1. Inflammation Imbalance
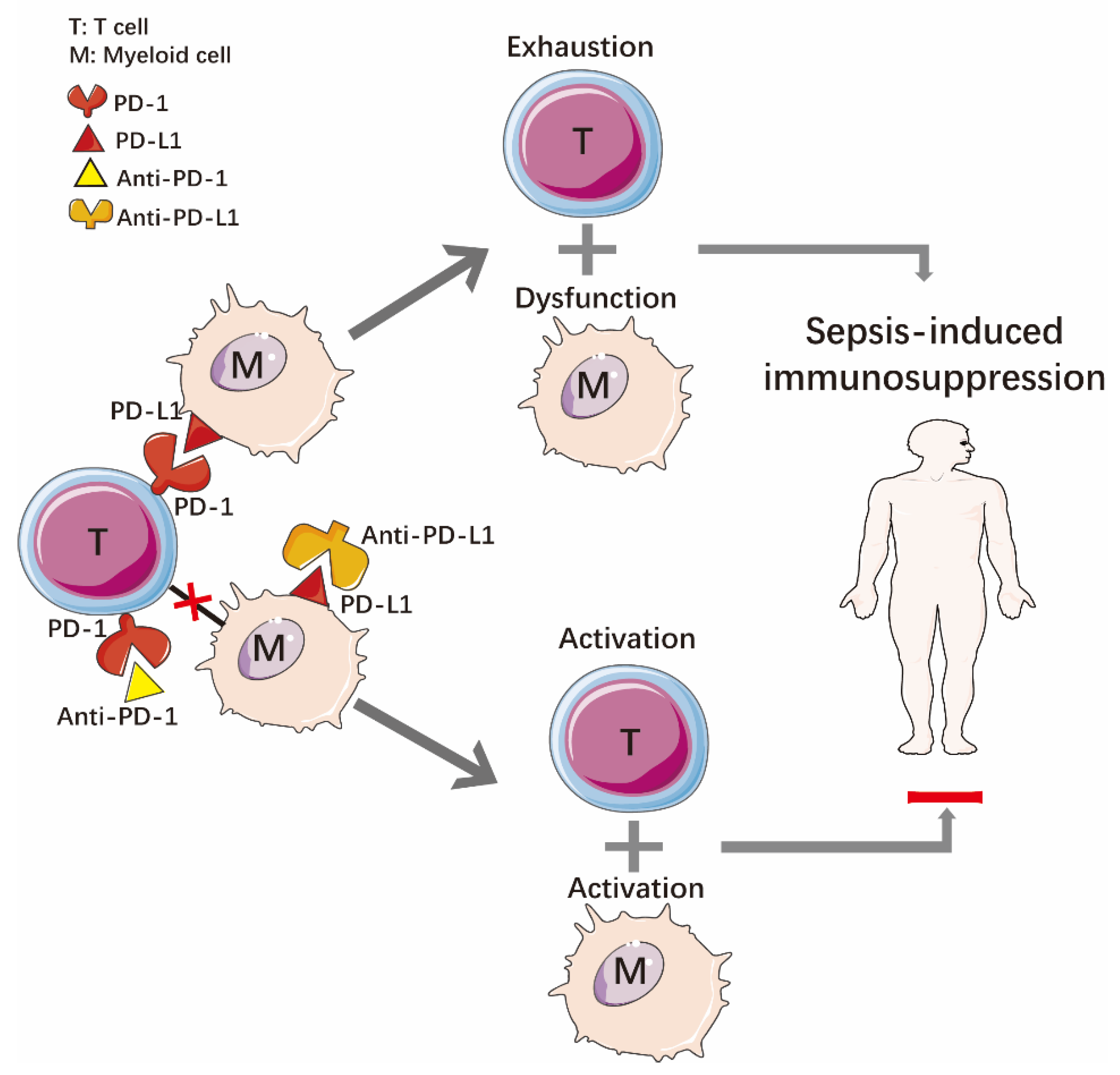
2.2. Immune Dysfunction
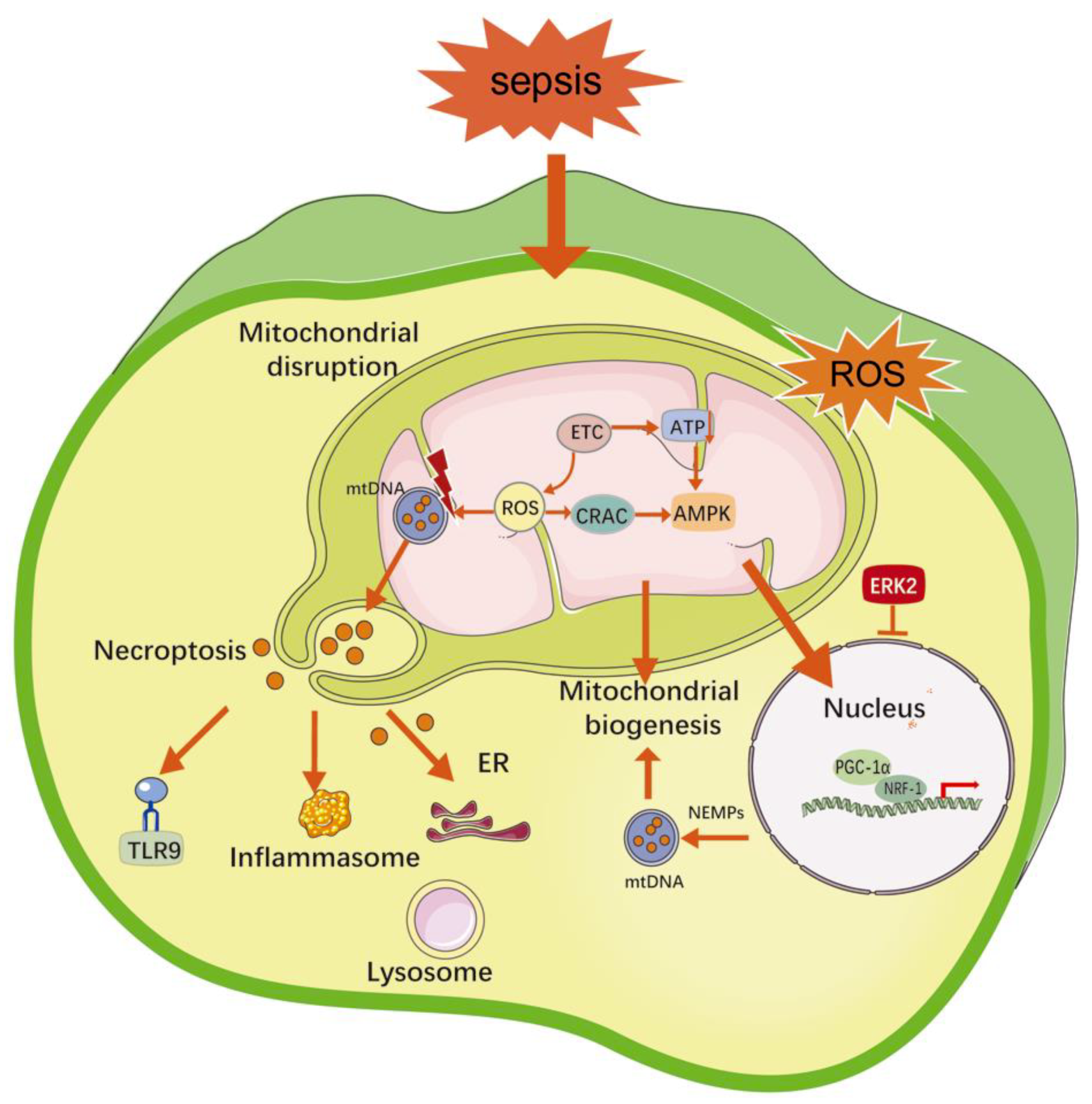
2.3. Mitochondrial Damage
2.4. Coagulation Disorders
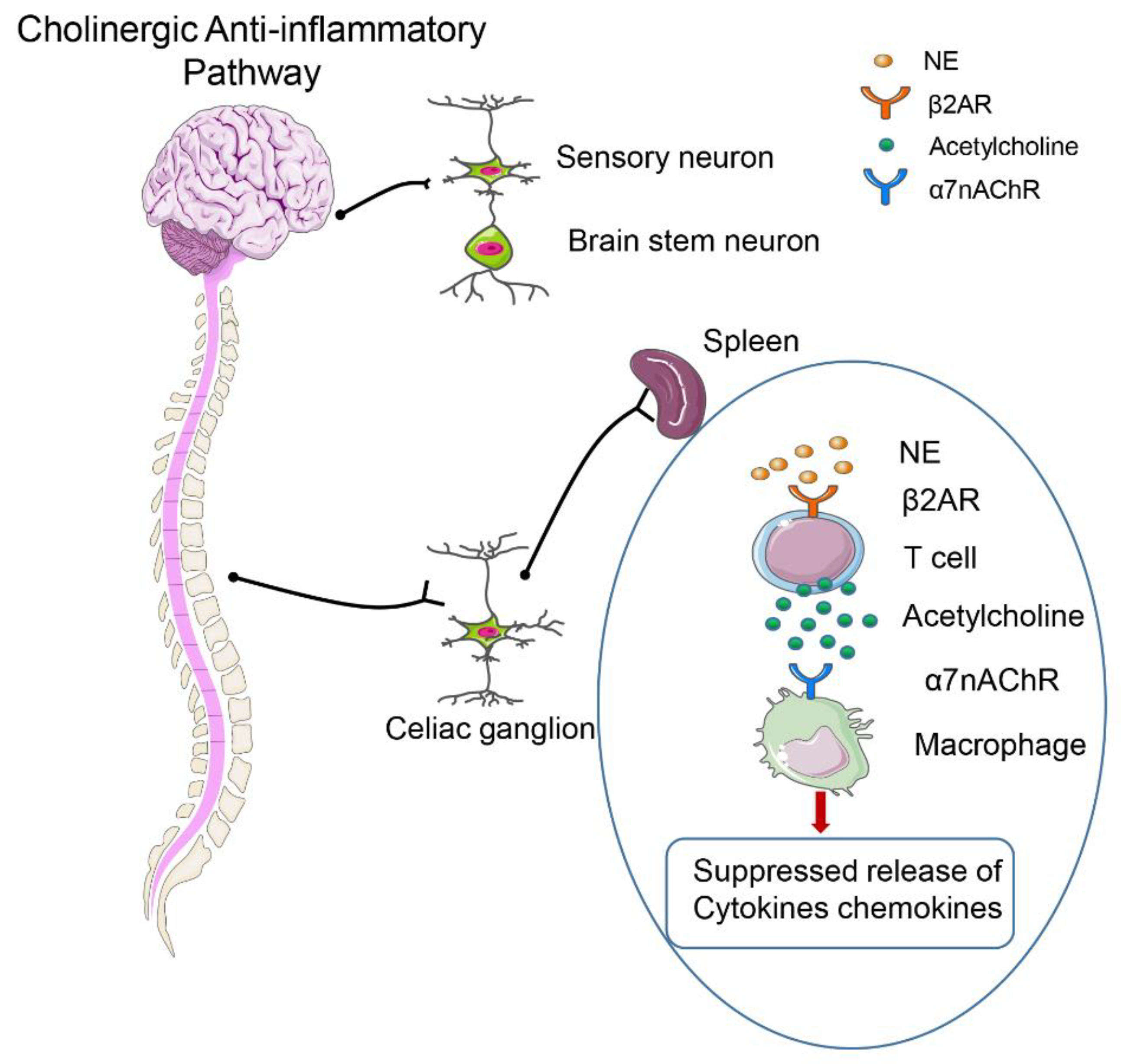
2.5. Neuroendocrine–Immune Network Abnormalities
2.6. Endoplasmic Reticulum Stress
2.7. Autophagy
3. Biomarkers of Sepsis
3.1. Infection-Related Biomarkers
3.1.1. Procalcitonin (PCT)
3.1.2. C-Reactive Protein (CRP)
3.1.3. Cytokines (TNF-α/IL-6)
3.2. Biomarkers Related to Inflammation Activation and Immune Imbalance
3.2.1. Monocyte Chemoattractant Protein-1 (MCP-1)
3.2.2. Programmed Death Receptor-1 and Programmed Death Ligand-1 (PD-1/PD-L1)
3.2.3. Soluble Triggering Receptor Expressed on Myeloid Cells-1 (sTREM-1)
3.2.4. Complement Pathway
3.2.5. Neutrophil Surface Receptor (CD64)
3.2.6. MicroRNA (miRNA)
3.2.7. Plasma Cell-Free DNA
3.2.8. Presepsin (sCD14-ST)
3.3. Biomarkers Related to Organ Dysfunction
3.3.1. Angiopoietin (Ang)
3.3.2. Matrix Metalloproteinases (MMPs)
3.4. Challenge
4. Specific Drugs for Treating Sepsis
4.1. Drugs Targeting Inflammatory Imbalance
4.1.1. Cytokine Antagonists
4.1.2. PRR Antagonist
4.1.3. Pathogen-Associated Molecular Antagonists
4.2. Drugs for Coagulopathy
4.2.1. Recombinant Human APC (rhAPC)
4.2.2. Recombinant Human Soluble Thrombosis Regulators
4.2.3. Pentoxifylline
4.3. Drugs Against Immune Function Inhibition
4.3.1. Cytokines
4.3.2. Co-Inhibiting Molecular Inhibitors
5. Future
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| ACTH | Adrenocorticotrophic hormone |
| AKI | Acute kidney injury |
| Ang | Angiopoietin |
| AP-1 | Adaptor protein 1 |
| APC | Activated protein C |
| ATF6 | Activating transcription factor 6 |
| AVP | Arginine vasopressin |
| C3 | Complement protein 3 |
| C5a | Complement component 5a |
| CAP | Cholinergic anti-inflammatory pathway |
| CHOP | CEBP homologous protein |
| CLP | Cecal ligation and puncture |
| CLRs | C-type lectin receptors |
| Cpb1 | Carboxypeptidase b1 |
| CRP | C-reaction protein |
| DAMPs | Damage-associated molecular patterns |
| DCs | Dendritic cells |
| DIC | Disseminated intravascular coagulation |
| ER | Endoplasmic reticulum |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ETC | Electron transfer chain |
| FcγR1 | Fc-γ receptor-1 |
| G-CSF | The cytokines granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GRP94 | Glucose-regulated protein 94 |
| HMGB-1 | High-mobility group box-1 |
| HPA | Hypothalamic-pituitary-adrenal |
| IL-1 | Interleukin-1 |
| iNOS | Inducible nitric oxide synthase |
| IRAK-M | Interleukin-1 receptor-associated kinase-M |
| IRE1 | Inositol-requiring enzyme 1 |
| IRF7 | Interferon regulatory factor 7 |
| JNK | c-Jun N-terminal kinase |
| LODS | Logistic Organ Dysfunction Score |
| LPS | Lipopolysaccharide |
| MAPK | p38 mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| miRNA | MicroRNA |
| MMPs | Matrix metalloproteinases |
| NA | Noradrenaline |
| NF-κB | Nuclear factor-κB |
| NK cells | Natural killer cells |
| NLRs | NOD-like receptors |
| NRF-1 | Nuclear respiratory factor-1 |
| NS | Neonatal sepsis |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PAMPs | Pathogen-associated molecular patterns |
| PCT | Procalcitonin |
| PD-1/PD-L1 | Programmed death receptor-1 and programmed death ligand-1 |
| PERK | PKR-like endoplasmic reticulum kinase |
| PMX-HP | Polymyxin B hemoperfusion |
| PRRs | Pattern-recognition receptors |
| qSOFA | Quick Sequential Organ Failure Assessment |
| rhAPC | Recombinant human APC |
| RLRs | RIG-I like receptors |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| rTM | Recombinant TM |
| SIGIRR | Single Ig IL-1R-related molecule |
| SIRS | Systemic Inflammatory Response Syndrome |
| SOCS1 | Suppressor of cytokine signaling 1 |
| SOFA | Sequential Organ Failure Assessment |
| ST2 | Stimulation expressed gene 2 |
| sTREM-1 | Soluble triggering receptor expressed on myeloid cells-1 |
| TAK-242 | Resatorvid |
| TFAM | Transcriptional activator of mitochondrial transcription factor A |
| TLRs | Toll-like receptors |
| TM | Thrombomodulin |
| TNF-α | Tumor necrosis factor-α |
| TOLLIP | Toll interacting protein |
| t-PA | Tissue plasminogen activator |
| u-PA | Urokinase-type plasminogen activator |
| α7nAChR | α7 nicotinic ACh receptor |
References
- Rocheteau, P.; Chatre, L.; Briand, D.; Mebarki, M.; Jouvion, G.; Bardon, J.; Crochemore, C.; Serrani, P.; Lecci, P.P.; Latil, M.; et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat. Commun. 2015, 6, 10145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and Costs of Sepsis in the United States-An Analysis Based on Timing of Diagnosis and Severity Level. Crit. Care Med. 2018, 46, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Cooke, C.R.; Wunsch, H.; Kahn, J.M. Population burden of long-term survivorship after severe sepsis in older Americans. J. Am. Geriatr. Soc. 2012, 60, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013, 41, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Barie, P.S. World Sepsis Day: September 13, 2012. Surg. Infect. (Larchmt) 2012, 13, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Majno, G. The ancient riddle of sigma eta psi iota sigma (sepsis). J. Infect. Dis. 1991, 163, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. Int. Immunopharmacol. 2018, 58, 173–185. [Google Scholar] [CrossRef]
- Stone, M.J. Regulation of Chemokine-Receptor Interactions and Functions. Int. J. Mol. Sci. 2017, 18, 2415. [Google Scholar] [CrossRef]
- Hawiger, J.; Veach, R.A.; Zienkiewicz, J. New paradigms in sepsis: From prevention to protection of failing microcirculation. J. Thromb Haemost. 2015, 13, 1743–1756. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- David, S.; Brunkhorst, F.M. Sepsis-3: What has been confirmed in therapy? Internist. (Berl) 2017, 58, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task, F. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Peach, B.C. Implications of the new sepsis definition on research and practice. J. Crit. Care. 2017, 38, 259–262. [Google Scholar] [CrossRef]
