Quantitative Phosphoproteomics Reveals System-Wide Phosphorylation Network Altered by Spry in Mouse Mammary Stromal Fibroblasts
Abstract
1. Introduction
2. Results
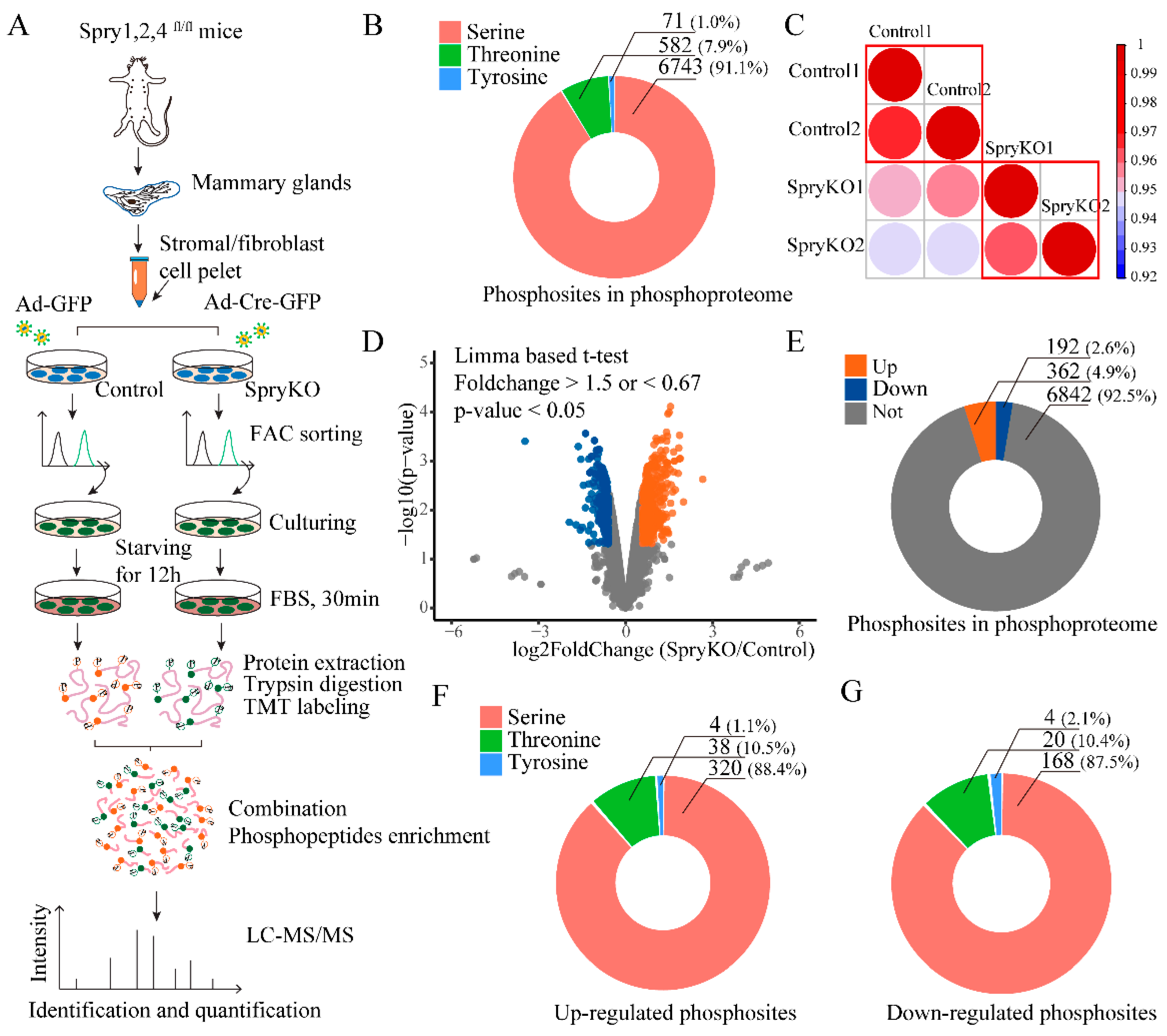
2.1. Phosphoproteomic Profiling and Quantification of Sprouty (SPRY)-Deficient Mouse Mammary Stromal Fibroblasts
2.2. The Functional Enrichment Analysis of SPRY-Altered Phosphoproteins in Mammary Stromal Fibroblasts
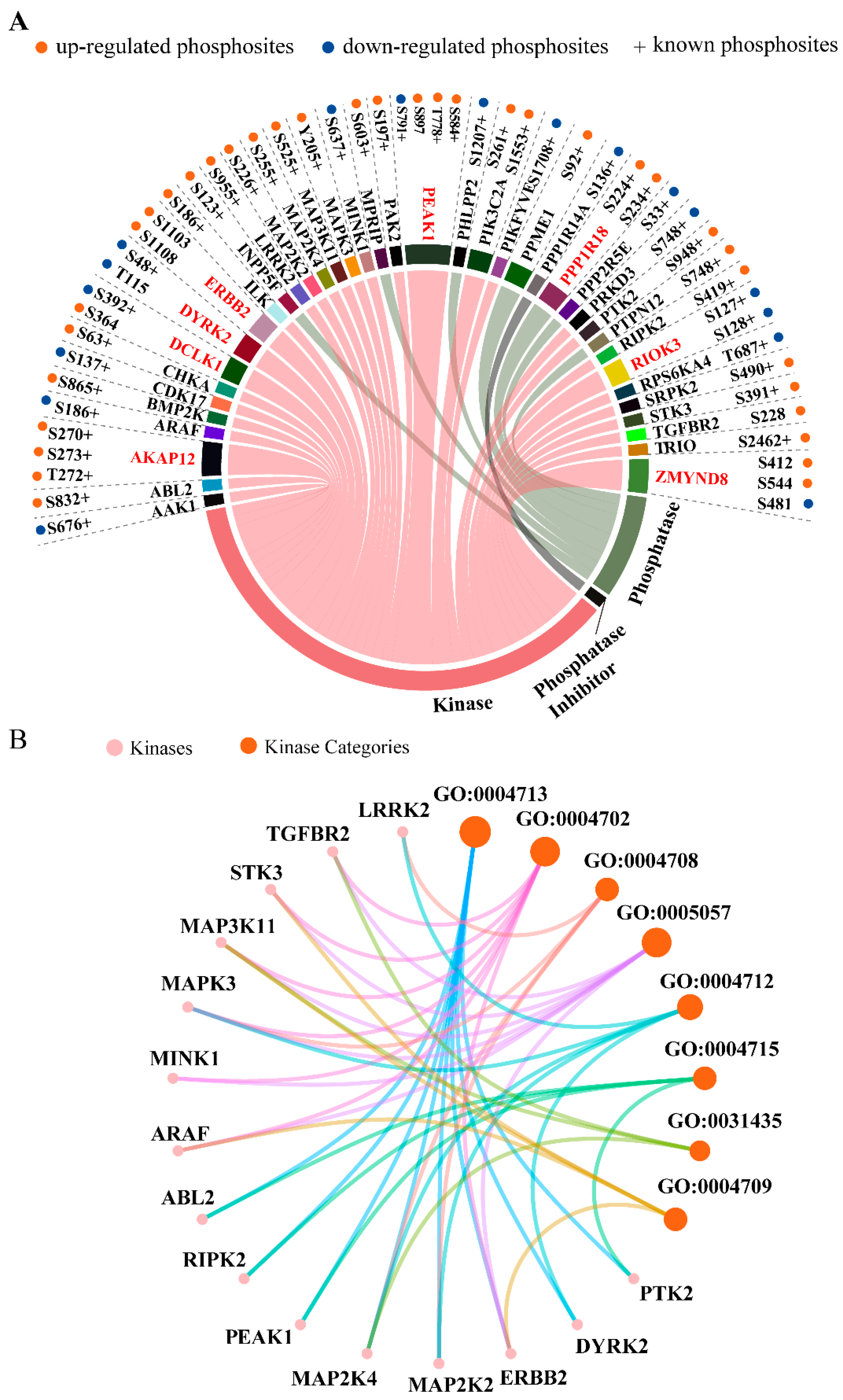
2.3. Kinases, Phosphatases, and Phosphatase Inhibitors Regulated by SPRY in Mammary Stromal Fibroblasts
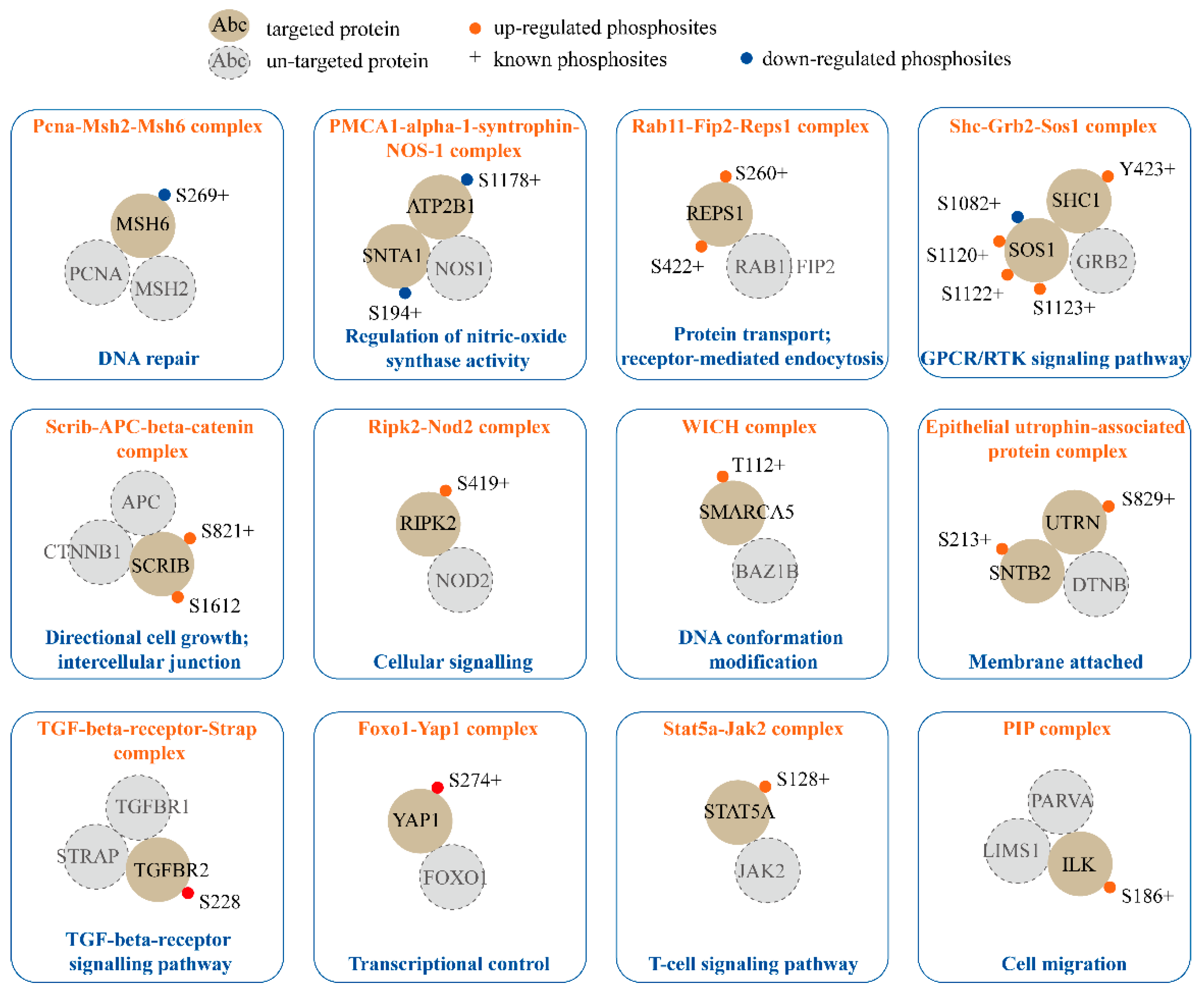
2.4. Phosphorylation in Established Protein Complexes Regulated by SPRY in Mammary Stromal Fibroblasts
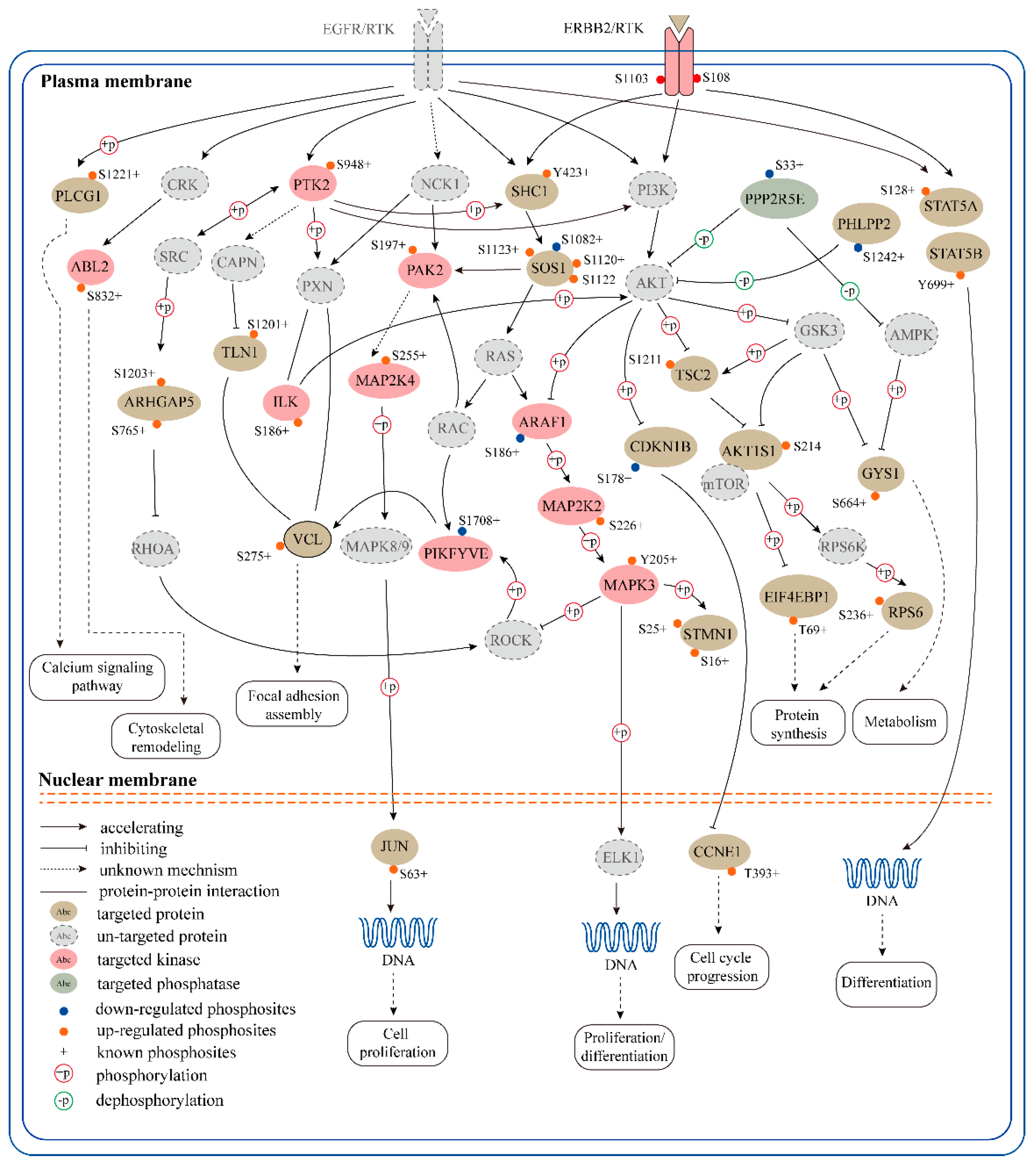
2.5. An Receptor Tyrosine Kinase (RTK)-Centric Signaling Network Regulated by SPRY in Mammary Stromal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Mice and Cells
4.2. Isolation of Primary Mouse Mammary Stromal Fibroblasts
4.3. Adenovirus Infection and Fluorescence-Activated Cell Sorting (FACS)
4.4. Cell lysate Preparation, Protein Digestion, and Peptide Desalting
4.5. Tandem Mass Tag (TMT) Labeling
4.6. Phosphopeptide Enrichment
4.7. Mass Spectrometry and Data Processing
4.8. Bioinformatics Analysis, Statistics and Data Visualization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAK1 | AP2 associated kinase 1 |
| ABL2 | v-abl Abelson murine leukemia viral oncogene 2 (arg, Abelson-related gene) |
| Ad | adenovirus |
| AGC | automatic gain control |
| AKAP12 | A kinase (PRKA) anchor protein (gravin) 12 |
| AKT1S1 | AKT1 substrate 1 (proline-rich) |
| ARAF | Araf proto-oncogene, serine/threonine kinase |
| ARHGAP42 | Rho GTPase activating protein 42 |
| ARHGAP5 | Rho GTPase activating protein 5 |
| ARHGEF12 | Rho guanine nucleotide exchange factor (GEF) 12 |
| ARHGEF40 | Rho guanine nucleotide exchange factor (GEF) 40 |
| ATP2B1 | ATPase, Ca++ transporting, plasma membrane 1 |
| BMP2K | BMP2 inducible kinase |
| BP | biological process |
| CAFs | cancer-associated fibroblasts |
| CC | cellular component |
| CCNE1 | cyclin E1 |
| CDK17 | cyclin-dependent kinase 17 |
| CDKN1B | cyclin-dependent kinase inhibitor 1B |
| CHKA | choline kinase alpha |
| CORUM | the comprehensive resource of mammalian protein complexes |
| Cre | cyclization recombination enzyme |
| DAVID | the database for annotation, visualization and integrated discovery |
| DCLK1 | doublecortin-like kinase 1 |
| DOCK9 | dedicator of cytokinesis 9 |
| DTT | Dithiothreitol |
| DYRK2 | dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 2 |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| EIF4EBP1 | eukaryotic translation initiation factor 4E binding protein 1 |
| ERBB2 | erb-b2 receptor tyrosine kinase 2 |
| ERK | extracellular regulated kinase |
| FACS | fluorescence activated cell sorting |
| FARP1 | FERM, RhoGEF (Arhgef) and pleckstrin domain protein 1 (chondrocyte-derived) |
| FBS | fetal bovine serum |
| FDR | false discovery rate |
