Composition of the Reconstituted Cell Wall in Protoplast-Derived Cells of Daucus Is Affected by Phytosulfokine (PSK)
Abstract
1. Introduction
2. Results
2.1. Protoplast Development
2.2. Reconstitution of the Cell Wall in the Early Stages of the Protoplast-Derived Cell Cultures
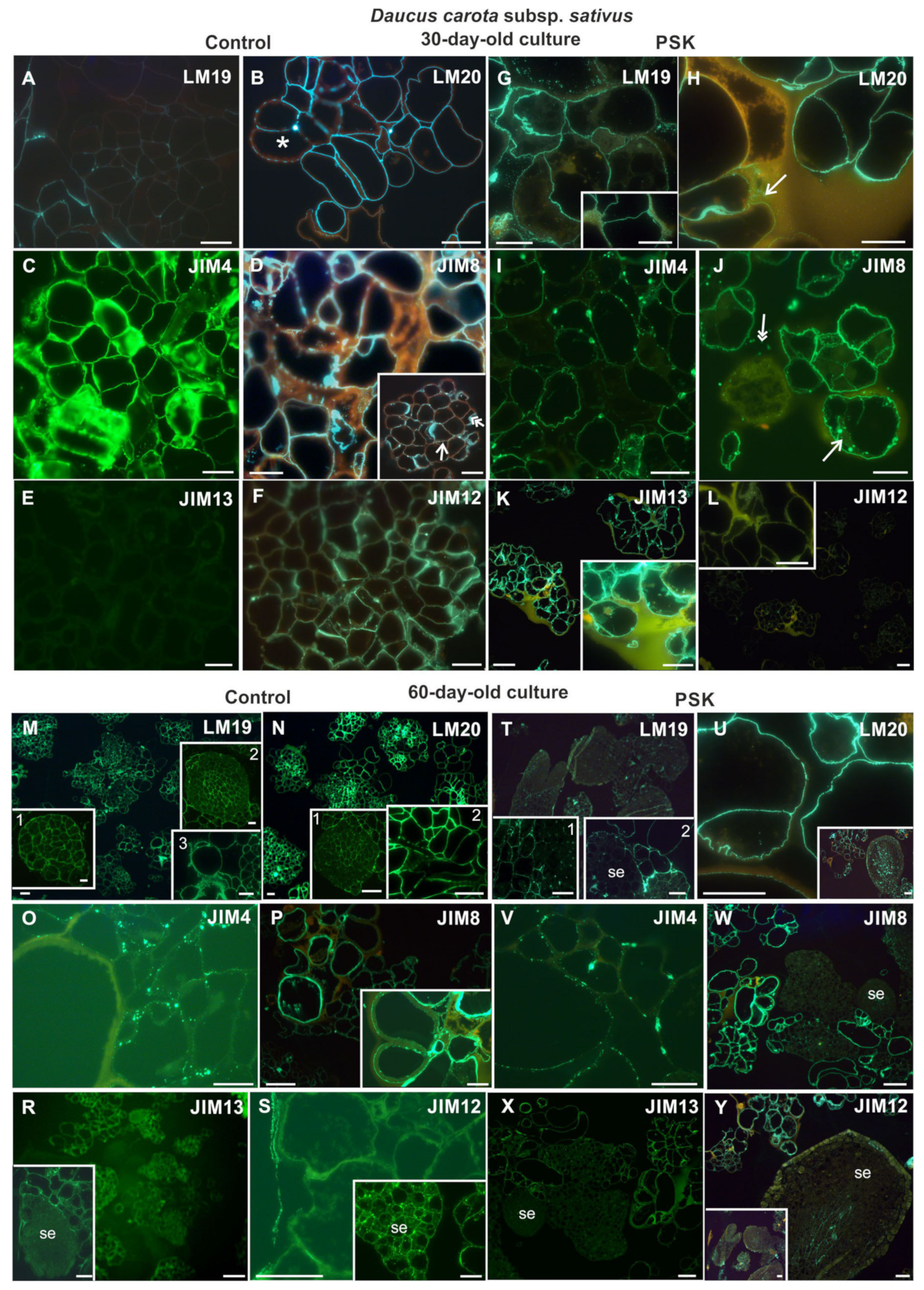
2.2.1. Daucus Carota Subsp. sativus
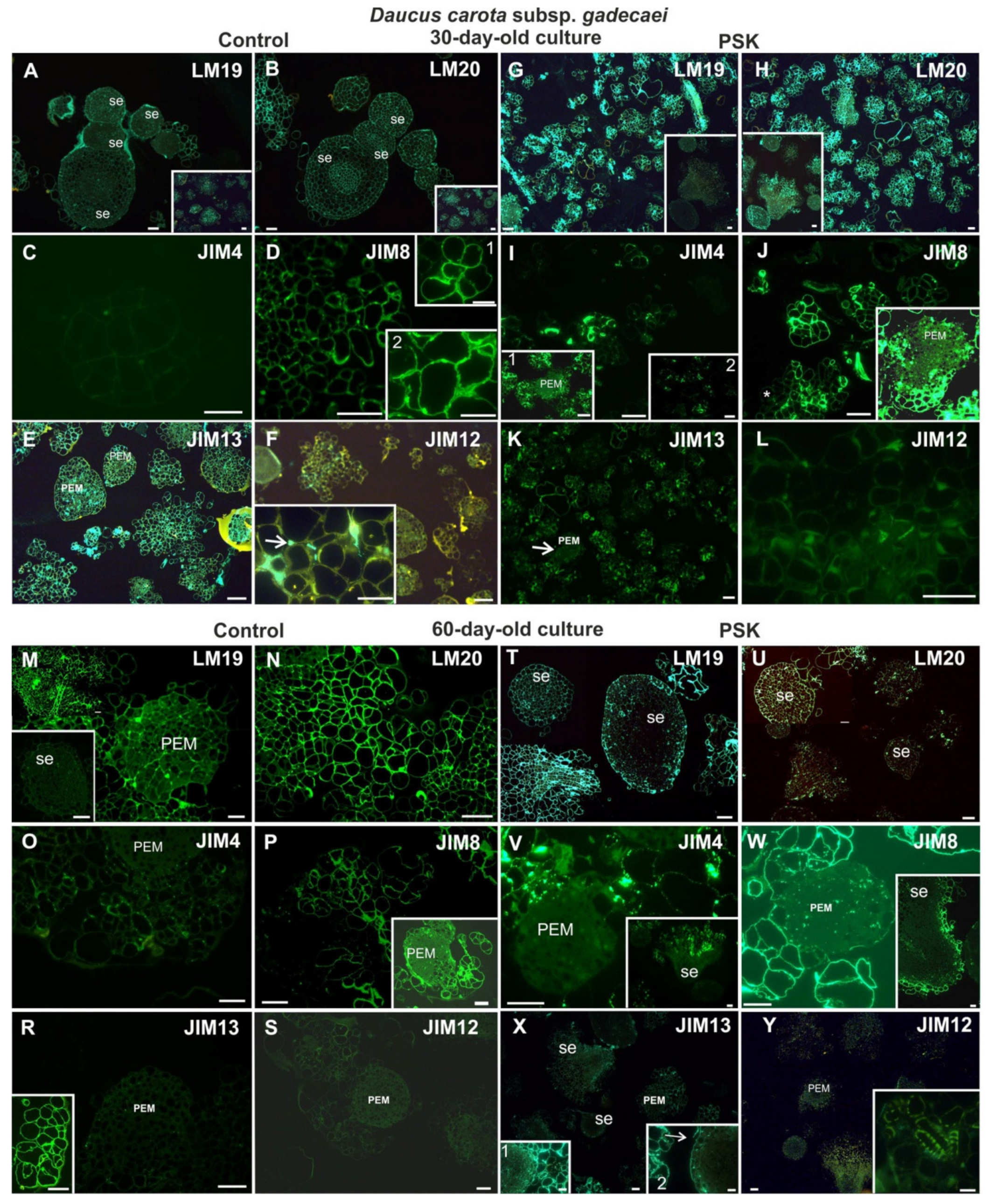
2.2.2. Daucus Carota Subsp. gadecaei
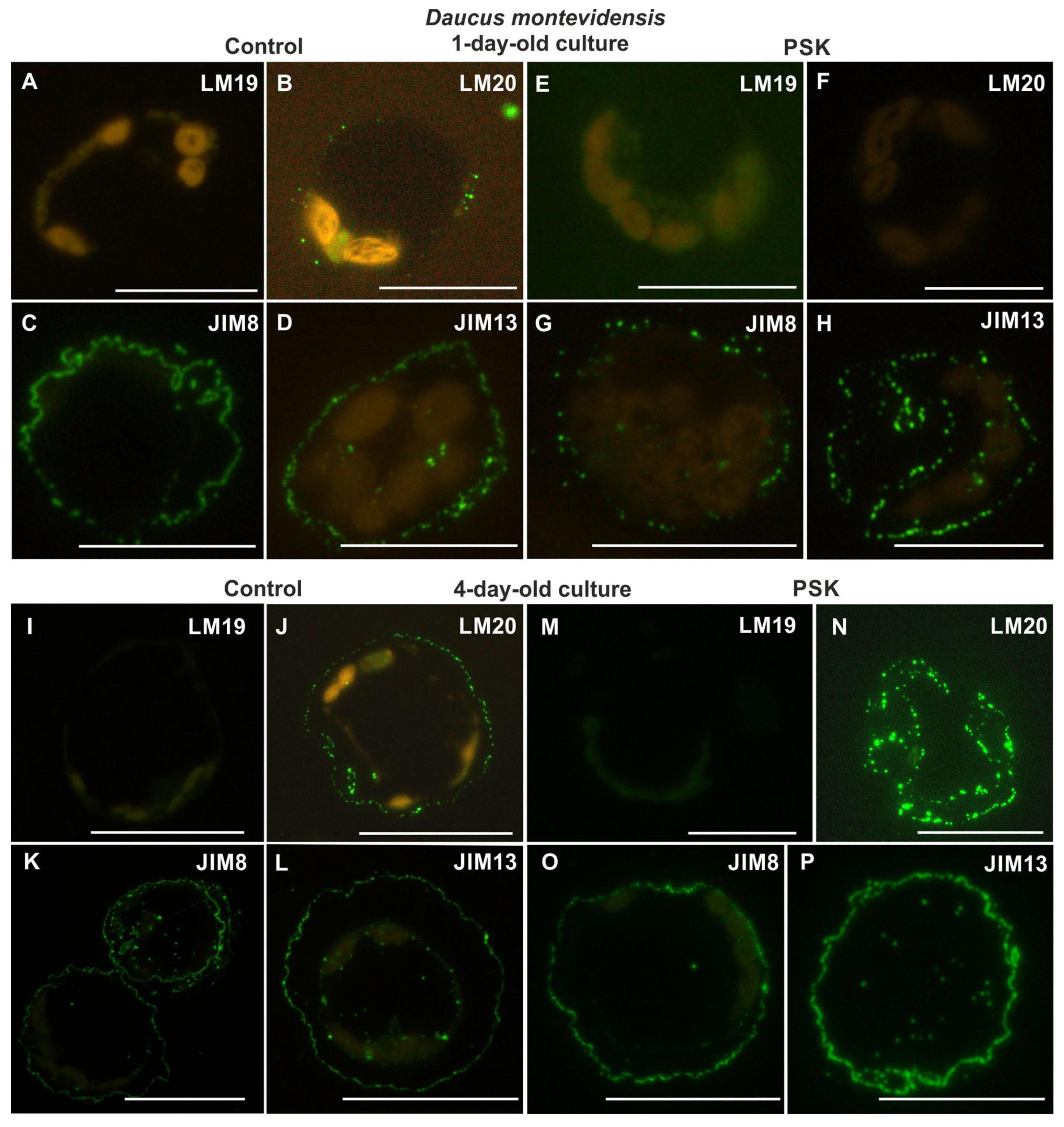
2.2.3. Daucus Montevidensis
2.3. Cell Wall Composition in the Middle Stages of the Culture
2.3.1. Daucus Carota Subsp. sativus
2.3.2. Daucus Carota Subsp. gadecaei
2.3.3. Daucus montevidensis
2.4. Distribution of Pectic, AGP and Extensin Epitopes in Mature Cultures
2.4.1. Daucus Carota Subsp. sativus
2.4.2. Daucus Carota Subsp. gadecaei
2.5. Summarizing
3. Discussion
3.1. Distribution of Pectins with Different Levels of Esterification Change in a Diverse Manner Depends on the Culture Condition and the Species
3.2. Expression and Localization of AGPs in Protoplast-Derived Cells and Cell Aggregates are Time and Tissue Specific and Enhanced by PSK
3.3. The Increase in the Extensin Level is Characteristic for Middle-Aged Cultures
3.4. PSK and Wall Modification on the Molecular Level
4. Materials and Methods
4.1. Plant Material
4.2. Protoplast Isolation
4.3. Protoplast Embedding in Alginate Matrix and Culture
4.4. Evaluation of Culture Development and Data Analysis
4.5. Sampling, Fixation and Embeding of Plant Materials
4.6. Immunological Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGPs | Arabinogalactan proteins |
| CPP | Carrot petiole protoplast medium |
| HG | Homogalaturonan |
| HRGPs | Hydroxyproline-rich glycoproteins |
| mAbs | Monoclonal antibodies |
| MS | Murashige and Skoog medium (1962) |
| PEM | Proembryonic mass |
| PME | Pectic methyl-esterase activity |
| PSK | Phytosulfokine-α |
| TAL | Thin alginate layer |
| R | Regeneration medium |
References
- Plunkett, G.M.; Pimenov, M.G.; Reduron, J.-P.; Kljuykov, E.V.; van Wyk, B.-E.; Ostroumova, T.A.; Henwood, M.J.; Tilney, P.M.; Spalik, K.; Watson, M.F.; et al. Apiaceae. In Flowering Plants. Eudicots: Apiales, Gentianales (Except Rubiaceae); Kadereit, J.W., Bittrich, V., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 9–206. ISBN 978-3-319-93605-5. [Google Scholar]
- Spooner, D.M. Daucus: Taxonomy, Phylogeny, Distribution. In The Carrot Genome. Compendium of Plant Genomes; Simon, P., Iorizzo, M., Grzebelus, D., Baranski, R., Eds.; Springer: Berlin, Germany, 2019; pp. 9–26. [Google Scholar]
- Banasiak, Ł.; Wojewódzka, A.; Baczyński, J.; Reduron, J.-P.; Piwczyński, M.; Kurzyna-Młynik, R.; Gutaker, R.; Czarnocka-Cieciura, A.; Kosmala-Grzechnik, S.; Spalik, K. Phylogeny of Apiaceae subtribe Daucinae and the taxonomic delineation of its genera. Taxon 2016, 65, 563–585. [Google Scholar] [CrossRef]