- Park, H.K.; Kim, W.Y.; Kim, M.C.; Jung, W.; Ko, B.S. Quick sequential organ failure assessment compared to systemic inflammatory response syndrome for predicting sepsis in emergency department. J. Crit. Care. 2017, 42, 12–17. [Google Scholar] [CrossRef]
- Simpson, S.Q. New Sepsis Criteria: A Change We Should Not Make. Chest 2016, 149, 1117–1118. [Google Scholar] [CrossRef]
- Churpek, M.M.; Snyder, A.; Han, X.; Sokol, S.; Pettit, N.; Howell, M.D.; Edelson, D.P. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am. J. Respir. Crit. Care Med. 2017, 195, 906–911. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- D’Elia, R.V.; Harrison, K.; Oyston, P.C.; Lukaszewski, R.A.; Clark, G.C. Targeting the “cytokine storm” for therapeutic benefit. Clin. Vaccine Immunol. 2013, 20, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.L.; Holden, D.C.; Mira, J.C.; Stortz, J.A.; Loftus, T.J.; Mohr, A.M.; Moldawer, L.L.; Moore, F.A.; Larson, S.D.; Efron, P.A. Microbial recognition and danger signals in sepsis and trauma. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M. Emerging inflammasome effector mechanisms. Nat. Rev. Immunol. 2011, 11, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Basith, S.; Choi, S. Negative regulatory approaches to the attenuation of Toll-like receptor signaling. Exp. Mol. Med. 2013, 45, e11. [Google Scholar] [CrossRef] [PubMed]
- Arens, C.; Bajwa, S.A.; Koch, C.; Siegler, B.H.; Schneck, E.; Hecker, A.; Weiterer, S.; Lichtenstern, C.; Weigand, M.A.; Uhle, F. Sepsis-induced long-term immune paralysis--results of a descriptive, explorative study. Crit. Care. 2016, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Broz, P.; Newton, K.; Lamkanfi, M.; Mariathasan, S.; Dixit, V.M.; Monack, D.M. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 2010, 207, 1745–1755. [Google Scholar] [CrossRef]
- Qiu, Z.; He, Y.; Ming, H.; Lei, S.; Leng, Y.; Xia, Z.Y. Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes. J. Diabetes Res. 2019, 2019, 8151836. [Google Scholar] [CrossRef]
- Xing, K.; Murthy, S.; Liles, W.C.; Singh, J.M. Clinical utility of biomarkers of endothelial activation in sepsis--a systematic review. Crit. Care. 2012, 16, R7. [Google Scholar] [CrossRef]
- Moser, J.; Heeringa, P.; Jongman, R.M.; Zwiers, P.J.; Niemarkt, A.E.; Yan, R.; de Graaf, I.A.; Li, R.; Ravasz Regan, E.; Kumpers, P.; et al. Intracellular RIG-I Signaling Regulates TLR4-Independent Endothelial Inflammatory Responses to Endotoxin. J. Immunol. 2016, 196, 4681–4691. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Richter, R.; Robert, S.; Kong, M. Viral Sepsis in Children. Front. Pediatr. 2018, 6, 252. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.K.; Ramos, H.J.; Wu, X.; Aggarwal, S.; Shrestha, B.; Gorman, M.; Kim, K.Y.; Suthar, M.S.; Atkinson, J.P.; Gale, M., Jr.; et al. Deficient IFN signaling by myeloid cells leads to MAVS-dependent virus-induced sepsis. Plos Pathog. 2014, 10, e1004086. [Google Scholar] [CrossRef]
- Cinel, I.; Dellinger, R.P. Advances in pathogenesis and management of sepsis. Curr. Opin. Infect. Dis. 2007, 20, 345–352. [Google Scholar] [CrossRef]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49, 740–753.e7. [Google Scholar] [CrossRef] [Green Version]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Shen, X.F.; Cao, K.; Jiang, J.P.; Guan, W.X.; Du, J.F. Neutrophil dysregulation during sepsis: An overview and update. J. Cell Mol. Med. 2017, 21, 1687–1697. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Q.; Zhang, K.; Cheng, B.; Xie, G.; Wu, X.; Luo, C.; Chen, L.; Liu, H.; Zhao, B.; et al. Sphingosine 1-phosphate Receptor 2 Signaling Suppresses Macrophage Phagocytosis and Impairs Host Defense against Sepsis. J. Am. Soc. Anesthesiol. 2015, 123, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Efron, P.A.; Martins, A.; Minnich, D.; Tinsley, K.; Ungaro, R.; Bahjat, F.R.; Hotchkiss, R.; Clare-Salzler, M.; Moldawer, L.L. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J. Immunol. 2004, 173, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Scicluna, B.P.; Arts, R.J.; Gresnigt, M.S.; Lachmandas, E.; Giamarellos-Bourboulis, E.J.; Kox, M.; Manjeri, G.R.; Wagenaars, J.A.; Cremer, O.L.; et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 2016, 17, 406–413. [Google Scholar] [CrossRef]
- Guo, Y.; Patil, N.K.; Luan, L.; Bohannon, J.K.; Sherwood, E.R. The biology of natural killer cells during sepsis. Immunology 2018, 153, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Pepin, D.; Godeny, M.; Russell, D.; Mehta, P.; Lie, W.R. Profiling of soluble immune checkpoint proteins as potential non-invasive biomarkers in colorectal cancer and sepsis. J. Immunol. 2018, 200, 174.43. [Google Scholar]
- Honma, K.; Udono, H.; Kohno, T.; Yamamoto, K.; Ogawa, A.; Takemori, T.; Kumatori, A.; Suzuki, S.; Matsuyama, T.; Yui, K. Interferon regulatory factor 4 negatively regulates the production of proinflammatory cytokines by macrophages in response to LPS. Proc. Natl. Acad. Sci. USA 2005, 102, 16001–16006. [Google Scholar] [CrossRef] [Green Version]
- Negishi, H.; Ohba, Y.; Yanai, H.; Takaoka, A.; Honma, K.; Yui, K.; Matsuyama, T.; Taniguchi, T.; Honda, K. Negative regulation of Toll-like-receptor signaling by IRF-4. Proc. Natl. Acad. Sci. USA 2005, 102, 15989–15994. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.; Herance, R.; Rovira, S.; Hernandez-Mijares, A.; Victor, V.M. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect. Disord Drug Targets. 2012, 12, 161–178. [Google Scholar] [CrossRef]
- Quoilin, C.; Mouithys-Mickalad, A.; Lecart, S.; Fontaine-Aupart, M.P.; Hoebeke, M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim. Biophys. Acta. 2014, 1837, 1790–1800. [Google Scholar] [CrossRef] [Green Version]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.G.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Carchman, E.H.; Whelan, S.; Loughran, P.; Mollen, K.; Stratamirovic, S.; Shiva, S.; Rosengart, M.R.; Zuckerbraun, B.S. Experimental sepsis-induced mitochondrial biogenesis is dependent on autophagy, TLR4, and TLR9 signaling in liver. Faseb. J. 2013, 27, 4703–4711. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yao, Y.M.; Lu, Z.Q. Mitochondrial quality control mechanisms as potential therapeutic targets in sepsis-induced multiple organ failure. J. Mol. Med. (Berl) 2019, 97, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, Y.W.; Yao, Y.M. Potential therapy strategy: Targeting mitochondrial dysfunction in sepsis. Mil. Med. Res. 2018, 5, 41. [Google Scholar] [CrossRef]