| FGD1 | FYVE, RhoGEF and PH domain containing 1 |
| FGF | fibroblast growth factor |
| GDP | guanosine diphosphate |
| GEO | gene expression omnibus |
| GFP | green fluorescent protein |
| GO | Gene Ontology |
| GPCR | G protein-coupled receptors |
| GSEA | gene Set Enrichment Analysis |
| GTP | guanosine triphosphate |
| GYS1 | glycogen synthase 1, muscle |
| HCD | higher-energy collisional dissociation |
| IAM | Iodoacetamide |
| ILK | integrin linked kinase |
| INPP5F | inositol polyphosphate-5-phosphatase F |
| ITS | insulin transferrin selenium |
| JUN | jun proto-oncogene |
| KEGG | kyoto encyclopedia of genes and genomes |
| KLHL32 | kelch-like 32 |
| KSR | kinase-substrate relationships |
| LC-MS/MS | large-scale liquid chromatography tandem mass spectrometry |
| LRRK2 | leucine-rich repeat kinase 2 |
| MAP1B | microtubule-associated protein 1B |
| MAP2K2 | mitogen-activated protein kinase kinase 2 |
| MAP2K4 | mitogen-activated protein kinase kinase 4 |
| MAP3K11 | mitogen-activated protein kinase kinase kinase 11 |
| MAPK | mitogen-activated protein kinase |
| MAPK3 | mitogen-activated protein kinase 3 |
| MF | molecular function |
| MINK1 | misshapen-like kinase 1 (zebrafish) |
| MMPs | matrix metalloproteinases |
| MPRIP | myosin phosphatase Rho interacting protein |
| MS | mass spectrometry |
| MSH6 | mutS homolog 6 |
| mTOR | mammalian target of rapamycin |
| MYOT | myotilin |
| PAK2 | p21 (RAC1) activated kinase 2 |
| PBS | phosphate buffered saline |
| PDGF | platelet-derived growth factor |
| PEAK1 | pseudopodium-enriched atypical kinase 1 |
| PHLPP2 | PH domain and leucine rich repeat protein phosphatase 2 |
| PIK3C2A | phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2 alpha |
| PIKFYVE | phosphoinositide kinase, FYVE type zinc finger containing |
| PIP complex | PINCH-1/2/ILK/α-parvin complex |
| PLCG1 | phospholipase C, gamma 1 |
| PPME1 | protein phosphatase methylesterase 1 |
| PPP1R14A | protein phosphatase 1, regulatory inhibitor subunit 14A |
| PPP1R18 | protein phosphatase 1, regulatory subunit 18 |
| PPP2R5E | protein phosphatase 2, regulatory subunit B’, epsilon |
| PRKD3 | protein kinase D3 |
| PTK2 | PTK2 protein tyrosine kinase 2 |
| PTPN12 | protein tyrosine phosphatase, non-receptor type 12 |
| RAB11FIP5 | RAB11 family interacting protein 5 (class I) |
| RAB3IL1 | RAB3A interacting protein (rabin3)-like 1 |
| RAB3IP | RAB3A interacting protein |
| REPS1 | RalBP1 associated Eps domain containing protein |
| RIOK3 | RIO kinase 3 |
| RIPK2 | receptor (TNFRSF)-interacting serine-threonine kinase 2 |
| RPS6 | ribosomal protein S6 |
| RPS6KA4 | ribosomal protein S6 kinase, polypeptide 4 |
| RTK | receptor tyrosine kinase |
| S | serine |
| T | Threonine |
| SCRIB | scribbled planar cell polarity |
| SHC1 | src homology 2 domain-containing transforming protein C1 |
| SMARCA5 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 5 |
| SMCR8 | Smith-Magenis syndrome chromosome region, candidate 8 homolog (human) |
| SNTA1 | syntrophin, acidic 1 |