- Heywood, V.H. The socio-economic importance of the Apiales. J. Fac. Pharm. Istambul 2014, 44, 113–130. [Google Scholar]
- Gaurtheret, R.J. Sur la possibilite de realiser La culture indefinite des tissues de tubercules de carotte. CR Hebd. Seances Acad. Sci. 1939, 208, 118–121. [Google Scholar]
- Steward, F.C. Growth and organized development of cultured cells. III. Interpretations of the growth from free cell to carrot plant. Am. J. Bot. 1958, 45, 709–713. [Google Scholar] [CrossRef]
- Eeckhaut, T.; Lakshmanan, P.S.; Deryckere, D.; Van Bockstaele, E.; Van Huylenbroeck, J. Progress in plant protoplast research. Planta 2013, 238, 991–1003. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Wang, Y. Protoplast fusion for crop improvement and breeding in China. Plant Cell Tissue Organ. Cult. 2013, 112, 131–142. [Google Scholar] [CrossRef]
- Kameya, T.; Uchimiya, H. Embryoids derived from isolated protoplasts of carrot. Planta 1972, 103, 356–360. [Google Scholar] [CrossRef]
- Grambow, H.J.; Kao, K.N.; Miller, R.A.; Gamborg, O.L. Cell division and plant development from protoplasts of carrot cell suspension cultures. Planta 1972, 103, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Arbizu, C.; Reitsma, K.R.; Simon, P.W.; Spooner, D.M. Morphometrics of Daucus (Apiaceae): A counterpart to a phylogenomic study. Am. J. Bot. 2014, 101, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Dudits, D.; Hadlaczky, G.; Lévi, E.; Fejér, O.; Haydu, Z.; Lazar, G. Somatic hybridisation of Daucus carota and D. capillifolius by protoplast fusion. Theor. Appl. Genet. 1977, 51, 127–132. [Google Scholar] [CrossRef]
- Ichikawa, H.; Tanno-Suenaga, L.; Imamura, J. Selection of Daucus cybrids based on metabolic complementation between X-irradiated D. capillifolius and iodoacetamide-treated D. carota by somatic cell fusion. Theor. Appl. Genet. 1987, 74, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Dirks, R.; Sidorov, V.; Tulmans, C. A new protoplast culture system in Daucus carota L. and its applications for mutant selection and transformation. Theor. Appl. Genet. 1996, 93, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Grzebelus, E.; Szklarczyk, M.; Baranski, R. An improved protocol for plant regeneration from leaf-and hypocotyl-derived protoplasts of carrot. Plant Cell Tissue Organ. Cult. 2012, 109, 101–109. [Google Scholar] [CrossRef]
- Maćkowska, K.; Jarosz, A.; Grzebelus, E. Plant regeneration from leaf-derived protoplasts within the Daucus genus: Effect of different conditions in alginate embedding and phytosulfokine application. Plant Cell Tissue Organ. Cult. 2014, 117, 241–252. [Google Scholar] [CrossRef]
- Davey, M.R.; Anthony, P.; Power, J.B.; Lowe, K.C. Plant protoplasts: Status and biotechnological perspectives. Biotechnol. Adv. 2005, 23, 131–171. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S. Protoplast Culture and Somatic Hybridization, a Promising Frontier of Plant Biotechnology. J. Arts Sci. Teach. 2016, 2, 46–54. [Google Scholar]
