RETRACTED: Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Cognitive Function
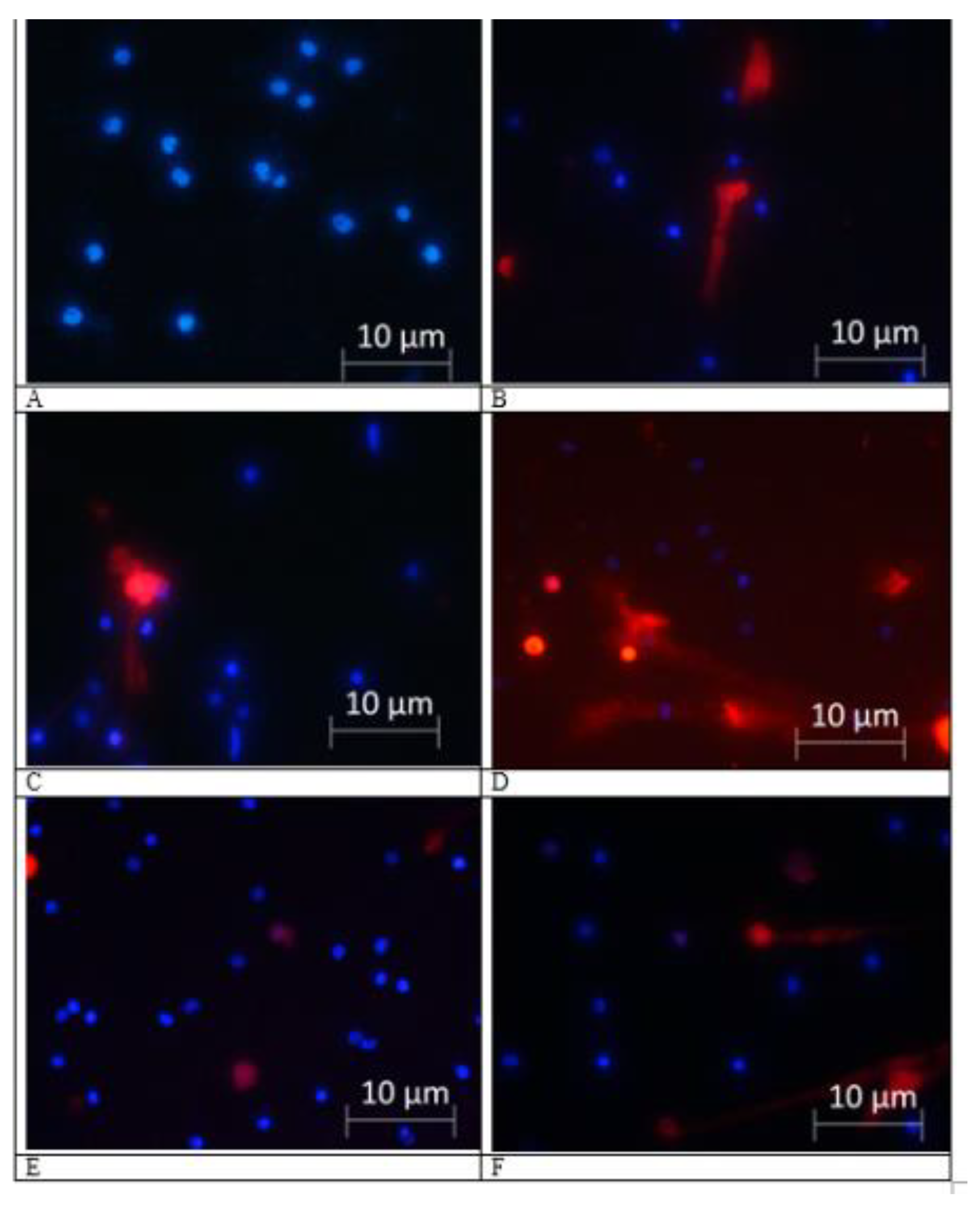
2.2. NETs Formation
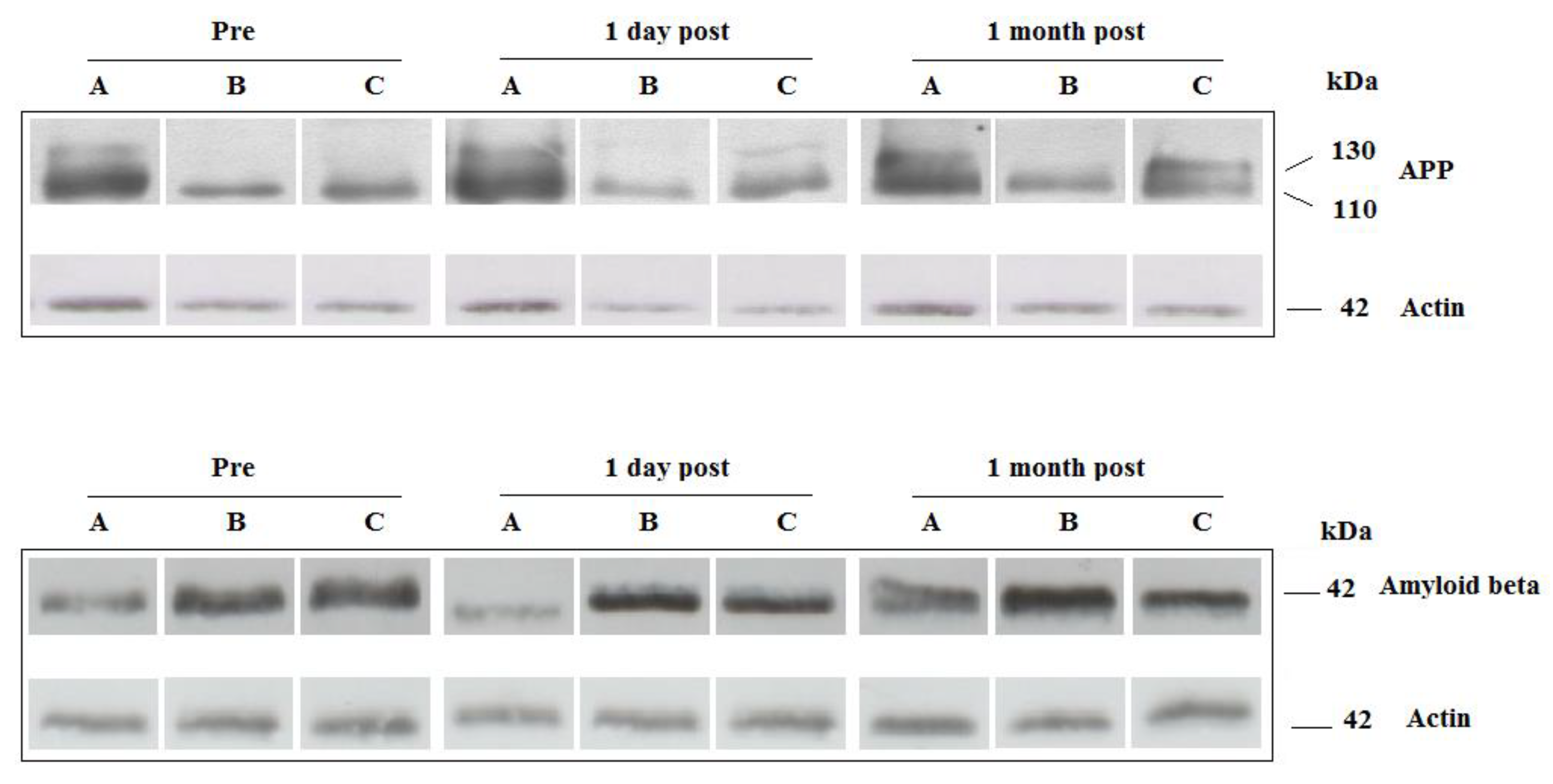
2.3. Platelets Amyloid Precursor Proteins (APP) and Amyloid Beta (Aβ)
2.4. Expression of miR-29a and lncRNA BACE-AS in Platelets
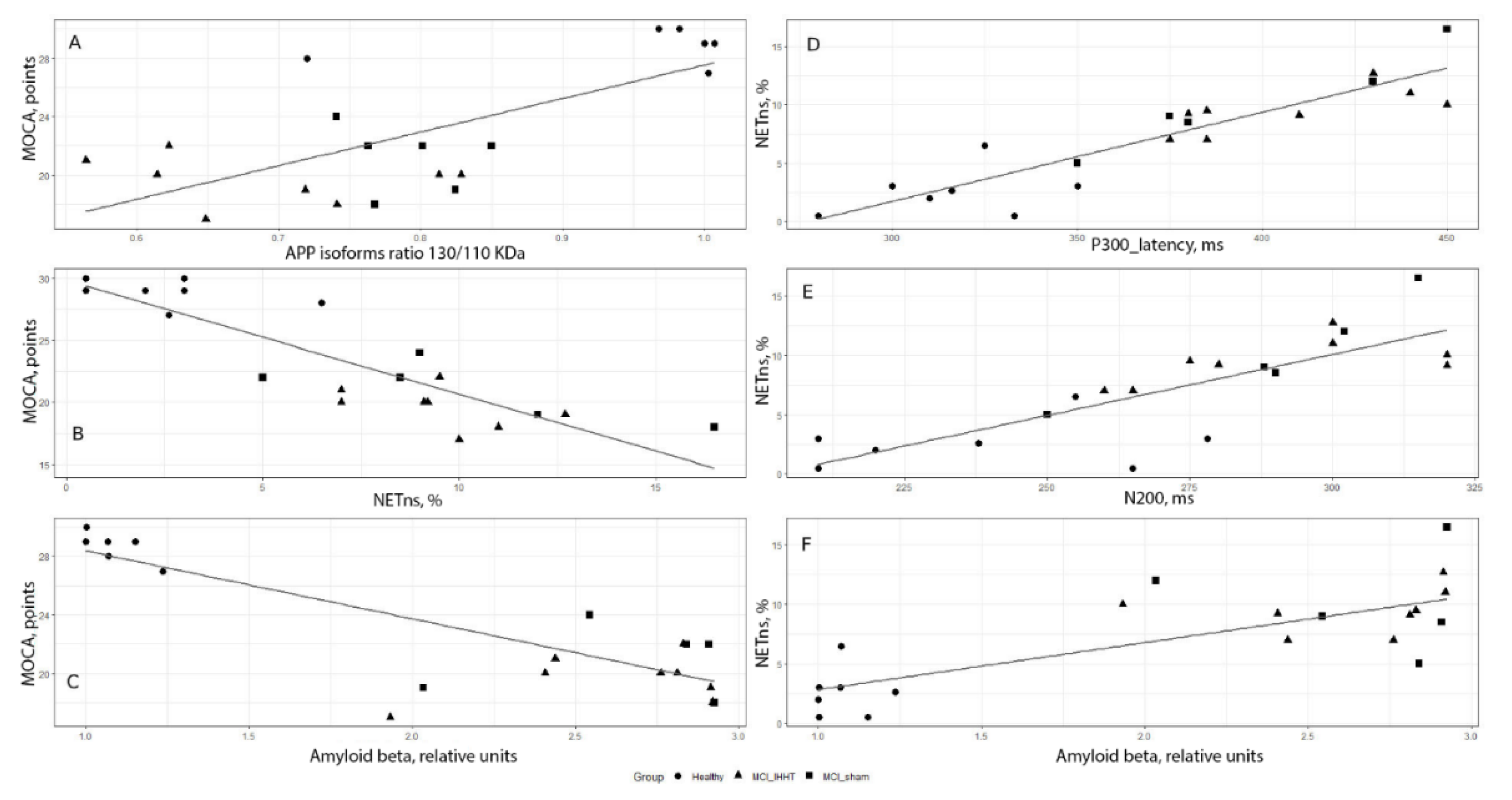
2.5. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Characteristics of Participants
4.2. Protocol of IHHT
4.3. Cognitive Function Assessment
4.4. Neutrophil Isolation and NETs Evaluation
4.5. Platelets Isolation
4.6. Western Blot Analysis
4.7. RNA Isolation and Real-Time Polymerase Chain Reaction (PCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid beta |
| ACK | Ammonium-Chloride-Potassium |
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| BACE1 | Beta-site amyloid precursor protein cleaving enzyme 1 |
| dB HL | decibels Hearing Level |
| EEG | Electroencephalography |
| ERPs | Event-Related Potentials |
| FIO2 | Fractional concentration of oxygen in the inspired gas |
| HBSS | Hank’s Balanced Salt Solution |
| IHHT | Intermittent Hypoxia-Hyperoxia Training |
| IHT | Intermittent Hypoxia-Normoxia Training |
| lncRNA | Long noncoding RNA |
| MCI | Mild Cognitive Impairment |
| MoCA | Montreal Cognitive Assessment |
| NETns | Non-stimulated neutrophil extracellular traps |
| NETs | Neutrophil extracellular traps |
| OSA | Obstructive Sleep Apnea |
| PBS | Phosphate Buffered Saline |
| PCR | Polymerase Chain Reaction |
| PMA | Phorbol myristate acetate |
| PMN | Polymorphonuclear leukocyte |
| ROS | Reactive oxygen species |
References
- Dong, Y.; Lagarde, J.; Xicota, L.; Corne, H.; Chantran, Y.; Chaigneau, T.; Crestani, B.; Bottlaender, M.; Potier, M.-C.; Aucouturier, P.; et al. Neutrophil hyperactivation correlates with Alzheimer’s disease progression. Ann. Neurol. 2018, 83, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Evin, G.; Li, Q.X. Platelets and Alzheimer’s disease: Potential of APP as a biomarker. World J. Psychiatry 2012, 2, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavykh, L.Y.; Davila-Rodriguez, J.; Rivera-Aponte, D.E.; Zueva, L.V.; Washington, A.V.; Sanabria, P.; Inyushin, M.Y. Platelets are responsible for the accumulation of beta-amyloid in blood clots inside and around blood vessels in mouse brain after thrombosis. Brain Res. Bull. 2017, 128, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Muller, U.C.; Zheng, H. Physiological functions of APP family proteins. Cold Spring Harb. Perspect. Med. 2012, 2, a006288. [Google Scholar] [CrossRef] [PubMed]
- Visconte, C.; Canino, J.; Guidetti, G.F.; Zarà, M.; Seppi, C.; Abubaker, A.A.; Pula, G.; Torti, M.; Canobbio, I. Amyloid precursor protein is required for in vitro platelet adhesion to amyloid peptides and potentiation of thrombus formation. Cell Signal. 2018, 52, 95–102. [Google Scholar] [CrossRef]