- Protti, A.; Fortunato, F.; Artoni, A.; Lecchi, A.; Motta, G.; Mistraletti, G.; Novembrino, C.; Comi, G.P.; Gattinoni, L. Platelet mitochondrial dysfunction in critically ill patients: Comparison between sepsis and cardiogenic shock. Crit. Care. 2015, 19, 39. [Google Scholar] [CrossRef]
- Tranca, S.D.; Petrisor, C.L.; Hagau, N. Biomarkers in polytrauma induced systemic inflammatory response syndrome and sepsis - a narrative review. Rom. J. Anaesth. Intensive Care. 2014, 21, 118–122. [Google Scholar]
- Liu, Y.; Hou, J.H.; Li, Q.; Chen, K.J.; Wang, S.N.; Wang, J.M. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: A systematic review and meta-analysis. Springerplus. 2016, 5, 2091. [Google Scholar] [CrossRef]
- Hedegaard, S.S.; Wisborg, K.; Hvas, A.M. Diagnostic utility of biomarkers for neonatal sepsis--a systematic review. Infect. Dis. 2015, 47, 117–124. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Wu, F.; Ji, W.; Zhang, J.; Wang, X. The Bidirectional Interactions Between Inflammation and Coagulation in Fracture Hematoma. Tissue Eng. Part. B. Rev. 2018. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38, S26–S34. [Google Scholar] [CrossRef]
- Levi, M.; Poll, T. Coagulation in patients with severe sepsis. Semin. Thromb Hemost. 2015, 41, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Biemond, B.J.; Levi, M.; Ten Cate, H.; Van der Poll, T.; Buller, H.R.; Hack, C.E.; Ten Cate, J.W. Plasminogen activator and plasminogen activator inhibitor I release during experimental endotoxaemia in chimpanzees: Effect of interventions in the cytokine and coagulation cascades. Clin. Sci. (Lond) 1995, 88, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Peres Wingeyer, S.D.; Cunto, E.R.; Nogueras, C.M.; San Juan, J.A.; Gomez, N.; de Larranaga, G.F. Biomarkers in sepsis at time zero: Intensive care unit scores, plasma measurements and polymorphisms in Argentina. J. Infect. Dev. Ctries. 2012, 6, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Tracey, K.J. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 2012, 30, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2015, 43, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sonneville, R.; Verdonk, F.; Rauturier, C.; Klein, I.F.; Wolff, M.; Annane, D.; Chretien, F.; Sharshar, T. Understanding brain dysfunction in sepsis. Ann. Intensive Care. 2013, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Kanczkowski, W.; Sue, M.; Zacharowski, K.; Reincke, M.; Bornstein, S.R. The role of adrenal gland microenvironment in the HPA axis function and dysfunction during sepsis. Mol. Cell Endocrinol. 2015, 408, 241–248. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Vizi, E.S. Receptor-mediated local fine-tuning by noradrenergic innervation of neuroendocrine and immune systems. Ann. N Y. Acad. Sci. 1998, 851, 388–396. [Google Scholar] [CrossRef]
- Tynan, R.J.; Weidenhofer, J.; Hinwood, M.; Cairns, M.J.; Day, T.A.; Walker, F.R. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav. Immun. 2012, 26, 469–479. [Google Scholar] [CrossRef]
- Herve, J.; Haurogne, K.; Bacou, E.; Pogu, S.; Allard, M.; Mignot, G.; Bach, J.M.; Lieubeau, B. beta2-adrenergic stimulation of dendritic cells favors IL-10 secretion by CD4( + ) T cells. Immunol. Res. 2017, 65, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.J.; Fu, H.; Tong, J.; Li, Y.H.; Qu, L.F.; Wang, P.; Shen, F.M. Cholinergic anti-inflammatory pathway inhibits neointimal hyperplasia by suppressing inflammation and oxidative stress. Redox Biol. 2018, 15, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Kohoutova, M.; Horak, J.; Jarkovska, D.; Martinkova, V.; Tegl, V.; Nalos, L.; Vistejnova, L.; Benes, J.; Sviglerova, J.; Kuncova, J.; et al. Vagus Nerve Stimulation Attenuates Multiple Organ Dysfunction in Resuscitated Porcine Progressive Sepsis. Crit. Care Med. 2019, 47, e461–e469. [Google Scholar] [CrossRef]
- Khan, M.M.; Yang, W.L.; Wang, P. Endoplasmic Reticulum Stress in Sepsis. Shock 2015, 44, 294–304. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta. Biochim. Biophys. Sin. (Shanghai) 2015, 47, 146–147. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, X.; Cui, Y.; Cui, L.; Zhao, P. Macrophage migration inhibitory factor knockout attenuates endotoxin-induced cardiac dysfunction in mice. Kardiol Pol 2018, 76, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Garcia de la Cadena, S.; Massieu, L. Caspases and their role in inflammation and ischemic neuronal death. Focus on caspase-12. Apoptosis 2016, 21, 763–777. [Google Scholar] [CrossRef]
- Jiao, G.; Hao, L.; Wang, M.; Zhong, B.; Yu, M.; Zhao, S.; Wang, P.; Feng, R.; Tan, S.; Chen, L. Upregulation of endoplasmic reticulum stress is associated with diaphragm contractile dysfunction in a rat model of sepsis. Mol. Med. Rep. 2017, 15, 366–374. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, Y.; Zhang, J. Review: The Role and Mechanisms of Macrophage Autophagy in Sepsis. Inflammation 2019, 42, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Reyes-Robles, T.; Alonzo, F., 3rd; Durbin, J.; Torres, V.J.; Cadwell, K. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host. Microbe. 2015, 17, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Mizumura, K.; Choi, A.M. The impact of autophagy on cell death modalities. Int. J. Cell Biol. 2014, 2014, 502676. [Google Scholar] [CrossRef]
- Schafer, S.T.; Franken, L.; Adamzik, M.; Schumak, B.; Scherag, A.; Engler, A.; Schonborn, N.; Walden, J.; Koch, S.; Baba, H.A.; et al. Mitochondrial DNA: An Endogenous Trigger for Immune Paralysis. J. Am. Soc. Anesthesiol. 2016, 124, 923–933. [Google Scholar] [CrossRef]