- Majewska-Sawka, A.; Münster, A. Cell-wall antigens in mesophyll cells and mesophyll-derived protoplasts of sugar beet: Possible implication in protoplast recalcitrance? Plant Cell Rep. 2003, 21, 946–954. [Google Scholar] [CrossRef]
- Sala, K.; Potocka, I.; Kurczynska, E. Spatio-temporal distribution and methyl-esterification of pectic epitopes provide evidence of developmental regulation of pectins during somatic embryogenesis in Arabidopsis thaliana. Biol. Plant. 2013, 57, 410–416. [Google Scholar] [CrossRef]
- Potocka, I.; Godel, K.; Dobrowolska, I.; Kurczyńska, E.U. Spatio-temporal localization of selected pectic and arabinogalactan protein epitopes and the ultrastructural characteristics of explant cells that accompany the changes in the cell fate during somatic embryogenesis in Arabidopsis thaliana. Plant Physiol. Biochem. 2018, 127, 573–589. [Google Scholar] [CrossRef]
- Malinowski, R.; Filipecki, M. The role of cell wall in plant embryogenesis. Cell. Mol. Biol. Lett. 2002, 7, 1137–1152. [Google Scholar]
- David, H.; Savy, C.; Miannay, N.; Dargent, R.; David, A. Supporting matrix influences protoplast-derived colony formation: Structural analysis. Protoplasma 1994, 179, 111–120. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Yokoyama, R.; Nishitani, K. A proteomic approach to apoplastic proteins involved in cell wall regeneration in protoplasts of Arabidopsis suspension-cultured cells. Plant Cell Physiol. 2005, 46, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tu, L.; Zhu, L.; Fu, L.; Min, L.; Zhang, X. Expression profile analysis of genes involved in cell wall regeneration during protoplast culture in cotton by suppression subtractive hybridization and macroarray. J. Exp. Bot. 2008, 59, 3661–3674. [Google Scholar] [CrossRef] [PubMed]
- Betekhtin, A.; Rojek, M.; Jaskowiak, J.; Milewska-Hendel, A.; Kwasniewska, J.; Kostyukova, Y.; Kurczynska, E.; Rumyantseva, N.; Hasterok, R. Nuclear genome stability in long-term cultivated callus lines of Fagopyrum tataricum (L.) Gaertn. PLoS ONE 2017, 12, e0173537. [Google Scholar] [CrossRef]
- Betekhtin, A.; Rojek, M.; Nowak, K.; Pinski, A.; Milewska-Hendel, A.; Kurczynska, E.; Doonan, J.; Hasterok, R. Cell wall epitopes and endoploidy as reporters of embryogenic potential in Brachypodium distachyon callus culture. Int. J. Mol. Sci. 2018, 19, 3811. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Niklas, A.; Rodriguez-Garcia, M.I.; Majewska-Sawka, A. Involvement of JIM13-and JIM8-responsive carbohydrate epitopes in early stages of cell wall formation. J. Plant Res. 1999, 112, 107–116. [Google Scholar] [CrossRef]