- Silva, J.V.; Yoon, S.; Domingues, S.; Guimarães, S.C.; Goltsev, A.V.; Silva, E.F.D.C.E.; Mendes, J.F.F.; Silva, O.A.B.D.C.E.; Fardilha, M. Amyloid precursor protein interaction network in human testis: Sentinel proteins for male reproduction. BMC Bioinform. 2015, 16, 12. [Google Scholar] [CrossRef]
- Borroni, B.; Agosti, C.; Marcello, E.; Di Luca, M.; Padovani, A. Blood cell markers in Alzheimer Disease: Amyloid Precursor Protein form ratio in platelets. Exp. Gerontol. 2010, 45, 53–56. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Platelets, lymphocytes and erythrocytes from Alzheimer’s disease patients: The quest for blood cell-based biomarkers. Folia Neuropathol. 2018, 56, 14–20. [Google Scholar] [CrossRef]
- Canobbio, I.; Guidetti, G.F.; Oliviero, B.; Manganaro, D.; Vara, D.; Torti, M.; Pula, G. Amyloid beta-peptide-dependent activation of human platelets: Essential role for Ca2+ and ADP in aggregation and thrombus formation. Biochem. J. 2014, 462, 513–523. [Google Scholar] [CrossRef]
- Gowert, N.S.; Donner, L.; Chatterjee, M.; Eisele, Y.S.; Towhid, S.T.; Munzer, P.; Walker, B.; Ogorek, I.; Borst, O.; Grandoch, M.; et al. Blood platelets in the progression of Alzheimer’s disease. PLoS ONE 2014, 9, e90523. [Google Scholar] [CrossRef]
- Branitzki-Heinemann, K.; Mollerherm, H.; Vollger, L.; Husein, D.M.; de Buhr, N.; Blodkamp, S.; Reuner, F.; Brogden, G.; Naim, H.; von Kockritz-Blickwede, M. Formation of Neutrophil Extracellular Traps under Low Oxygen Level. Front. Immunol. 2016, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Kazzaz, N.M.; Sule, G.; Knight, J.S. Intercellular Interactions as Regulators of NETosis. Front. Immunol. 2016, 7, 1532. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018, 371, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Kniewallner, K.M.; Foidl, B.M.; Humpel, C. Platelets isolated from an Alzheimer mouse damage healthy cortical vessels and cause inflammation in an organotypic ex vivo brain slice model. Sci. Rep. 2018, 8, 15483. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef]
- Pietronigro, E.C.; Della Bianca, V.; Zenaro, E.; Constantin, G. NETosis in Alzheimer’s Disease. Front. Immunol. 2017, 8, 159. [Google Scholar] [CrossRef]
- Azevedo, E.P.C.; Guimarães-Costa, A.B.; Torezani, G.S.; Braga, C.A.; Palhano, F.L.; Kelly, J.W.; Saraiva, E.M.; Foguel, D. Amyloid Fibrils Trigger the Release of Neutrophil Extracellular Traps (NETs), Causing Fibril Fragmentation by NET-associated Elastase. J. Boil. Chem. 2012, 287, 37206–37218. [Google Scholar] [CrossRef]
- Duyckaerts, C.; Delatour, B.; Potier, M.-C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef]
- Gomez-Isla, T.; Spires, T.; De Calignon, A.; Hyman, B.T. Neuropathology of Alzheimer’s Disease. Dementias 2008, 89, 233–243. [Google Scholar]
- Basavaraju, M.; de Lencastre, A. Alzheimer’s disease: Presence and role of microRNAs. Biomol. Concepts 2016, 7, 241–252. [Google Scholar] [CrossRef]