- Pundiche, M.; Sarbu, V.; Unc, O.; Grasa, C.; Martinescu, A.; Bădărău, V.; Durbală, I.; Sapte, E.; Pasăre, R.; Voineagu, L. Role of procalcitonin in monitoring the antibiotic therapy in septic surgical patients. Chirurgia (Buchar. Rom. 1990) 2012, 107, 71–78. [Google Scholar]
- Pontrelli, G.; De Crescenzo, F.; Buzzetti, R.; Jenkner, A.; Balduzzi, S.; Calo Carducci, F.; Amodio, D.; De Luca, M.; Chiurchiu, S.; Davies, E.H.; et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: A meta-analysis. Bmc Infect. Dis. 2017, 17, 302. [Google Scholar] [CrossRef]
- Vijayan, A.L.; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; Manoj, G. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care. 2017, 5, 51. [Google Scholar] [CrossRef]
- Arora, S.; Singh, P.; Singh, P.M.; Trikha, A. Procalcitonin Levels in Survivors and Nonsurvivors of Sepsis: Systematic Review and Meta-Analysis. Shock 2015, 43, 212–221. [Google Scholar] [CrossRef]
- Nunnally, M.E.; Patel, A. Sepsis - What’s new in 2019? Curr. Opin. Anaesthesiol. 2019, 32, 163–168. [Google Scholar] [CrossRef]
- Memar, M.Y.; Alizadeh, N.; Varshochi, M.; Kafil, H.S. Immunologic biomarkers for diagnostic of early-onset neonatal sepsis. J. Matern. Fetal Neonatal Med. 2019, 32, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Sager, R.; Kutz, A.; Mueller, B.; Schuetz, P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. Bmc. Med. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Jiwaji, Z.; Brady, S.; McIntyre, L.A.; Gray, A.; Walsh, T.S. Emergency department management of early sepsis: A national survey of emergency medicine and intensive care consultants. Emerg. Med. J. 2014, 31, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Park, Y.; Cho, Y.; Kim, H.S. Diagnostic utilities of procalcitonin and C-reactive protein for the prediction of bacteremia determined by blood culture. Clin. Chim. Acta. 2012, 413, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell. Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef]
- Lelubre, C.; Anselin, S.; Zouaoui Boudjeltia, K.; Biston, P.; Piagnerelli, M. Interpretation of C-reactive protein concentrations in critically ill patients. Biomed Res. Int. 2013, 2013, 124021. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathy, S.; Jyoti, A.; Singh, S.G. Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosens Bioelectron. 2019, 124–125, 205–215. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, E.; Kaushik, S.; Kumar Srivastava, V.; Mehta, S.K.; Jyoti, A. Evaluation of oxidative stress and antioxidant status: Correlation with the severity of sepsis. Scand. J. Immunol. 2018, 87, e12653. [Google Scholar] [CrossRef] [Green Version]
- Iking-Konert, C.; Bartz-Bazzanella, P.; Falagan, D.; Hofman, M.W.; Schwarting, A.; Dorner, T. Interleukin-6 inhibition as a potential therapeutic target in rheumatic diseases. Z Rheumatol. 2014, 73, 269–276. [Google Scholar] [CrossRef]
- Tschaikowsky, K.; Hedwig-Geissing, M.; Braun, G.G.; Radespiel-Troeger, M. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J. Crit. Care. 2011, 26, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Shahkar, L.; Keshtkar, A.; Mirfazeli, A.; Ahani, A.; Roshandel, G. The role of IL-6 for predicting neonatal sepsis: A systematic review and meta-analysis. Iran J Pediatr 2011, 21, 411–417. [Google Scholar] [PubMed]
- Hou, T.; Huang, D.; Zeng, R.; Ye, Z.; Zhang, Y. Accuracy of serum interleukin (IL)-6 in sepsis diagnosis: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 15238–15245. [Google Scholar] [PubMed]
- Kurt, A.; Aygun, A.D.; Godekmerdan, A.; Kurt, A.; Dogan, Y.; Yilmaz, E. Serum IL-1β, IL-6, IL-8, and TNF-α levels in early diagnosis and management of neonatal sepsis. Mediat. Inflamm. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Liao, X.; Feng, T.; Wu, Q.; Zhang, J.; Cao, X.; Li, H. Plasma monocyte chemoattractant protein 1 as a predictive marker for sepsis prognosis: A prospective cohort study. Tohoku J. Exp. Med. 2017, 241, 139–147. [Google Scholar] [CrossRef]
- Cavalcanti, N.V.; Torres, L.C.; da Matta, M.C.; Lindoso, C.D.; LN, A.C.; Duarte, M.C.; Correia, J.B. Chemokine Patterns in Children with Acute Bacterial Infections. Scand. J. Immunol. 2016, 84, 338–343. [Google Scholar] [CrossRef]
- Hong, T.H.; Chang, C.H.; Ko, W.J.; Lin, C.F.; Liu, H.H.; Chow, L.P.; Huang, C.T.; Yu, S.L.; Chen, Y.S. Biomarkers of early sepsis may be correlated with outcome. J. Transl. Med. 2014, 12, 146. [Google Scholar] [CrossRef]
- Holub, M.; Dzupova, O.; Ruzkova, M.; Stranikova, A.; Bartakova, E.; Maca, J.; Benes, J.; Herwald, H.; Beran, O. Selected Biomarkers Correlate with the Origin and Severity of Sepsis. Mediat. Inflamm. 2018, 2018, 7028267. [Google Scholar] [CrossRef]
- Turnis, M.E.; Andrews, L.P.; Vignali, D.A. Inhibitory receptors as targets for cancer immunotherapy. Eur. J. Immunol. 2015, 45, 1892–1905. [Google Scholar] [CrossRef]
- Chang, K.; Svabek, C.; Vazquez-Guillamet, C.; Sato, B.; Rasche, D.; Wilson, S.; Robbins, P.; Ulbrandt, N.; Suzich, J.; Green, J.; et al. Targeting the programmed cell death 1: Programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit. Care. 2014, 18, R3. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Francisco, L.M.; Sharpe, A.H. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 2007, 19, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D., 2nd; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Fang, Y.; Yu, H.; Zhao, L.; Jiang, Z.; Li, C.-S. Monocyte programmed death ligand-1 expression after 3–4 days of sepsis is associated with risk stratification and mortality in septic patients: A prospective cohort study. Crit. Care. 2016, 20, 124. [Google Scholar] [CrossRef]
- Larsen, F.F.; Petersen, J.A. Novel biomarkers for sepsis: A narrative review. Eur. J. Intern Med. 2017, 45, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; She, D.; Feng, D.; Jia, Y.; Xie, L. Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) reflect sepsis severity and can predict prognosis: A prospective study. BMC Infect. Dis. 2011, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Fan, X.; Bao, R.; Bo, L.; Li, J.; Deng, X. Accuracy of plasma sTREM-1 for sepsis diagnosis in systemic inflammatory patients: A systematic review and meta-analysis. Crit. Care. 2012, 16, R229. [Google Scholar] [CrossRef]