- Wiśniewska, E.; Majewska-Sawka, A. The differences in cell wall composition in leaves and regenerating protoplasts of Beta vulgaris and Nicotiana tabacum. Biol. Plant. 2008, 52, 634–641. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Takagi, L.; Sakagami, Y. Phytosulfokine-α, a sulfated pentapeptide, stimulates the proliferation of rice cells by means of specific high-and low-affinity binding sites. Proc. Natl. Acad. Sci. USA 1997, 94, 13357–13362. [Google Scholar] [CrossRef]
- Grzebelus, E.; Szklarczyk, M.; Greń, J.; Śniegowska, K.; Jopek, M.; Kacińska, I.; Mrożek, K. Phytosulfokine stimulates cell divisions in sugar beet (Beta vulgaris L.) mesophyll protoplast cultures. Plant Growth Regul. 2012, 67, 93–100. [Google Scholar] [CrossRef]
- Davey, M.R.; An, P.; Power, J.B.; Lowe, K.C. Plant protoplast technology: Current status. Acta Physiol. Plant. 2005, 27, 117–129. [Google Scholar] [CrossRef]
- Davey, M.R.; Anthony, P.; Power, J.B.; Lowe, K.C. 2004 SIVB congress symposium proceedings “Thinking outside the cell”: Plant protoplast technology: Status and applications. Vitr. Cell. Dev. Biol. 2005, 41, 202–212. [Google Scholar] [CrossRef]
- Grzebelus, E.; Maćkowska, K.; Macko-Podgorni, A.; Kiełkowska, A.; Szklarczyk, M.; Baranski, R.; Grzebelus, D. Application of protoplast technology to Apiaceaae species. Acta Hortic. 2019, in press. [Google Scholar]
- Dudits, D.; Maroy, E.; Praznovszky, T.; Olah, Z.; Gyorgyey, J.; Cella, R. Transfer of resistance traits from carrot into tobacco by asymmetric somatic hybridization: Regeneration of fertile plants. Proc. Natl. Acad. Sci. USA 1987, 84, 8434–8438. [Google Scholar] [CrossRef] [PubMed]
- Kisaka, H.; Kisaka, M.; Kanno, A.; Kameya, T. Production and analysis of plants that are somatic hybrids of barley (Hordeum vulgare L.) and carrot (Daucus carota L.). Theor. Appl. Genet. 1997, 94, 221–226. [Google Scholar] [CrossRef]
- Han, L.; Zhou, C.; Shi, J.; Zhi, D.; Xia, G. Ginsenoside Rb 1 in asymmetric somatic hybrid calli of Daucus carota with Panax quinquefolius. Plant Cell Rep. 2009, 28, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Liepman, A.H.; Wightman, R.; Geshi, N.; Turner, S.R.; Scheller, H.V. Arabidopsis—A powerful model system for plant cell wall research. Plant J. 2010, 61, 1107–1121. [Google Scholar] [CrossRef]
- Namasivayam, P.; Skepper, J.N.; Hanke, D. Distribution of arabinogalactan protein (AGP) epitopes on the anther-derived embryoid cultures of Brassica napus. Pertanika J. Trop. Agric. Sci. 2010, 33, 303–313. [Google Scholar]
- Wiszniewska, A.; Piwowarczyk, B. Studies on cell wall regeneration in protoplast culture of legumes—The effect of organic medium additives on cell wall components. Czech J. Genet. Plant Breed. 2014, 50, 84–91. [Google Scholar] [CrossRef]