- Gupta, P.; Bhattacharjee, S.; Sharma, A.R.; Sharma, G.; Lee, S.-S.; Chakraborty, C.; Gupta, S.B.P. miRNAs in Alzheimer Disease-A Therapeutic Perspective. Curr. Alzheimer Res. 2017, 14, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.H.; Liu, Y.N. Downregulated serum miR-223 servers as biomarker in Alzheimer’s disease. Cell Biochem. Funct. 2016, 34, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. MicroRNAs in Plasma and Cerebrospinal Fluid as Potential Markers for Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 39, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Huang, Q.; Hu, Y.; Stromberg, A.J.; Nelson, P.T. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: White matter versus gray matter. Acta. Neuropathol. 2011, 121, 193–205. [Google Scholar] [CrossRef]
- Yang, G.; Song, Y.; Zhou, X.; Deng, Y.; Liu, T.; Weng, G.; Yu, D.; Pan, S. MicroRNA-29c targets β-site amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Mol. Med. Rep. 2015, 12, 3081–3088. [Google Scholar] [CrossRef]
- Feng, L.; Liao, Y.-T.; He, J.-C.; Xie, C.-L.; Chen, S.-Y.; Fan, H.-H.; Su, Z.-P.; Wang, Z. Plasma long non-coding RNA BACE1 as a novel biomarker for diagnosis of Alzheimer disease. BMC Neurol. 2018, 18, 4. [Google Scholar] [CrossRef]
- Dudnik, E.; Zagaynaya, E.; Glazachev, O.S.; Susta, D. Intermittent Hypoxia–Hyperoxia Conditioning Improves Cardiorespiratory Fitness in Older Comorbid Cardiac Outpatients without Hematological Changes: A Randomized Controlled Trial. High Alt. Med. Boil. 2018, 19, 339–343. [Google Scholar] [CrossRef]
- Serebrovska, T.V.; Portnychenko, A.G.; I Drevytska, T.; I Portnichenko, V.; Xi, L.; Egorov, E.; Gavalko, A.V.; Naskalova, S.; Chizhova, V.; Shatylo, V.B. Intermittent hypoxia training in prediabetes patients: Beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp. Boil. Med. 2017, 242, 1542–1552. [Google Scholar] [CrossRef]
- Shatilo, V.B.; Korkushko, O.V.; Ischuk, V.A.; Downey, H.F.; Serebrovskaya, T.V. Effects of Intermittent Hypoxia Training on Exercise Performance, Hemodynamics, and Ventilation in Healthy Senior Men. High Alt. Med. Boil. 2008, 9, 43–52. [Google Scholar] [CrossRef]
- Glazachev, O.; Kopylov, P.; Susta, D.; Dudnik, E.; Zagaynaya, E. Adaptations following an intermittent hypoxia-hyperoxia training in coronary artery disease patients: A controlled study. Clin. Cardiol. 2017, 40, 370–376. [Google Scholar] [CrossRef]
- Susta, D.; Dudnik, E.; Glazachev, O.S. A programme based on repeated hypoxia-hyperoxia exposure and light exercise enhances performance in athletes with overtraining syndrome: A pilot study. Clin. Physiol. Funct. Imaging 2017, 37, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Mallet, R.T.; Manukhina, E.B.; Ruelas, S.S.; Caffrey, J.L.; Downey, H.F. Cardioprotection by intermittent hypoxia conditioning: Evidence, mechanisms, and therapeutic potential. Am. J. Physiol. Circ. Physiol. 2018, 315, H216–H232. [Google Scholar] [CrossRef] [PubMed]