- Saldir, M.; Tunc, T.; Cekmez, F.; Cetinkaya, M.; Kalayci, T.; Fidanci, K.; Babacan, O.; Erdem, G.; Kocak, N.; Sari, E.; et al. Endocan and Soluble Triggering Receptor Expressed on Myeloid Cells-1 as Novel Markers for Neonatal Sepsis. Pediatr Neonatol. 2015, 56, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Karasu, E.; Nilsson, B.; Kohl, J.; Lambris, J.D.; Huber-Lang, M. Targeting Complement Pathways in Polytrauma- and Sepsis-Induced Multiple-Organ Dysfunction. Front. Immunol. 2019, 10, 543. [Google Scholar] [CrossRef]
- Helling, H.; Stephan, B.; Pindur, G. Coagulation and complement system in critically ill patients. Clin. Hemorheol Microcirc. 2015, 61, 185–193. [Google Scholar] [CrossRef]
- Williams, A.L.; Gullipalli, D.; Ueda, Y.; Sato, S.; Zhou, L.; Miwa, T.; Tung, K.S.; Song, W.C. C5 inhibition prevents renal failure in a mouse model of lethal C3 glomerulopathy. Kidney Int. 2017, 91, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, X.; Zhao, T.; Xu, Q.; Peng, Q.; Hu, R.; Quan, S.; Zhou, Y.; Xing, G. Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice. Clin. Exp. Immunol. 2017, 189, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napier, B.A.; Brubaker, S.W.; Sweeney, T.E.; Monette, P.; Rothmeier, G.H.; Gertsvolf, N.A.; Puschnik, A.; Carette, J.E.; Khatri, P.; Monack, D.M. Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. J. Exp. Med. 2016, 213, 2365–2382. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Huang, J.; Yuan, H.; Yan, W.; Hu, G.; Wang, J. Tumor necrosis factor-alpha as a diagnostic marker for neonatal sepsis: A meta-analysis. Sci. World J. 2014, 2014, 471463. [Google Scholar] [CrossRef]
- Wei, Z.M.; Wang, Z.; Wan, X.J.; Li, X.J.; Li, Y.X.; Bai, Y.; Yang, X.; Yang, Y.; Jiao, S.C.; Liu, Z.F. FcRgamma deficiency improves survival in experimental sepsis by down-regulating TLR4 signaling pathway. Immunol. Res. 2018. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.-Y.; Zeng, L.; Zhang, A.-Q.; Pan, W.; Gu, W.; Jiang, J.-X. Neutrophil CD64 expression as a diagnostic marker for sepsis in adult patients: A meta-analysis. Crit. Care. 2015, 19, 245. [Google Scholar] [CrossRef]
- Li, W.; Qiu, X.; Jiang, H.; Han, Y.; Wei, D.; Liu, J. Downregulation of miR-181a protects mice from LPS-induced acute lung injury by targeting Bcl-2. Biomed Pharm. 2016, 84, 1375–1382. [Google Scholar] [CrossRef]
- Wang, H.; Meng, K.; jun Chen, W.; Feng, D.; Jia, Y.; Xie, L. Serum miR-574-5p: A prognostic predictor of sepsis patients. Shock 2012, 37, 263–267. [Google Scholar] [CrossRef]
- Sheedy, F.J.; Palsson-McDermott, E.; Hennessy, E.J.; Martin, C.; O’Leary, J.J.; Ruan, Q.; Johnson, D.S.; Chen, Y.; O’Neill, L.A. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010, 11, 141–147. [Google Scholar] [CrossRef]
- Zhou, M.H.; Zhang, L.; Song, M.J.; Sun, W.J. MicroRNA-218 prevents lung injury in sepsis by inhibiting RUNX2. Eur. Rev. Med. Pharm. Sci. 2018, 22, 8438–8446. [Google Scholar] [CrossRef]
- McHugh, L.; Seldon, T.A.; Brandon, R.A.; Kirk, J.T.; Rapisarda, A.; Sutherland, A.J.; Presneill, J.J.; Venter, D.J.; Lipman, J.; Thomas, M.R.; et al. A Molecular Host Response Assay to Discriminate Between Sepsis and Infection-Negative Systemic Inflammation in Critically Ill Patients: Discovery and Validation in Independent Cohorts. Plos Med. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, B.P.; Klein Klouwenberg, P.M.; van Vught, L.A.; Wiewel, M.A.; Ong, D.S.; Zwinderman, A.H.; Franitza, M.; Toliat, M.R.; Nurnberg, P.; Hoogendijk, A.J.; et al. A molecular biomarker to diagnose community-acquired pneumonia on intensive care unit admission. Am. J. Respir. Crit. Care. Med. 2015, 192, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Hotz, M.J.; Qing, D.; Shashaty, M.G.; Zhang, P.; Faust, H.; Sondheimer, N.; Rivella, S.; Worthen, G.S.; Mangalmurti, N.S. Red Blood Cells Homeostatically Bind Mitochondrial DNA through TLR9 to Maintain Quiescence and to Prevent Lung Injury. Am. J. Respir. Crit. Care Med. 2018, 197, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Kyung S.-Y., K.; Rogers, A.J.; Gazourian, L.; Youn, S.; Massaro, A.F.; Quintana, C.; Osorio, J.C.; Wang, Z.; Zhao, Y.; et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: Derivation and validation. Plos Med. 2013, 10, e1001577, discussion e1001577. [Google Scholar] [CrossRef]
- Arnalich, F.; Maldifassi, M.C.; Ciria, E.; Codoceo, R.; Renart, J.; Fernandez-Capitan, C.; Herruzo, R.; Garcia-Rio, F.; Lopez-Collazo, E.; Montiel, C. Plasma levels of mitochondrial and nuclear DNA in patients with massive pulmonary embolism in the emergency department: A prospective cohort study. Crit. Care. 2013, 17, R90. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, Y.; Gong, Y.; Sun, R.; Su, L.; Lin, X.; Shen, A.; Zhou, J.; Caiji, Z.; Wang, X. Diagnosis of sepsis with cell-free DNA by next-generation sequencing technology in ICU patients. Arch. Med Res. 2016, 47, 365–371. [Google Scholar] [CrossRef]
- Gould, T.J.; Vu, T.T.; Stafford, A.R.; Dwivedi, D.J.; Kim, P.Y.; Fox-Robichaud, A.E.; Weitz, J.I.; Liaw, P.C. Cell-Free DNA Modulates Clot Structure and Impairs Fibrinolysis in Sepsis. Arter. Thromb. Vasc. Biol. 2015, 35, 2544–2553. [Google Scholar] [CrossRef] [Green Version]
- Kung, C.T.; Hsiao, S.Y.; Tsai, T.C.; Su, C.M.; Chang, W.N.; Huang, C.R.; Wang, H.C.; Lin, W.C.; Chang, H.W.; Lin, Y.J.; et al. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J. Transl. Med. 2012, 10, 130. [Google Scholar] [CrossRef]