- Majewska-Sawka, A.; Münster, A.; Rodríguez-García, M.I. Guard cell wall: Immunocytochemical detection of polysaccharide components. J. Exp. Bot. 2002, 53, 1067–1079. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Verhoef, R.; Schols, H.A.; McCleary, B.V.; McKee, L.; Gilbert, H.J.; Paul Knox, J. Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J. 2009, 59, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan methyl-esterification and plant development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Mock, H.; Emmerling, M.; Seitz, H.U. Cell wall synthesis in carrot cells: Comparison of suspension-cultured cells and regenerating protoplasts. Physiol. Plant. 1990, 79, 347–353. [Google Scholar] [CrossRef]
- Zhao, J.; Mollet, J.-C.; Lord, E.M. Lily (Lilium longiflorum L.) pollen protoplast adhesion is increased in the presence of the peptide SCA. Sex. Plant Reprod. 2004, 16, 227–233. [Google Scholar] [CrossRef]
- Moustacas, A.; Nari, J.; Diamantidis, G.; Noat, G.; Crasnier, M.; Borel, M.; Ricard, J. Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells: 2. The role of pectin methyl esterase in the modulation of electrostatic effects in soybean cell walls. Eur. J. Biochem. 1986, 155, 191–197. [Google Scholar] [CrossRef] [PubMed]
- David, H.; Bade, P.; David, A.; Savy, C.; Demazy, C.; Van Cutsem, P. Pectins in walls of protoplast-derived cells imbedded in agarose and alginate beads. Protoplasma 1995, 186, 122–130. [Google Scholar] [CrossRef]
- Shea, E.M.; Gibeaut, D.M.; Carpita, N.C. Structural analysis of the cell walls regenerated by carrot protoplasts. Planta 1989, 179, 293–308. [Google Scholar] [CrossRef]
- Hanai, H.; Matsuno, T.; Yamamoto, M.; Matsubayashi, Y.; Kobayashi, T.; Kamada, H.; Sakagami, Y. A secreted peptide growth factor, phytosulfokine, acting as a stimulatory factor of carrot somatic embryo formation. Plant Cell Physiol. 2000, 41, 27–32. [Google Scholar] [CrossRef]
- Rodakowska, E.; Kasprowicz, A.; Łapa, A.; Łuczak, M.; Derba, M.; Wojtaszek, P. Ściany komórkowe jako źródło sygnałów regulujących procesy rozwojowe komórek roślin. Biotechnologia 2006, 4, 18–35. [Google Scholar]
- Stührwohldt, N.; Dahlke, R.I.; Kutschmar, A.; Peng, X.; Sun, M.; Sauter, M. Phytosulfokine peptide signaling controls pollen tube growth and funicular pollen tube guidance in Arabidopsis thaliana. Physiol. Plant. 2015, 153, 643–653. [Google Scholar] [CrossRef]
- Hartmann, J.; Stührwohldt, N.; Dahlke, R.I.; Sauter, M. Phytosulfokine control of growth occurs in the epidermis, is likely to be non-cell autonomous and is dependent on brassinosteroids. Plant J. 2013, 73, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell Online 1991, 3, 1317–1326. [Google Scholar] [CrossRef]
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early position, but not cell type, in the root apical carota L. Development 1989, 106, 47–56. [Google Scholar]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef]