- Manukhina, E.B.; Downey, H.F.; Shi, X.; Mallet, R.T. Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease. Exp. Boil. Med. 2016, 241, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Mateika, J.H.; Komnenov, D. Intermittent hypoxia initiated plasticity in humans: A multipronged therapeutic approach to treat sleep apnea and overlapping co-morbidities. Exp. Neurol. 2017, 287, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, T.V.; Manukhina, E.B.; Smith, M.L.; Downey, H.F.; Mallet, R.T. Intermittent Hypoxia: Cause of or Therapy for Systemic Hypertension? Exp. Boil. Med. 2008, 233, 627–650. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Xi, L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: Practical analysis on methods and equipment. Exp. Biol. Med. 2016, 241, 1708–1723. [Google Scholar] [CrossRef]
- Bayer, U.; Likar, R.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S.; Glazachev, O.; Burtscher, M. Intermittent hypoxic-hyperoxic training on cognitive performance in geriatric patients. Alzheimer’s Dement. : Transl. Res. Clin. Interv. 2017, 3, 114–122. [Google Scholar] [CrossRef]
- Bayer, U.; Likar, R.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S.; Glazachev, O.; Burtscher, M. Effects of intermittent hypoxia-hyperoxia on mobility and perceived health in geriatric patients performing a multimodal training intervention: A randomized controlled trial. BMC Geriatr. 2019, 19, 167. [Google Scholar] [CrossRef]
- Schega, L.; Peter, B.; Törpel, A.; Mutschler, H.; Isermann, B.; Hamacher, D. Effects of Intermittent Hypoxia on Cognitive Performance and Quality of Life in Elderly Adults: A Pilot Study. Gerontol 2013, 59, 316–323. [Google Scholar] [CrossRef]
- Dougherty, B.; Terada, J.; Springborn, S.; Vinit, S.; Macfarlane, P.; Mitchell, G. Daily acute intermittent hypoxia improves breathing function with acute and chronic spinal injury via distinct mechanisms. Respir. Physiol. Neurobiol. 2018, 256, 50–57. [Google Scholar] [CrossRef]
- Ju, Y.-E.S.; Ooms, S.J.; Sutphen, C.; Macauley, S.L.; Zangrilli, M.A.; Jerome, G.; Fagan, A.M.; Mignot, E.; Zempel, J.M.; Claassen, J.A.; et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 2017, 140, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Mercuri, N.B.; Izzi, F.; Romigi, A.; Cordella, A.; Sancesario, G.; Placidi, F. Obstructive Sleep Apnea is Associated With Early but Possibly Modifiable Alzheimer’s Disease Biomarkers Changes. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Struble, R.G.; Ala, T.; Patrylo, P.R.; Brewer, G.J.; Yan, X.-X. Is brain amyloid production a cause or a result of dementia of the Alzheimer’s type? J. Alzheimer’s Dis. 2010, 22, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.F.; Ledo, A.; Barbosa, R.M.; Laranjinha, J. Neurovascular uncoupling in the triple transgenic model of Alzheimer’s disease: Impaired cerebral blood flow response to neuronal-derived nitric oxide signaling. Exp. Neurol. 2017, 291, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef] [PubMed]