- Scherberich, J.E.; Nockher, W.A. Blood monocyte phenotypes and soluble endotoxin receptor CD14 in systemic inflammatory diseases and patients with chronic renal failure. Nephrol. Dial. Transpl. 2000, 15, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Klouche, K.; Cristol, J.P.; Devin, J.; Gilles, V.; Kuster, N.; Larcher, R.; Amigues, L.; Corne, P.; Jonquet, O.; Dupuy, A.M. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann. Intensive Care. 2016, 6, 59. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Fravega, M.; Fanos, V. Soluble CD14 subtype presepsin (sCD14-ST) and lipopolysaccharide binding protein (LBP) in neonatal sepsis: New clinical and analytical perspectives for two old biomarkers. J. Matern. Fetal Neonatal Med. 2011, 24, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Caironi, P.; Spanuth, E.; Thomae, R.; Panigada, M.; Sangiorgi, G.; Fumagalli, R.; Mauri, T.; Isgrò, S.; Fanizza, C. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: Data from the Albumin Italian Outcome Sepsis trial. Crit. Care. 2014, 18, R6. [Google Scholar] [CrossRef] [PubMed]
- Brodska, H.; Valenta, J.; Pelinkova, K.; Stach, Z.; Sachl, R.; Balik, M.; Zima, T.; Drabek, T. Diagnostic and prognostic value of presepsin vs. established biomarkers in critically ill patients with sepsis or systemic inflammatory response syndrome. Clin. Chem. Lab. Med. (Cclm) 2018, 56, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Massaccesi, L.; De Vecchi, E.; Banfi, G.; Romanelli, M.M.C. Clinical application of presepsin as diagnostic biomarker of infection: Overview and updates. Clin. Chem. Lab. Med. (Cclm) 2019. [Google Scholar] [CrossRef]
- Endo, S.; Suzuki, Y.; Takahashi, G.; Shozushima, T.; Ishikura, H.; Murai, A.; Nishida, T.; Irie, Y.; Miura, M.; Iguchi, H. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J. Infect. Chemother. 2012, 18, 891–897. [Google Scholar] [CrossRef]
- Bamba, Y.; Moro, H.; Aoki, N.; Koizumi, T.; Ohshima, Y.; Watanabe, S.; Sakagami, T.; Koya, T.; Takada, T.; Kikuchi, T. Increased presepsin levels are associated with the severity of fungal bloodstream infections. PLoS ONE 2018, 13, e0206089. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.K.; Guo, Y.; Luan, L.; Sherwood, E.R. Targeting Immune Cell Checkpoints during Sepsis. Int. J. Mol. Sci. 2017, 18, 2413. [Google Scholar] [CrossRef]
- Okamura, Y.; Yokoi, H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin. Chim. Acta. 2011, 412, 2157–2161. [Google Scholar] [CrossRef]
- Yeh, C.-F.; Wu, C.-C.; Liu, S.-H.; Chen, K.-F. Comparison of the accuracy of neutrophil CD64, procalcitonin, and C-reactive protein for sepsis identification: A systematic review and meta-analysis. Ann. Intensive Care. 2019, 9, 5. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lan, H.-M.; Han, S.-T.; Chaou, C.-H.; Yeh, C.-F.; Liu, S.-H.; Li, C.-H.; Blaney, G.N.; Liu, Z.-Y.; Chen, K.-F. Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: A systematic review and meta-analysis. Ann. Intensive Care. 2017, 7, 91. [Google Scholar] [CrossRef]
- Takahashi, G.; Shibata, S.; Ishikura, H.; Miura, M.; Fukui, Y.; Inoue, Y.; Endo, S. Presepsin in the prognosis of infectious diseases and diagnosis of infectious disseminated intravascular coagulation: A prospective, multicentre, observational study. Eur. J. Anaesthesiol. 2015, 32, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Fitrou, G.; Pergialiotis, V.; Thomakos, N.; Perrea, D.N.; Daskalakis, G. The diagnostic accuracy of presepsin in neonatal sepsis: A meta-analysis. Eur. J. Pediatrics. 2018, 177, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Chenevier-Gobeaux, C.; Trabattoni, E.; Roelens, M.; Borderie, D.; Claessens, Y.E. Presepsin (sCD14-ST) in emergency department: The need for adapted threshold values? Clin. Chim. Acta. 2014, 427, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, D.; Brkic, S.; Uvelin, A.; Draskovic, B.; Vrsajkov, V. Use of presepsin and procalcitonin for prediction of SeptiFast results in critically ill patients. J. Crit. Care. 2017, 40, 197–201. [Google Scholar] [CrossRef]
- Shozushima, T.; Takahashi, G.; Matsumoto, N.; Kojika, M.; Okamura, Y.; Endo, S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J. Infect. Chemother. 2011, 17, 764–769. [Google Scholar] [CrossRef]
- Kim, H.; Hur, M.; Moon, H.W.; Yun, Y.M.; Di Somma, S.; Network, G. Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann. Intensive Care. 2017, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Gyawali, N.; Sanjana, R.K. Bacteriological profile and antibiogram of neonatal septicemia. Indian J. Pediatr 2013, 80, 371–374. [Google Scholar] [CrossRef]
- Milam, K.E.; Parikh, S.M. The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barriers. 2015, 3, e957508. [Google Scholar] [CrossRef]
- Parikh, S.M. The Angiopoietin-Tie2 Signaling Axis in Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 1973–1982. [Google Scholar] [CrossRef]
- Fang, Y.; Li, C.; Shao, R.; Yu, H.; Zhang, Q. The role of biomarkers of endothelial activation in predicting morbidity and mortality in patients with severe sepsis and septic shock in intensive care: A prospective observational study. Thromb Res. 2018, 171, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Ricciuto, D.R.; dos Santos, C.C.; Hawkes, M.; Toltl, L.J.; Conroy, A.L.; Rajwans, N.; Lafferty, E.I.; Cook, D.J.; Fox-Robichaud, A.; Kahnamoui, K.; et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit. Care Med. 2011, 39, 702–710. [Google Scholar] [CrossRef]
- Fisher, J.; Douglas, J.J.; Linder, A.; Boyd, J.H.; Walley, K.R.; Russell, J.A. Elevated Plasma Angiopoietin-2 Levels Are Associated With Fluid Overload, Organ Dysfunction, and Mortality in Human Septic Shock. Crit. Care Med. 2016, 44, 2018–2027. [Google Scholar] [CrossRef]
- Luanraksa, S.; Jindatanmanusan, P.; Boonsiri, T.; Nimmanon, T.; Chaovanalikit, T.; Arnutti, P. An MMP/TIMP ratio scoring system as a potential predictive marker of diabetic foot ulcer healing. J. Wound Care. 2018, 27, 849–855. [Google Scholar] [CrossRef]
- Lauhio, A.; Hastbacka, J.; Pettila, V.; Tervahartiala, T.; Karlsson, S.; Varpula, T.; Varpula, M.; Ruokonen, E.; Sorsa, T.; Kolho, E. Serum MMP-8, -9 and TIMP-1 in sepsis: High serum levels of MMP-8 and TIMP-1 are associated with fatal outcome in a multicentre, prospective cohort study. Hypothetical impact of tetracyclines. Pharm. Res. 2011, 64, 590–594. [Google Scholar] [CrossRef]