- Schindler, T.; Bergfeld, R.; Schopfer, P. Arabinogalactan proteins in maize coleoptiles: Developmental relationship to cell death during xylem differentiation but not to extension growth. Plant J. 1995, 7, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Sala, K.; Malarz, K.; Barlow, P.W.; Kurczyńska, E.U. Distribution of some pectic and arabinogalactan protein epitopes during Solanum lycopersicum (L.) adventitious root development. BMC Plant Biol. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Stacey, N.J.; Roberts, K.; Knox, J.P. Patterns of expression of the JIM4 arabinogalactan-protein epitope in cell cultures and during somatic embryogenesis in Daucus carota L. Planta 1990, 180, 285–292. [Google Scholar] [CrossRef]
- Šamaj, J.; Baluška, F.; Bobák, M.; Volkmann, D. Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep. 1999, 18, 369–374. [Google Scholar] [CrossRef]
- Serpe, M.D.; Nothnagel, E.A. Effects of Yariv phenylglycosides on Rosa cell suspensions: Evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta 1994, 193, 542–550. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P. A role for arabinogalactan-proteins in plant cell expansion: Evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J. 1996, 9, 919–925. [Google Scholar] [CrossRef]
- Majewska-Sawka, A.; Nothnagel, E.A. The multiple roles of arabinogalactan proteins in plant development. Plant Physiol. 2000, 122, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, E.; Majewska-Sawka, A. Arabinogalactan-proteins stimulate the organogenesis of guard cell protoplasts-derived callus in sugar beet. Plant Cell Rep. 2007, 26, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Kreuger, M.; van Holst, G.-J. Arabinogalactan proteins are essential in somatic embryogenesis of Daucus carota L. Planta 1993, 189, 243–248. [Google Scholar] [CrossRef]
- Jauh, G.Y.; Lord, E.M. Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 1996, 199, 251–261. [Google Scholar] [CrossRef]
- Pereira, A.M.; Pereira, L.G.; Coimbra, S. Arabinogalactan proteins: Rising attention from plant biologists. Plant Reprod. 2015, 28, 1–15. [Google Scholar] [CrossRef]
- Pennell, R.I.; Roberts, K. Sexual development in the pea is presaged by altered expression of arabinogalactan protein. Nature 1990, 344, 547. [Google Scholar] [CrossRef]
- Betekhtin, A.; Rojek, M.; Milewska-Hendel, A.; Gawecki, R.; Karcz, J.; Kurczynska, E.; Hasterok, R. Spatial distribution of selected chemical cell wall components in the embryogenic callus of Brachypodium distachyon. PLoS ONE 2016, 11, e0167426. [Google Scholar] [CrossRef]
- Shibaya, T.; Sugawara, Y. Induction of multinucleation by β-glucosyl Yariv reagent in regenerated cells from Marchantia polymorpha protoplasts and involvement of arabinogalactan proteins in cell plate formation. Planta 2009, 230, 581–588. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Kieliszewski, M.J.; Chen, Y.; Cannon, M.C. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011, 156, 11–19. [Google Scholar] [CrossRef]