- Arkhipenko, Y.V.; Sazontova, T.G.; Zhukova, A.G. Adaptation to periodic hypoxia and hyperoxia improves resistance of membrane structures in heart, liver, and brain. Bull. Exp. Boil. Med. 2005, 140, 278–281. [Google Scholar] [CrossRef]
- Lukyanova, L.D.; Dudchenko, A.M.; Tsybina, T.A.; Germanova, E.L.; Tkachuk, E.N.; Erenburg, I.V. Effect of intermittent normobaric hypoxia on kinetic properties of mitochondrial enzymes. Bull. Exp. Boil. Med. 2007, 144, 795–801. [Google Scholar] [CrossRef]
- Lukyanova, L.D.; Kirova, Y.I. Mitochondria-controlled signaling mechanisms of brain protection in hypoxia. Front. Neurosci. 2015, 9, 320. [Google Scholar] [CrossRef]
- Mashina, S.Y.; Aleksandrin, V.V.; Goryacheva, A.V.; Vlasova, M.A.; Vanin, A.F.; Malyshev, I.Y.; Manukhina, E.B. Adaptation to hypoxia prevents disturbances in cerebral blood flow during neurodegenerative process. Bull. Exp. Boil. Med. 2006, 142, 169–172. [Google Scholar] [CrossRef]
- Haider, T.; Casucci, G.; Linser, T.; Faulhaber, M.; Gatterer, H.; Ott, G.; Linser, A.; Ehrenbourg, I.; Tkatchouk, E.; Burtscher, M.; et al. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J. Hypertens. 2009, 27, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Manukhina, E.B.; Belkina, L.M.; Terekhina, O.L.; Abramochkin, D.V.; A Smirnova, E.; Budanova, O.P.; Mallet, R.T.; Downey, H.F. Normobaric, intermittent hypoxia conditioning is cardio- and vasoprotective in rats. Exp. Boil. Med. 2013, 238, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Manukhina, E.B.; Tseilikman, V.E.; Tseilikman, O.B.; Komelkova, M.V.; Kondashevskaya, M.V.; Goryacheva, A.V.; Lapshin, M.S.; Platkovskii, P.O.; Alliluev, A.V.; Downey, H.F. Intermittent hypoxia improves behavioral and adrenal gland dysfunction induced by posttraumatic stress disorder in rats. J. Appl. Physiol. 2018, 125, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Sazontova, T.G.; Glazachev, O.S.; Bolotova, A.V.; Dudnik, E.N.; Striapko, N.V.; Bedareva, I.V.; A Anchishkina, N.; Arkhipenko, I.V. Adaptation to hypoxia and hyperoxia improves physical endurance: The role of reactive oxygen species and redox-signaling. Ross. Fiziol. zhurnal Im. IM Sechenova 2012, 98, 793–807. [Google Scholar]
- Zhu, X.-H.; Yan, H.-C.; Zhang, J.; Qu, H.-D.; Qiu, X.-S.; Chen, L.; Li, S.-J.; Cao, X.; Bean, J.C.; Qin, X.-H.; et al. Intermittent Hypoxia Promotes Hippocampal Neurogenesis and Produces Antidepressant-Like Effects in Adult Rats. J. Neurosci. 2010, 30, 12653–12663. [Google Scholar] [CrossRef]
- Mähler, A.; Balogh, A.; Csizmadia, I.; Klug, L.; Kleinewietfeld, M.; Steiniger, J.; Šušnjar, U.; Müller, D.N.; Boschmann, M.; Paul, F. Metabolic, Mental and Immunological Effects of Normoxic and Hypoxic Training in Multiple Sclerosis Patients: A Pilot Study. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Burtscher, M. High-altitude cerebral effects: Risks and mechanisms. Lancet Neurol. 2009, 8, 604–605. [Google Scholar] [CrossRef]
- Burtscher, M.; Pachinger, O.; Ehrenbourg, I.; Mitterbauer, G.; Faulhaber, M.; Pühringer, R.; Tkatchouk, E. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int. J. Cardiol. 2004, 96, 247–254. [Google Scholar] [CrossRef]