| SNTB2 | syntrophin, basic 2 |
| SOS1 | SOS Ras/Rac guanine nucleotide exchange factor 1 |
| SPRY | Sprouty |
| SpryKO | Spry1, 2 and 4 triple knock out |
| SRPK2 | serine/arginine-rich protein specific kinase 2 |
| STAT5A | signal transducer and activator of transcription 5A |
| STAT5B | signal transducer and activator of transcription 5B |
| STK3 | serine/threonine kinase 3 |
| STMN1 | stathmin 1 |
| TBC1D10A | TBC1 domain family, member 10a |
| TEAB | triethyl ammonium bicarbonate |
| TFA | trifluoroacetic acid |
| TGFBR2 | transforming growth factor, beta receptor II |
| TLN1 | talin 1 |
| TMT | tandem mass tag |
| TRIO | triple functional domain (PTPRF interacting) |
| TSC2 | TSC complex subunit 2 |
| UTRN | Utrophin |
| VCL | Vinculin |
| VEGF | vascular endothelial growth factor |
| WICH complex | WSTF-ISWI chromatin remodeling complex |
| Y | Tyrosine |
| YAP1 | yes-associated protein 1 |
| ZBTB11 | zinc finger and BTB domain containing 11 |
| ZMYND8 | zinc finger, MYND-type containing 8 |
References
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Koledova, Z.; Zhang, X.; Streuli, C.; Clarke, R.B.; Klein, O.D.; Werb, Z.; Lu, P. SPRY1 regulates mammary epithelial morphogenesis by modulating EGFR-dependent stromal paracrine signaling and ECM remodeling. Proc. Natl. Acad. Sci. USA 2016, 113, E5731–E5740. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Kim, J.B.; Stein, R.; O’Hare, M.J. Tumour-stromal interactions in breast cancer: The role of stroma in tumourigenesis. Tumour Biol. 2005, 26, 173–185. [Google Scholar] [CrossRef]
- Schor, S.L.; Schor, A.M. Phenotypic and genetic alterations in mammary stroma: Implications for tumour progression. Breast Cancer Res. 2001, 3, 373–379. [Google Scholar] [CrossRef]
- Aboussekhra, A. Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int. J. Dev. Biol. 2011, 55, 841–849. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J. Tumor stroma as targets for cancer therapy. Pharmacol. Ther. 2013, 137, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Brechbuhl, H.M.; Finlay-Schultz, J.; Yamamoto, T.M.; Gillen, A.E.; Cittelly, D.M.; Tan, A.C.; Sams, S.B.; Pillai, M.M.; Elias, A.D.; Robinson, W.A.; et al. Fibroblast Subtypes Regulate Responsiveness of Luminal Breast Cancer to Estrogen. Clin. Cancer Res. 2017, 23, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Tabassum, D.P.; Janiszewska, M.; Place, A.E.; Trinh, A.; Rozhok, A.I.; Pyne, S.; Guerriero, J.L.; Shu, S.; Ekram, M.; et al. Spatial Proximity to Fibroblasts Impacts Molecular Features and Therapeutic Sensitivity of Breast Cancer Cells Influencing Clinical Outcomes. Cancer Res. 2016, 76, 6495–6506. [Google Scholar] [CrossRef]
- Fantl, W.J.; Johnson, D.E.; Williams, L.T. Signalling by receptor tyrosine kinases. Annu. Rev. Biochem. 1993, 62, 453–481. [Google Scholar] [CrossRef]
- Casaletto, J.B.; McClatchey, A.I. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer 2012, 12, 387–400. [Google Scholar] [CrossRef]