- Hoffmann, U.; Bertsch, T.; Dvortsak, E.; Liebetrau, C.; Lang, S.; Liebe, V.; Huhle, G.; Borggrefe, M.; Brueckmann, M. Matrix-metalloproteinases and their inhibitors are elevated in severe sepsis: Prognostic value of TIMP-1 in severe sepsis. Scand. J. Infect. Dis. 2006, 38, 867–872. [Google Scholar] [CrossRef]
- Fang, Y.; Li, C.; Shao, R.; Yu, H.; Zhang, Q.; Zhao, L. Prognostic significance of the angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for early sepsis in an emergency department. Crit. Care. 2015, 19, 367. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Pan, Y.; Wang, C.; Liu, Y.; Shi, M.; Ding, G. HMGB1 Turns Renal Tubular Epithelial Cells into Inflammatory Promoters by Interacting with TLR4 During Sepsis. J. Interferon Cytokine Res. 2016, 36, 9–19. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.L. Sepsis biomarkers: A review. Crit. Care. 2010, 14, R15. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Kumar, A.; Gangadharan, S. Biomarkers and newer laboratory investigations in the diagnosis of sepsis. J. R. Coll. Physicians Edinb. 2019, 49, 207–216. [Google Scholar] [CrossRef]
- Lippi, G. Sepsis biomarkers: Past, present and future. Clin. Chem. Lab. Med. 2019, 57, 1281–1283. [Google Scholar] [CrossRef]
- Sharma, D.; Farahbakhsh, N.; Shastri, S.; Sharma, P. Biomarkers for diagnosis of neonatal sepsis: A literature review. J. Matern Fetal Neonatal Med. 2018, 31, 1646–1659. [Google Scholar] [CrossRef]
- Klompas, M.; Calandra, T.; Singer, M. Antibiotics for Sepsis-Finding the Equilibrium. JAMA 2018, 320, 1433–1434. [Google Scholar] [CrossRef]
- Rello, J.; Valenzuela-Sanchez, F.; Ruiz-Rodriguez, M.; Moyano, S. Sepsis: A review of advances in management. Adv. in Ther. 2017, 34, 2393–2411. [Google Scholar] [CrossRef]
- Vincent, J.-L. The clinical challenge of sepsis identification and monitoring. Plos Med 2016, 13, e1002022. [Google Scholar] [CrossRef]
- Pammi, M.; Flores, A.; Versalovic, J.; Leeflang, M.M. Molecular assays for the diagnosis of sepsis in neonates. Cochrane Database Syst. Rev. 2017, 2, CD011926. [Google Scholar] [CrossRef]
- Jordan, J.A.; Durso, M.B. Real-time polymerase chain reaction for detecting bacterial DNA directly from blood of neonates being evaluated for sepsis. J. Mol. Diagn. 2005, 7, 575–581. [Google Scholar] [CrossRef]
- Qiu, P.; Cui, X.; Barochia, A.; Li, Y.; Natanson, C.; Eichacker, P.Q. The evolving experience with therapeutic TNF inhibition in sepsis: Considering the potential influence of risk of death. Expert Opin. Investig. Drugs. 2011, 20, 1555–1564. [Google Scholar] [CrossRef]
- Marshall, J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014, 20, 195–203. [Google Scholar] [CrossRef]
- Reinhart, K.; Karzai, W. Anti-tumor necrosis factor therapy in sepsis: Update on clinical trials and lessons learned. Crit. Care Med. 2001, 29, S121–S125. [Google Scholar] [CrossRef]
- Kotsaki, A.; Giamarellos-Bourboulis, E.J. Emerging drugs for the treatment of sepsis. Expert Opin. Emerg. Drugs. 2012, 17, 379–391. [Google Scholar] [CrossRef]
- Panacek, E.A.; Marshall, J.C.; Albertson, T.E.; Johnson, D.H.; Johnson, S.; MacArthur, R.D.; Miller, M.; Barchuk, W.T.; Fischkoff, S.; Kaul, M.; et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit. Care Med. 2004, 32, 2173–2182. [Google Scholar] [CrossRef]
- Conrad, U.; Plagmann, I.; Malchow, S.; Sack, M.; Floss, D.M.; Kruglov, A.A.; Nedospasov, S.A.; Rose-John, S.; Scheller, J. ELPylated anti-human TNF therapeutic single-domain antibodies for prevention of lethal septic shock. Plant Biotechnol. J. 2011, 9, 22–31. [Google Scholar] [CrossRef]
- Shirey, K.A.; Lai, W.; Scott, A.J.; Lipsky, M.; Mistry, P.; Pletneva, L.M.; Karp, C.L.; McAlees, J.; Gioannini, T.L.; Weiss, J.; et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 2013, 497, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharm. 2011, 79, 34–41. [Google Scholar] [CrossRef]
- Opal, S.M.; Laterre, P.F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef]
- Ii, M.; Matsunaga, N.; Hazeki, K.; Nakamura, K.; Takashima, K.; Seya, T.; Hazeki, O.; Kitazaki, T.; Iizawa, Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharm. 2006, 69, 1288–1295. [Google Scholar] [CrossRef]
- Sha, T.; Iizawa, Y.; Ii, M. Combination of imipenem and TAK-242, a Toll-like receptor 4 signal transduction inhibitor, improves survival in a murine model of polymicrobial sepsis. Shock 2011, 35, 205–209. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Raftogiannis, M. The immune response to severe bacterial infections: Consequences for therapy. Expert Rev. Anti. Infect. 2012, 10, 369–380. [Google Scholar] [CrossRef]
- Davies, B.; Cohen, J. Endotoxin removal devices for the treatment of sepsis and septic shock. Lancet Infect. Dis. 2011, 11, 65–71. [Google Scholar] [CrossRef]
- Hanasawa, K.; Tani, T.; Oka, T.; Yoshioka, T.; Aoki, H.; Endo, Y.; Kodama, M. Selective removal of endotoxin from the blood by extracorporeal hemoperfusion with polymyxin B immobilized fiber. Prog Clin. Biol. Res. 1988, 264, 337–341. [Google Scholar]
- Nemoto, H.; Nakamoto, H.; Okada, H.; Sugahara, S.; Moriwaki, K.; Arai, M.; Kanno, Y.; Suzuki, H. Newly developed immobilized polymyxin B fibers improve the survival of patients with sepsis. Blood Purif. 2001, 19, 361–368, discussion 368–369. [Google Scholar] [CrossRef]
- Cutuli, S.L.; Artigas, A.; Fumagalli, R.; Monti, G.; Ranieri, V.M.; Ronco, C.; Antonelli, M.; Group, E.C. Polymyxin-B hemoperfusion in septic patients: Analysis of a multicenter registry. Ann. Intensive Care. 2016, 6, 77. [Google Scholar] [CrossRef]
- Li Bassi, G.; Marti, J.D.; Xiol, E.A.; Comaru, T.; De Rosa, F.; Rigol, M.; Terraneo, S.; Rinaudo, M.; Fernandez, L.; Ferrer, M.; et al. The effects of direct hemoperfusion using a polymyxin B-immobilized column in a pig model of severe Pseudomonas aeruginosa pneumonia. Ann. Intensive Care. 2016, 6, 58. [Google Scholar] [CrossRef]
- Koga, Y.; Oba, U.; Takimoto, T.; Suminoe, A.; Takada, H.; Hara, T. Polymyxin B-immobilized fiber column hemoperfusion therapy for septic shock. Shock 2013, 40, 233. [Google Scholar] [CrossRef]