- Findlay, M.D.; Dawson, J.; Dickie, D.A.; Forbes, K.P.; McGlynn, D.; Quinn, T.; Mark, P.B. Investigating the Relationship between Cerebral Blood Flow and Cognitive Function in Hemodialysis Patients. J. Am. Soc. Nephrol. 2019, 30, 147–158. [Google Scholar] [CrossRef]
- Gao, H.; Han, Z.; Huang, S.; Bai, R.; Ge, X.; Chen, F.; Lei, P. Intermittent hypoxia caused cognitive dysfunction relate to miRNAs dysregulation in hippocampus. Behav. Brain Res. 2017, 335, 80–87. [Google Scholar] [CrossRef]
- Montagna, E.; Dorostkar, M.M.; Herms, J. The Role of APP in Structural Spine Plasticity. Front. Mol. Neurosci. 2017, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Rahangdale, S.; Yeh, S.Y.; Novack, V.; Stevenson, K.; Barnard, M.R.; Furman, M.I.; Frelinger, A.L.; Michelson, A.D.; Malhotra, A. The Influence of Intermittent Hypoxemia on Platelet Activation in Obese Patients with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2011, 7, 172–178. [Google Scholar] [PubMed]
- Rocke, A.S.; Paterson, G.G.; Barber, M.T.; Jackson, A.I.R.; Main, S.; Stannett, C.; Schnopp, M.F.; Baillie, J.K.; Horne, E.H.; Moores, C.; et al. Thromboelastometry and Platelet Function during Acclimatization to High Altitude. Thromb. Haemost. 2018, 118, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Vij, A.G. Effect of prolonged stay at high altitude on platelet aggregation and fibrinogen levels. Platelets 2009, 20, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.W.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Dawes, P.; Pye, A.; Reeves, D.; Yeung, W.K.; Sheikh, S.; Thodi, C.; Charalambous, A.P.; Gallant, K.; Nasreddine, Z.; Leroi, I. Protocol for the development of versions of the Montreal Cognitive Assessment (MoCA) for people with hearing or vision impairment. BMJ Open 2019, 9, e026246. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H.; Bédirian, V. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Milani, S.A.; Marsiske, M.; Cottler, L.B.; Chen, X.; Striley, C.W. Optimal cutoffs for the Montreal Cognitive Assessment vary by race and ethnicity. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 773–781. [Google Scholar] [CrossRef]
- Morris, J.C. Clinical Dementia Rating: A Reliable and Valid Diagnostic and Staging Measure for Dementia of the Alzheimer Type. Int. Psychogeriatr 1997, 9, 173–176. [Google Scholar] [CrossRef]
- Hünerli, D.; Emek-Savaş, D.D.; Çavuşoğlu, B.; Çolakoğlu, B.D.; Ada, E.; Yener, G.G. Mild cognitive impairment in Parkinson’s disease is associated with decreased P300 amplitude and reduced putamen volume. Clin. Neurophysiol. 2019, 130, 1208–1217. [Google Scholar] [CrossRef]