- Ching, S.T.; Cunha, G.R.; Baskin, L.S.; Basson, M.A.; Klein, O.D. Coordinated activity of Spry1 and Spry2 is required for normal development of the external genitalia. Dev. Biol. 2014, 386, 1–11. [Google Scholar] [CrossRef]
- Shehata, H.M.; Khan, S.; Chen, E.; Fields, P.E.; Flavell, R.A.; Sanjabi, S. Lack of Sprouty 1 and 2 enhances survival of effector CD8(+) T cells and yields more protective memory cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8939–E8947. [Google Scholar] [CrossRef]
- Dikic, I.; Giordano, S. Negative receptor signalling. Curr. Opin. Cell Biol. 2003, 15, 128–135. [Google Scholar] [CrossRef]
- Qian, B.Z.; Zhang, H.; Li, J.; He, T.; Yeo, E.J.; Soong, D.Y.; Carragher, N.O.; Munro, A.; Chang, A.; Bresnick, A.R.; et al. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J. Exp. Med. 2015, 212, 1433–1448. [Google Scholar] [CrossRef]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Faratian, D.; Sims, A.H.; Mullen, P.; Kay, C.; Um, I.; Langdon, S.P.; Harrison, D.J. Sprouty 2 is an independent prognostic factor in breast cancer and may be useful in stratifying patients for trastuzumab therapy. PLoS ONE 2011, 6, e23772. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.; Balmanno, K.; Hadfield, K.; Weston, C.; Cook, S.J. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 2003, 278, 18811–18816. [Google Scholar] [CrossRef]
- Beltran, L.; Chaussade, C.; Vanhaesebroeck, B.; Cutillas, P.R. Calpain interacts with class IA phosphoinositide 3-kinases regulating their stability and signaling activity. Proc. Natl. Acad. Sci. USA 2011, 108, 16217–16222. [Google Scholar] [CrossRef]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef]
- Yi, T.; Zhai, B.; Yu, Y.; Kiyotsugu, Y.; Raschle, T.; Etzkorn, M.; Seo, H.C.; Nagiec, M.; Luna, R.E.; Reinherz, E.L.; et al. Quantitative phosphoproteomic analysis reveals system-wide signaling pathways downstream of SDF-1/CXCR4 in breast cancer stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E2182–E2190. [Google Scholar] [CrossRef]
- Matheron, L.; van den Toorn, H.; Heck, A.J.; Mohammed, S. Characterization of biases in phosphopeptide enrichment by Ti(4+)-immobilized metal affinity chromatography and TiO2 using a massive synthetic library and human cell digests. Anal. Chem. 2014, 86, 8312–8320. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef]
- Kronke, J.; Fink, E.C.; Hollenbach, P.W.; MacBeth, K.J.; Hurst, S.N.; Udeshi, N.D.; Chamberlain, P.P.; Mani, D.R.; Man, H.W.; Gandhi, A.K.; et al. Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature 2015, 523, 183–188. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yoon, S.O.; Poulogiannis, G.; Yang, Q.; Ma, X.M.; Villen, J.; Kubica, N.; Hoffman, G.R.; Cantley, L.C.; Gygi, S.P.; et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 2011, 332, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Bosbach, B.; Rossi, F.; Yozgat, Y.; Loo, J.; Zhang, J.Q.; Berrozpe, G.; Warpinski, K.; Ehlers, I.; Veach, D.; Kwok, A.; et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc. Natl. Acad. Sci. USA 2017, 114, E8448–E8457. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.P.; Kang, S.A.; Rameseder, J.; Zhang, Y.; Ottina, K.A.; Lim, D.; Peterson, T.R.; Choi, Y.; Gray, N.S.; Yaffe, M.B.; et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 2011, 332, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Huttlin, E.L.; Jedrychowski, M.P.; Elias, J.E.; Goswami, T.; Rad, R.; Beausoleil, S.A.; Villen, J.; Haas, W.; Sowa, M.E.; Gygi, S.P. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 2010, 143, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.M.; Graham, N.A.; Stoyanova, T.; Sedghi, A.; Goldstein, A.S.; Cai, H.; Smith, D.A.; Zhang, H.; Komisopoulou, E.; Huang, J.; et al. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc. Natl. Acad. Sci. USA 2012, 109, 1643–1648. [Google Scholar] [CrossRef]
- Masoumi-Moghaddam, S.; Amini, A.; Morris, D.L. The developing story of Sprouty and cancer. Cancer Metastasis Rev. 2014, 33, 695–720. [Google Scholar] [CrossRef]
- Raynor, J.; Chi, H. Sprouty branches out to control T cell memory. Proc. Natl. Acad. Sci. USA 2018, 115, 9339–9341. [Google Scholar] [CrossRef]
- Gross, I.; Bassit, B.; Benezra, M.; Licht, J.D. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J. Biol. Chem. 2001, 276, 46460–46468. [Google Scholar] [CrossRef]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef]
- Blumer, J.; Rey, J.; Dehmelt, L.; Mazel, T.; Wu, Y.W.; Bastiaens, P.; Goody, R.S.; Itzen, A. RabGEFs are a major determinant for specific Rab membrane targeting. J. Cell. Biol. 2013, 200, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Cherfils, J.; Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef] [PubMed]
- Wiza, C.; Nascimento, E.B.; Linssen, M.M.; Carlotti, F.; Herzfeld de Wiza, D.; van der Zon, G.C.; Maassen, J.A.; Diamant, M.; Guigas, B.; Ouwens, D.M. Proline-rich Akt substrate of 40-kDa contains a nuclear export signal. Cell. Signal. 2013, 25, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jiang, X.; Li, B.; Yang, H.J.; Miller, M.; Yang, A.; Dhar, A.; Pavletich, N.P. Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature 2017, 552, 368–373. [Google Scholar] [CrossRef]
- Fleissner, F.; Jazbutyte, V.; Fiedler, J.; Gupta, S.K.; Yin, X.; Xu, Q.; Galuppo, P.; Kneitz, S.; Mayr, M.; Ertl, G.; et al. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ. Res. 2010, 107, 138–143. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, D.; Dongiovanni, P.; Ozcan, L.; Nayak, S.; Ueberheide, B.; Valenti, L.; Auwerx, J.; Pajvani, U.B. Degradation of PHLPP2 by KCTD17, via a Glucagon-Dependent Pathway, Promotes Hepatic Steatosis. Gastroenterology 2017, 153, 1568–1580.e10. [Google Scholar] [CrossRef]
- Grzechnik, A.T.; Newton, A.C. PHLPPing through history: a decade in the life of PHLPP phosphatases. Biochem. Soc. Trans. 2016, 44, 1675–1682. [Google Scholar] [CrossRef]
- Slepak, T.I.; Salay, L.D.; Lemmon, V.P.; Bixby, J.L. Dyrk kinases regulate phosphorylation of doublecortin, cytoskeletal organization, and neuronal morphology. Cytoskeleton (Hoboken) 2012, 69, 514–527. [Google Scholar] [CrossRef]