- Smallwood, M.; Beven, A.; Donovan, N.; Neill, S.J.; Peart, J.; Roberts, K.; Knox, J.P. Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant J. 1994, 5, 237–246. [Google Scholar] [CrossRef]
- Parmentier, Y.; Durr, A.; Marbach, J.; Hirsinger, C.; Criqui, M.-C.; Fleck, J.; Jamet, E. A novel wound-inducible extensin gene is expressed early in newly isolated protoplasts of Nicotiana sylvestris. Plant Mol. Biol. 1995, 29, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Casero, P.J.; Casimiro, I.; Knox, J.P. Occurrence of cell surface arabinogalactan-protein and extensin epitopes in relation to pericycle and vascular tissue development in the root apex of four species. Planta 1998, 204, 252–259. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Bio. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, Y.; Liu, Y.; Li, Q.; Tang, G. Overexpression of phytosulfokine-α induces male sterility and cell growth by regulating cell wall development in Arabidopsis. Plant Cell Rep. 2016, 35, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; van der Does, D.; Ladwig, F.; Sticht, C.; Kolbeck, A.; Schürholz, A.K.; Augustin, S.; Keinath, N.; Rausch, T.; Greiner, S.; et al. A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 15261–15266. [Google Scholar] [CrossRef]
- Holzwart, E.; Huerta, A.I.; Glöckner, N.; Gómeza, B.G.; Wanke, F.; Augustin, S.; Askani, J.C.; Schürholz, A.K.; Harter, K.; Wolf, S. BRI1 controls vascular cell fate in the Arabidopsis root through RLP44 and phytosulfokine signaling. Proc. Natl. Acad. Sci. USA 2018, 115, 11838–11843. [Google Scholar] [CrossRef]
- Schürholz, A.K. Spatio-temporal control of cell wall properties and signalling networks in Arabidopsis meristems. Ph.D. Thesis, Ruperto Carola University Heidelberg, Heidelberg, Germany, 19 July 2019. [Google Scholar]
- Gómez, B.G. Phosphorylation of RLP44: Shifting between subcellular localization and receptor complexes. Ph.D. Thesis, Ruperto Carola University Heidelberg, Heidelberg, Germany, 17 November 2017. [Google Scholar]
- Gancheva, M.S.; Malovichkoa, Y.V.; Poliushkevich, L.O.; Dodueva, I.E.; Lutova, L.A. Plant peptide hormones. Russ. J. Plant Physiol. 2019, 66, 83–103. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Anthony, P.; Davey, M.R.; Power, J.B.; Lowe, K.C. Enhanced mitotic division of cultured Passiflora and Petuniaprotoplastsby oxygenated perfluorocarbon and haemoglobin. Biotechnol. Tech. 1997, 11, 581–584. [Google Scholar] [CrossRef]
- Pielach, A.; Leroux, O.; Domozych, D.S.; Knox, J.P.; Popper, Z.A. Arabinogalactan protein-rich cell walls, paramural deposits and ergastic globules define the hyaline bodies of rhinanthoid Orobanchaceae haustoria. Ann. Bot. 2014, 114, 1359–1373. [Google Scholar] [CrossRef]