- Lai, C.-L.; Lin, R.-T.; Liou, L.-M.; Liu, C.-K. The role of event-related potentials in cognitive decline in Alzheimer’s disease. Clin. Neurophysiol. 2010, 121, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Vaitkevicius, A.; Kaubrys, G.; Audronytė, E. Distinctive Effect of Donepezil Treatment on P300 and N200 Subcomponents of Auditory Event-Related Evoked Potentials in Alzheimer Disease Patients. Med. Sci. Monit. 2015, 21, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Pashevin, D.O.; Nagibin, V.S.; Tumanovska, L.V.; Moibenko, A.A.; Dosenko, V.E. Proteasome Inhibition Diminishes the Formation of Neutrophil Extracellular Traps and Prevents the Death of Cardiomyocytes in Coculture with Activated Neutrophils during Anoxia-Reoxygenation. Pathobiol 2015, 82, 290–298. [Google Scholar] [CrossRef] [PubMed]
| Group | Healthy Control (n = 7) | MCI + Sham (n = 6) | MCI + IHHT (n = 8) | Group Main Effect | Time Effect 3 Time-Points | Group × Time Interaction |
|---|---|---|---|---|---|---|
| MoCA, score | ||||||
| Pre- | 28.9 ± 1.06 | 21.2 ± 2.12 # | 19.6 ±1.59 # | F = 50.031 | F = 13.207 | F = 5.029 |
| 1-day Post | 29.3 ± 0.75 | 20.3 ± 2.1 # | 22.1 ± 1.68 #,* | P = 0.000 | P = 0.000 | P = 0.03 |
| 1-month Post | 29.4 ± 1.13 | 20.0 ± 1.75 # | 21.3 ± 1.57 # | |||
| P300, ms | ||||||
| Pre- | 319 ± 24.5 | 398 ± 27.7 # | 407 ± 29.7 # | F = 15.233 | F = 5.535 | F = 3.904 |
| 1-day Post | 317 ± 19.5 | 396 ± 29.73 # | 391 ± 29.7 # | P = 0.000 | P = 0.008 | P = 0.01 |
| 1-month Post | 318 ± 22.8 | 398 ± 27.97 # | 392 ± 28.4 # | |||
| N200, ms | ||||||
| Pre- | 239 ± 23.3 | 280 ± 21.7 # | 290 ± 21.4 # | F = 7.285 | F = 11.087 | F = 7.254 |
| 1-day Post | 242 ± 24.3 | 284 ± 21.9 # | 273 ± 20.2 # | P = 0.005 | P = 0.000 | P = 0.000 |
| 1-month Post | 238 ± 26.6 | 285 ± 22.4 # | 275 ± 20.1 # |
| Group | Healthy Control (n = 7) | MCI + Sham (n = 6) | MCI + IHHT (n = 8) | Group Main Effect | Time Effect 3 time- points | Group × Time Interaction |
|---|---|---|---|---|---|---|
| APP130, relative unit | ||||||
| Pre- | 1.04 ± 0.05 | 0.40 ± 0.07 # | 0.41 ± 0.11 # | F = 90.897 | F = 5.843 | F = 7.814 |
| 1-day Post | 1.04 ± 0.04 | 0.37 ± 0.03 # | 0.65 ± 0.12 #,*,^ | P = 0.000 | P = 0.007 | P = 0.000 |
| 1-month Post | 1.05 ± 0.05 | 0.36 ± 0.04 # | 0.58 ± 0.13 #,*,^ | |||
| APP110, relative unit | ||||||
| Pre- | 1.02 ± 0.04 | 0.47 ± 0.17 # | 0.56 ± 0.14 # | F = 83.45 | F = 4.063 | F = 2.137 |
| 1-day Post | 1.02 ± 0.03 | 0.49 ± 0.09 # | 0.73 ± 0.14 #,*,^ | P = 0.000 | P = 0.026 | P = 0.098 |
| 1-month Post | 1.05 ± 0.06 | 0.48 ± 0.08 # | 0.76 ± 0.11 #,*,^ | |||
| APP ratio | ||||||
| Pre- | 1,01 ± 0.10 | 0.82 ± 0.05 # | 0.73 ± 0.09 # | F = 14.622 | F = 0.857 | F = 1.426 |
| 1-day Post | 1.01 ± 0.11 | 0.77 ± 0.13 # | 0.89 ± 0.09 #,*,^ | P = 0.000 | P = 0.435 | P = 0.249 |
| 1-month Post | 1.00 ± 0.11 | 0.76 ± 0.06 # | 0.76 ± 0.1 # | |||
| Aβ, relative unit | ||||||
| Pre- | 1.08 ± 0.1 | 2.69 ± 0.35 # | 2.63 ± 0.34 # | F = 924.103 | F = 1.238 | F = 4.723 |
| 1-day Post | 1.17 ± 0.21 | 2.76 ± 0.35 # | 2.24 ± 0.43 #,*,^ | P = 0.000 | P = 0.302 | P = 0.004 |
| 1-month Post | 1.29 ± 0.5 | 2.82 ± 0.22 # | 2.08 ± 0.44 #,*,^ |