- Nishi, H.; Hashimoto, K.; Panchenko, A.R. Phosphorylation in protein-protein binding: effect on stability and function. Structure 2011, 19, 1807–1815. [Google Scholar] [CrossRef]
- Good, M.C.; Zalatan, J.G.; Lim, W.A. Scaffold proteins: Hubs for controlling the flow of cellular information. Science 2011, 332, 680–686. [Google Scholar] [CrossRef]
- Giurgiu, M.; Reinhard, J.; Brauner, B.; Dunger-Kaltenbach, I.; Fobo, G.; Frishman, G.; Montrone, C.; Ruepp, A. CORUM: The comprehensive resource of mammalian protein complexes-2019. Nucleic Acids Res. 2019, 47, D559–D563. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Tomura, M.; Yu, W.G.; Iwasaki, M.; Park, W.R.; Hamaoka, T.; Fujiwara, H. Requirement for distinct Janus kinases and STAT proteins in T cell proliferation versus IFN-gamma production following IL-12 stimulation. J. Immunol. 1998, 161, 5893–5900. [Google Scholar] [PubMed]
- Papin, J.A.; Hunter, T.; Palsson, B.O.; Subramaniam, S. Reconstruction of cellular signalling networks and analysis of their properties. Nat. Rev. Mol. Cell Biol. 2005, 6, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Straussman, R.; Morikawa, T.; Shee, K.; Barzily-Rokni, M.; Qian, Z.R.; Du, J.; Davis, A.; Mongare, M.M.; Gould, J.; Frederick, D.T.; et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012, 487, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, J.; Ryden, L.; Strell, C.; Frings, O.; Tobin, N.P.; Fornander, T.; Bergh, J.; Landberg, G.; Stal, O.; Ostman, A. High expression of stromal PDGFRbeta is associated with reduced benefit of tamoxifen in breast cancer. J. Pathol. Clin. Res. 2017, 3, 38–43. [Google Scholar] [CrossRef]
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010, 330, 827–830. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, W.; Strasner, A.; Grivennikov, S.; Cheng, J.Q.; Hoffman, R.M.; Karin, M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 2011, 470, 548–553. [Google Scholar] [CrossRef]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef]
- Denton, A.E.; Roberts, E.W.; Linterman, M.A.; Fearon, D.T. Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12139–12144. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef]
- Toullec, A.; Gerald, D.; Despouy, G.; Bourachot, B.; Cardon, M.; Lefort, S.; Richardson, M.; Rigaill, G.; Parrini, M.C.; Lucchesi, C.; et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol. Med. 2010, 2, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Benyahia, Z.; Dussault, N.; Cayol, M.; Sigaud, R.; Berenguer-Daize, C.; Delfino, C.; Tounsi, A.; Garcia, S.; Martin, P.M.; Mabrouk, K.; et al. Stromal fibroblasts present in breast carcinomas promote tumor growth and angiogenesis through adrenomedullin secretion. Oncotarget 2017, 8, 15744–15762. [Google Scholar] [CrossRef] [PubMed]
- Rasanen, K.; Vaheri, A. Activation of fibroblasts in cancer stroma. Exp. Cell Res. 2010, 316, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Evans, R.A.; Pettit, E.; Hallett, M.; Williams, J.D.; Steadman, R. Cellular activation through the ligation of intercellular adhesion molecule-1. J. Cell. Sci. 1998, 111 Pt 4, 443–453. [Google Scholar]