- Yates, E.A.; Valdor, J.-F.; Haslam, S.M.; Morris, H.R.; Dell, A.; Mackie, W.; Knox, J.P. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 1996, 6, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Milewska-Hendel, A.; Baczewska, A.H.; Sala, K.; Dmuchowski, W.; Brągoszewska, P.; Gozdowski, D.; Jozwiak, A.; Chojnacki, T.; Swiezewska, E.; Kurczynska, E. Quantitative and qualitative characteristics of cell wall components and prenyl lipids in the leaves of Tilia x euchlora trees growing under salt stress. PLoS ONE 2017, 12, e0172682. [Google Scholar] [CrossRef] [PubMed]
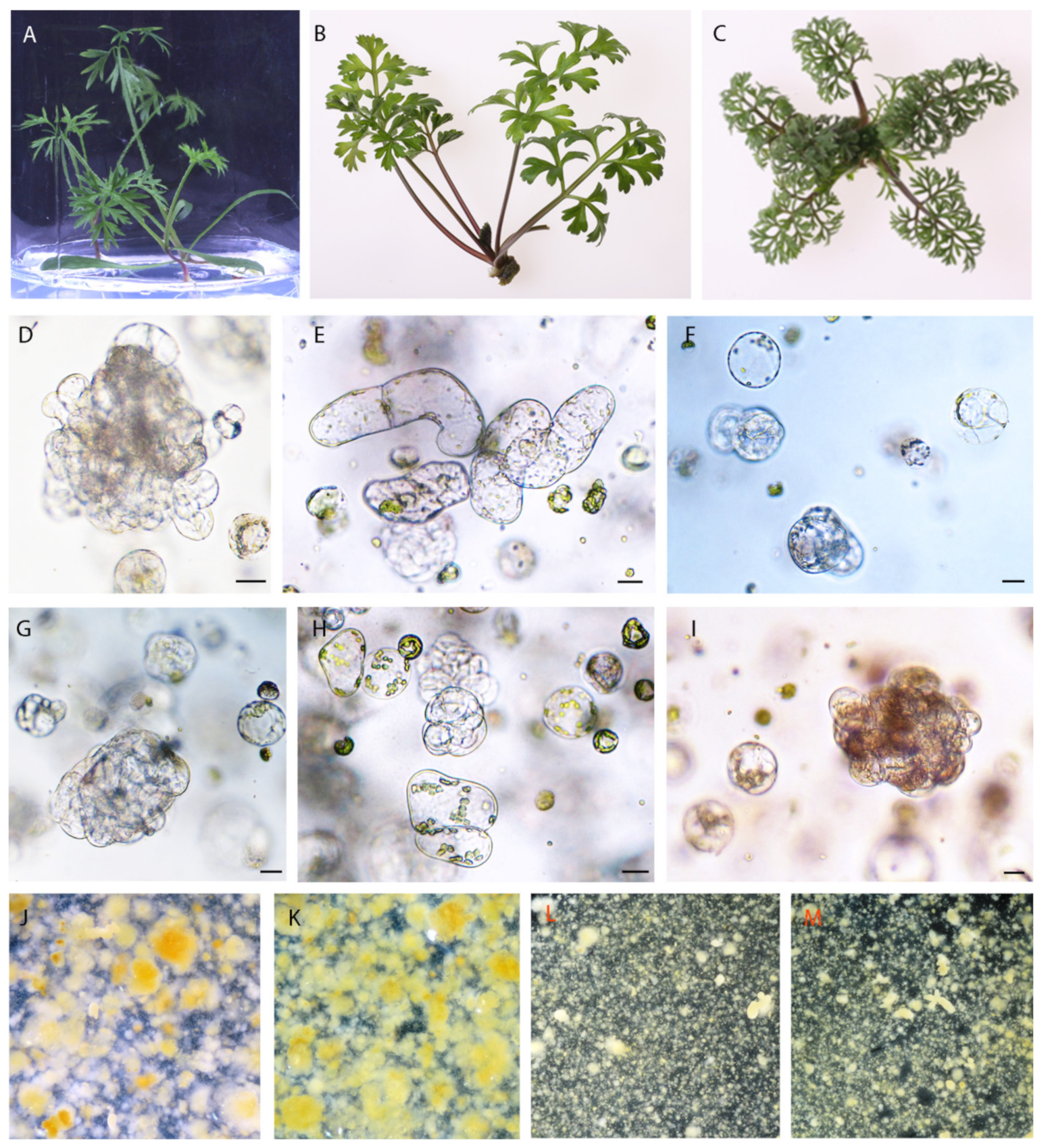








| Species | Protoplast Yield (× 106/g FW) | Protoplast Viability (%) | ||
|---|---|---|---|---|
| Mean ± SE | n | Mean ± SE | n | |
| D. carota subsp. sativus (cv. Dolanka) | 5.1 ± 0.9 a | 3 | 69.0 ± 2.3 a | 3 |
| D. carota subsp. gadecaei | 5.2 ± 1.6 a | 3 | 68.0 ± 1.3 a | 3 |
| D. montevidensis | 1.3 ± 0.4 b | 6 | 48.8 ± 2.7 b | 4 |
| Species | Origin, Seed Source | 2n | Time of Sampling for Epitope Analysis(day of pc) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 10 | 20 | 30 | 60 | |||
| D. carota subsp. sativus | Poland, OP, cv. Dolanka (Polan Spółka z o.o.) | 18 | + | + | + | + | + | + | + | + | + | + |
| D. carota subsp. gadecaei | UK, HRI 7160 | 18 | + | + | + | + | + | + | + | + | + | + |
| D. montevidensis | South America, JKI AS162 | 22 | + | + | + | + | + | + | + | + | - | - |
| Antibody | Epitope | References |
|---|---|---|
| Anti-pectin | ||
| LM19 | Low methyl-esterified HG | [58] |
| LM20 | High metyl-esterifed HG | [58] |
| Anti-AGP | ||
| JIM4 | AGP glycan (betaGlcA-(1,3)-alphaGalA-(1,2)-Rha) | [55,85] |
| JIM8 | Arabinogalactan (epitope structure unknown) | [86] |
| JIM13 | AGP glycan ((beta)GlcA1->3(alpha)GalA1->2Rha) | [86] |
| Anti-extensin | ||
| JIM12 | (epitope structure unknown) | [27] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godel-Jędrychowska, K.; Maćkowska, K.; Kurczyńska, E.; Grzebelus, E. Composition of the Reconstituted Cell Wall in Protoplast-Derived Cells of Daucus Is Affected by Phytosulfokine (PSK). Int. J. Mol. Sci. 2019, 20, 5490. https://doi.org/10.3390/ijms20215490
Godel-Jędrychowska K, Maćkowska K, Kurczyńska E, Grzebelus E. Composition of the Reconstituted Cell Wall in Protoplast-Derived Cells of Daucus Is Affected by Phytosulfokine (PSK). International Journal of Molecular Sciences. 2019; 20(21):5490. https://doi.org/10.3390/ijms20215490
Chicago/Turabian StyleGodel-Jędrychowska, Kamila, Katarzyna Maćkowska, Ewa Kurczyńska, and Ewa Grzebelus. 2019. "Composition of the Reconstituted Cell Wall in Protoplast-Derived Cells of Daucus Is Affected by Phytosulfokine (PSK)" International Journal of Molecular Sciences 20, no. 21: 5490. https://doi.org/10.3390/ijms20215490
APA StyleGodel-Jędrychowska, K., Maćkowska, K., Kurczyńska, E., & Grzebelus, E. (2019). Composition of the Reconstituted Cell Wall in Protoplast-Derived Cells of Daucus Is Affected by Phytosulfokine (PSK). International Journal of Molecular Sciences, 20(21), 5490. https://doi.org/10.3390/ijms20215490