| Group | Healthy Control (n = 7) | MCI +Sham (n = 6) | MCI+ IHHT (n = 8) | Group Main Effect | Time Effect 3 Time-Points | Group × Time Interaction |
|---|---|---|---|---|---|---|
| NETns, % | ||||||
| Pre- | 2.58 ± 2.22 | 9.62 ± 4.83 # | 9.47 ± 2.06 # | F = 119.799 | F = 3.689 | F = 4.170 |
| 1-day Post | 2.05 ± 1.79 | 9.22 ± 3.94 # | 4.48 ± 1.09 #,*,^ | P = 0.001 | P = 0.04 | P = 0.011 |
| 1-month Post | 3.02 ± 1.51 | 8.25 ± 2.04 # | 4.21 ± 1.28 *,^ | |||
| NETst, % | ||||||
| Pre- | 10.3 ± 4.68 | 12.4 ± 6.07 | 12.7 ± 6.18 | F = 0.573 | F = 5.316 | F = 0.803 |
| 1-day Post | 7.18 ± 3.04 | 10.20 ± 4.86 | 8.76 ± 3.34 | P = 0.577 | P = 0.012 | P = 0.534 |
| 1-month Post | 9.18 ± 2.80 | 11.16 ± 3.63 | 6.11 ± 3.50 *,^ |
| Group | Healthy Control (n = 7) | MCI + Sham (n = 6) | MCI + IHHT (n = 8) | Group Main Effect | Time Effect 3 Time-Points | Group × Time Interaction |
|---|---|---|---|---|---|---|
| lncRNA BACE1-AS, relative unit | ||||||
| Pre- | 13.6 ± 10.8 | 92.5 ± 45.5 # | 85.3 ± 55.6 # | F = 11.708 | F = 1.288 | F = 0.729 |
| 1-day Post | 12.6 ± 5.9 | 75.4 ± 57.3 # | 36.8 ± 34.6 * | P = 0.001 | P = 0.288 | P = 0.075 |
| 1-month Post | 10.6 ± 7.7 | 99.3 ± 70.4 # | 45.6 ± 32.8 # |
| Groups | Gender (Female/Male) | Age (Year) | BMI (kg/m2) | SBP (mmHg) | DBP (mmHg) |
|---|---|---|---|---|---|
| Healthy Control (n = 7) | 6/1 | 63.0 ± 10.0 | 26.5 ± 3.6 | 125.8 ± 15.8 | 81.0 ± 11.0 |
| MCI + Sham (n = 6) | 6/0 | 72.6 ± 6.9 | 26.3 ± 5.5 | 135.8 ± 18.6 | 81.4 ± 14.0 |
| MCI+ IHHT (n = 8) | 6/2 | 68.2 ± 7.2 | 27.7 ± 2.0 | 137.3 ± 13.4 | 83.7 ± 9.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serebrovska, Z.O.; Serebrovska, T.V.; Kholin, V.A.; Tumanovska, L.V.; Shysh, A.M.; Pashevin, D.A.; Goncharov, S.V.; Stroy, D.; Grib, O.N.; Shatylo, V.B.; et al. RETRACTED: Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study. Int. J. Mol. Sci. 2019, 20, 5405. https://doi.org/10.3390/ijms20215405
Serebrovska ZO, Serebrovska TV, Kholin VA, Tumanovska LV, Shysh AM, Pashevin DA, Goncharov SV, Stroy D, Grib ON, Shatylo VB, et al. RETRACTED: Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study. International Journal of Molecular Sciences. 2019; 20(21):5405. https://doi.org/10.3390/ijms20215405
Chicago/Turabian StyleSerebrovska, Zoya O., Tetiana V. Serebrovska, Viktor A. Kholin, Lesya V. Tumanovska, Angela M. Shysh, Denis A. Pashevin, Sergii V. Goncharov, Dmytro Stroy, Oksana N. Grib, Valeriy B. Shatylo, and et al. 2019. "RETRACTED: Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study" International Journal of Molecular Sciences 20, no. 21: 5405. https://doi.org/10.3390/ijms20215405
APA StyleSerebrovska, Z. O., Serebrovska, T. V., Kholin, V. A., Tumanovska, L. V., Shysh, A. M., Pashevin, D. A., Goncharov, S. V., Stroy, D., Grib, O. N., Shatylo, V. B., Bachinskaya, N. Y., Egorov, E., Xi, L., & Dosenko, V. E. (2019). RETRACTED: Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study. International Journal of Molecular Sciences, 20(21), 5405. https://doi.org/10.3390/ijms20215405