- Templeton, A.J.; Diez-Gonzalez, L.; Ace, O.; Vera-Badillo, F.; Seruga, B.; Jordan, J.; Amir, E.; Pandiella, A.; Ocana, A. Prognostic relevance of receptor tyrosine kinase expression in breast cancer: a meta-analysis. Cancer Treat. Rev. 2014, 40, 1048–1055. [Google Scholar] [CrossRef]
- Gruosso, T.; Gigoux, M.; Manem, V.S.K.; Bertos, N.; Zuo, D.; Perlitch, I.; Saleh, S.M.I.; Zhao, H.; Souleimanova, M.; Johnson, R.M.; et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Investig. 2019, 129, 1785–1800. [Google Scholar] [CrossRef]
- Bauer, M.; Su, G.; Casper, C.; He, R.; Rehrauer, W.; Friedl, A. Heterogeneity of gene expression in stromal fibroblasts of human breast carcinomas and normal breast. Oncogene 2010, 29, 1732–1740. [Google Scholar] [CrossRef]
- Wagner, J.; Rapsomaniki, M.A.; Chevrier, S.; Anzeneder, T.; Langwieder, C.; Dykgers, A.; Rees, M.; Ramaswamy, A.; Muenst, S.; Soysal, S.D.; et al. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell 2019, 177, 1330–1345.e18. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef]
- Basson, M.A.; Akbulut, S.; Watson-Johnson, J.; Simon, R.; Carroll, T.J.; Shakya, R.; Gross, I.; Martin, G.R.; Lufkin, T.; McMahon, A.P.; et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell 2005, 8, 229–239. [Google Scholar] [CrossRef]
- Shim, K.; Minowada, G.; Coling, D.E.; Martin, G.R. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev. Cell 2005, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.D.; Minowada, G.; Peterkova, R.; Kangas, A.; Yu, B.D.; Lesot, H.; Peterka, M.; Jernvall, J.; Martin, G.R. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev. Cell 2006, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Dudoit, S.; Luu, P.; Lin, D.M.; Peng, V.; Ngai, J.; Speed, T.P. Normalization for cDNA microarray data: A robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002, 30, e15. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.; Cowley, M.J.; Hoon, S.L.; Guilhaus, M.; Raftery, M.J.; Cavicchioli, R. Normalization and statistical analysis of quantitative proteomics data generated by metabolic labeling. Mol. Cell. Proteom. 2009, 8, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]

| SPRY1 | SPRY2 | SPRY3 | SPRY4 |
|---|---|---|---|
| bud elongation involved in lung branching; | branching morphogenesis of an epithelial tube; | axon development; | multicellular organism development; |
| EMT involved in cardiac fibroblast development; | bud elongation involved in lung branching; | multicellular organism development; | negative regulation of ERK1 and ERK2 cascade; |
| metanephros development; | cell fate commitment; | regulation of signal transduction. | regulation of signal transduction. |
| multicellular organism development; | cellular response to leukemia inhibitory factor | ||
| negative regulation of cell proliferation; | cellular response to VEGF stimulus; | ||