- Iwagami, M.; Yasunaga, H.; Doi, K.; Horiguchi, H.; Fushimi, K.; Matsubara, T.; Yahagi, N.; Noiri, E. Postoperative polymyxin B hemoperfusion and mortality in patients with abdominal septic shock: A propensity-matched analysis. Crit Care Med 2014, 42, 1187–1193. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and sepsis. Thromb Res. 2017, 149, 38–44. [Google Scholar] [CrossRef]
- Payen, D.M.; Guilhot, J.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Pottecher, J.; Joannes-Boyau, O.; Martin-Lefevre, L.; Jabaudon, M.; et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized control trial. Intensive Care Med. 2015, 41, 975–984. [Google Scholar] [CrossRef]
- Derhaschnig, U.; Reiter, R.; Knobl, P.; Baumgartner, M.; Keen, P.; Jilma, B. Recombinant human activated protein C (rhAPC; drotrecogin alfa [activated]) has minimal effect on markers of coagulation, fibrinolysis, and inflammation in acute human endotoxemia. Blood 2003, 102, 2093–2098. [Google Scholar] [CrossRef]
- Abraham, E.; Laterre, P.F.; Garg, R.; Levy, H.; Talwar, D.; Trzaskoma, B.L.; Francois, B.; Guy, J.S.; Bruckmann, M.; Rea-Neto, A.; et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N. Engl. J. Med. 2005, 353, 1332–1341. [Google Scholar] [CrossRef]
- Hagiwara, S.; Iwasaka, H.; Goto, K.; Ochi, Y.; Mizunaga, S.; Saikawa, T.; Noguchi, T. Recombinant thrombomodulin prevents heatstroke by inhibition of high-mobility group box 1 protein in sera of rats. Shock 2010, 34, 402–406. [Google Scholar] [CrossRef]
- Yamakawa, K.; Murao, S.; Aihara, M. Recombinant Human Soluble Thrombomodulin in Sepsis-Induced Coagulopathy: An Updated Systematic Review and Meta-Analysis. Thromb Haemost. 2019, 119, 56–65. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Yang, H.; Tong, Z. Recombinant human soluble thrombomodulin and short-term mortality of infection patients with DIC: A meta-analysis. Am. J. Emerg. Med. 2016, 34, 1876–1882. [Google Scholar] [CrossRef]
- Iba, T.; Gando, S.; Thachil, J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: The view from Japan. J. Thromb Haemost. 2014, 12, 1010–1019. [Google Scholar] [CrossRef]
- Yamakawa, K.; Ogura, H.; Fujimi, S.; Morikawa, M.; Ogawa, Y.; Mohri, T.; Nakamori, Y.; Inoue, Y.; Kuwagata, Y.; Tanaka, H.; et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: A multicenter propensity score analysis. Intensive Care Med. 2013, 39, 644–652. [Google Scholar] [CrossRef]
- Vincent, J.L.; Ramesh, M.K.; Ernest, D.; LaRosa, S.P.; Pachl, J.; Aikawa, N.; Hoste, E.; Levy, H.; Hirman, J.; Levi, M.; et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit. Care Med. 2013, 41, 2069–2079. [Google Scholar] [CrossRef]
- Adel, M.; Awad, H.A.; Abdel-Naim, A.B.; Al-Azizi, M.M. Effects of pentoxifylline on coagulation profile and disseminated intravascular coagulation incidence in Egyptian septic neonates. J. Clin. Pharm. 2010, 35, 257–265. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Bo, L.; Wang, F.; Zhu, J.; Li, J.; Deng, X. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: A meta-analysis. Crit. Care. 2011, 15, R58. [Google Scholar] [CrossRef]
- Stephens, D.P.; Thomas, J.H.; Higgins, A.; Bailey, M.; Anstey, N.M.; Currie, B.J.; Cheng, A.C. Randomized, double-blind, placebo-controlled trial of granulocyte colony-stimulating factor in patients with septic shock. Crit. Care Med. 2008, 36, 448–454. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Hutchins, N.A.; Unsinger, J.; Hotchkiss, R.S.; Ayala, A. The new normal: Immunomodulatory agents against sepsis immune suppression. Trends Mol. Med. 2014, 20, 224–233. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun. Rev. 2013, 12, 1091–1100. [Google Scholar] [CrossRef]
- Rossi, A.L.; Le, M.; Chung, C.S.; Chen, Y.; Fallon, E.A.; Matoso, A.; Xu, S.; Chun, T.T.; Erickson, C.P.; Ayala, A. A novel role for programmed cell death receptor ligand 2 in sepsis-induced hepatic dysfunction. Am. J. Physiol Gastrointest Liver Physiol. 2019, 316, G106–G114. [Google Scholar] [CrossRef]
- Wilson, J.K.; Zhao, Y.; Singer, M.; Spencer, J.; Shankar-Hari, M. Lymphocyte subset expression and serum concentrations of PD-1/PD-L1 in sepsis - pilot study. Crit. Care. 2018, 22, 95. [Google Scholar] [CrossRef]
- Huang, X.; Venet, F.; Wang, Y.L.; Lepape, A.; Yuan, Z.; Chen, Y.; Swan, R.; Kherouf, H.; Monneret, G.; Chung, C.S.; et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. USA 2009, 106, 6303–6308. [Google Scholar] [CrossRef] [Green Version]
- Brahmamdam, P.; Inoue, S.; Unsinger, J.; Chang, K.C.; McDunn, J.E.; Hotchkiss, R.S. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J. Leukoc. Biol. 2010, 88, 233–240. [Google Scholar] [CrossRef]
- Watanabe, E.; Thampy, L.K.; Hotchkiss, R.S. Immunoadjuvant therapy in sepsis: Novel strategies for immunosuppressive sepsis coming down the pike. Acute Med. Surg. 2018, 5, 309–315. [Google Scholar] [CrossRef]
- Kawamoto, E.; Masui-Ito, A.; Eguchi, A.; Soe, Z.Y.; Prajuabjinda, O.; Darkwah, S.; Park, E.J.; Imai, H.; Shimaoka, M. Integrin and PD-1 Ligand Expression on Circulating Extracellular Vesicles in Systemic Inflammatory Response Syndrome and Sepsis. Shock 2019, 52, 13–22. [Google Scholar] [CrossRef]
- Nelson, G.E.; Mave, V.; Gupta, A. Biomarkers for sepsis: A review with special attention to India. Biomed Res. Int. 2014, 2014, 264351. [Google Scholar] [CrossRef]
- Jacobs, L.; Wong, H.R. Emerging infection and sepsis biomarkers: Will they change current therapies? Expert Rev. Anti. Infect. 2016, 14, 929–941. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. https://doi.org/10.3390/ijms20215376
Huang M, Cai S, Su J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. International Journal of Molecular Sciences. 2019; 20(21):5376. https://doi.org/10.3390/ijms20215376
Chicago/Turabian StyleHuang, Min, Shaoli Cai, and Jingqian Su. 2019. "The Pathogenesis of Sepsis and Potential Therapeutic Targets" International Journal of Molecular Sciences 20, no. 21: 5376. https://doi.org/10.3390/ijms20215376
APA StyleHuang, M., Cai, S., & Su, J. (2019). The Pathogenesis of Sepsis and Potential Therapeutic Targets. International Journal of Molecular Sciences, 20(21), 5376. https://doi.org/10.3390/ijms20215376