| negative regulation of EGF receptor signaling pathway; | establishment of mitotic spindle orientation; | ||
| negative regulation of ERK1 and ERK2 cascade; | inner ear morphogenesis; | ||
| negative regulation of FGF receptor signaling pathway; | lung development; | ||
| negative regulation of GTPase activity; | lung growth; | ||
| negative regulation of MAP kinase activity; | lung morphogenesis; | ||
| negative regulation of neurotrophin TRK receptor signaling pathway; | multicellular organism development; | ||
| negative regulation of Ras protein signal transduction; | negative regulation of angiogenesis; | ||
| organ induction; | negative regulation of apoptotic process; | ||
| regulation of signal transduction; | negative regulation of cell projection organization; | ||
| ureteric bud development. | negative regulation of cell proliferation; | ||
| negative regulation of EGF receptor signaling pathway; | |||
| negative regulation of ERK1 and ERK2 cascade; | |||
| negative regulation of FGF receptor signaling pathway; | |||
| negative regulation of GTPase activity; | |||
| negative regulation of MAP kinase activity; | |||
| negative regulation of peptidyl; | |||
| negative regulation of Ras protein signal transduction; | |||
| negative regulation of VEGF signaling pathway; | |||
| positive regulation of cell migration; | |||
| positive regulation of ERK1 and ERK2 cascade; | |||
| positive regulation of gene expression; | |||
| positive regulation of peptidyl-serine phosphorylation; | |||
| positive regulation of protein kinase B signaling; | |||
| positive regulation of protein serine/threonine kinase activity; | |||
| regulation of cell differentiation; | |||
| regulation of cell proliferation; | |||
| regulation of signal transduction; | |||
| respiratory system development; | |||
| sensory perception of sound. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Yao, L.; Han, Y.; Hao, P.; Lu, P. Quantitative Phosphoproteomics Reveals System-Wide Phosphorylation Network Altered by Spry in Mouse Mammary Stromal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5400. https://doi.org/10.3390/ijms20215400
Shi T, Yao L, Han Y, Hao P, Lu P. Quantitative Phosphoproteomics Reveals System-Wide Phosphorylation Network Altered by Spry in Mouse Mammary Stromal Fibroblasts. International Journal of Molecular Sciences. 2019; 20(21):5400. https://doi.org/10.3390/ijms20215400
Chicago/Turabian StyleShi, Tiezhu, Linli Yao, Ying Han, Piliang Hao, and Pengfei Lu. 2019. "Quantitative Phosphoproteomics Reveals System-Wide Phosphorylation Network Altered by Spry in Mouse Mammary Stromal Fibroblasts" International Journal of Molecular Sciences 20, no. 21: 5400. https://doi.org/10.3390/ijms20215400
APA StyleShi, T., Yao, L., Han, Y., Hao, P., & Lu, P. (2019). Quantitative Phosphoproteomics Reveals System-Wide Phosphorylation Network Altered by Spry in Mouse Mammary Stromal Fibroblasts. International Journal of Molecular Sciences, 20(21), 5400. https://doi.org/10.3390/ijms20215400




