β-Xylosidases: Structural Diversity, Catalytic Mechanism, and Inhibition by Monosaccharides
Abstract
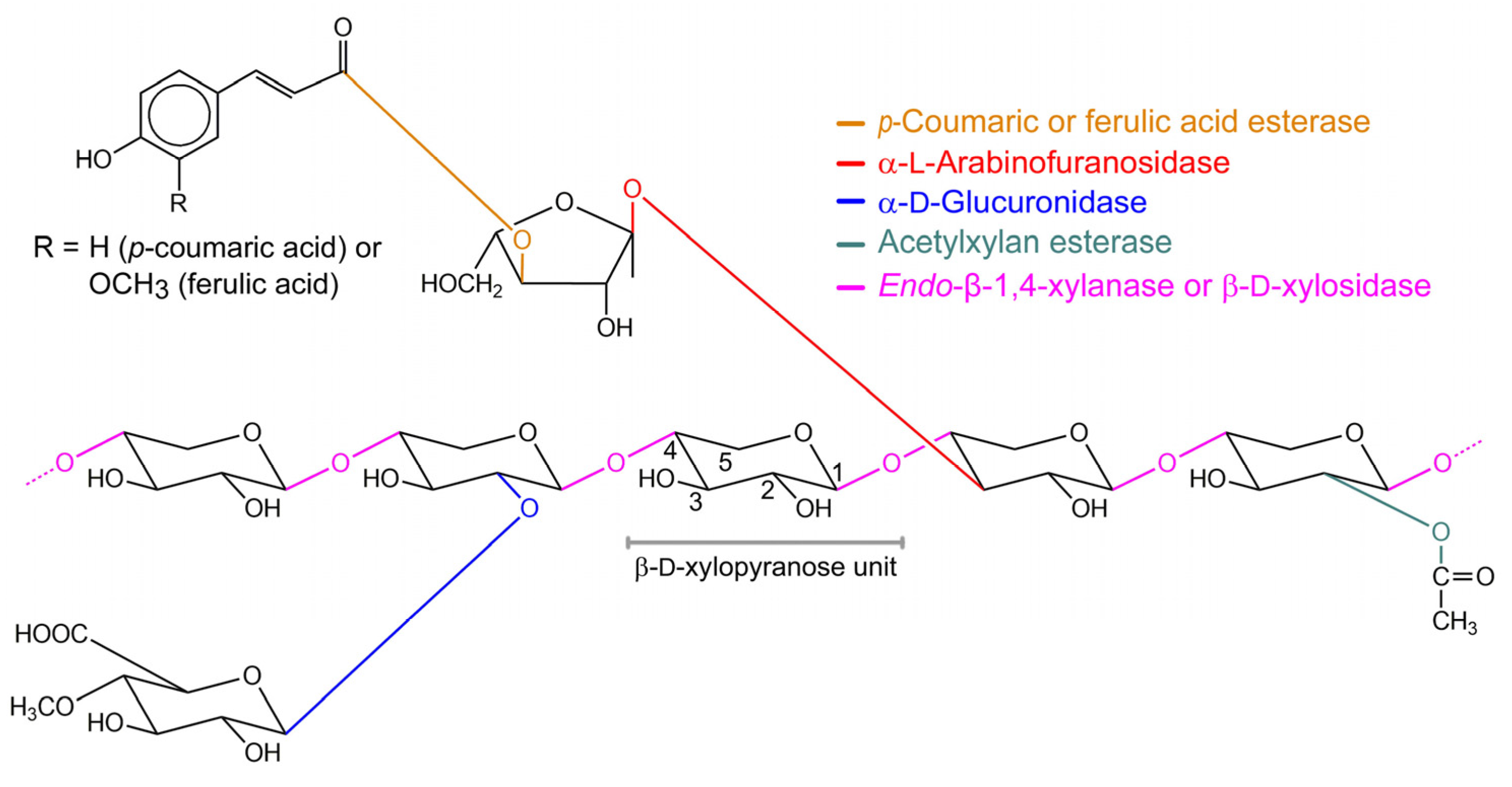
1. Introduction
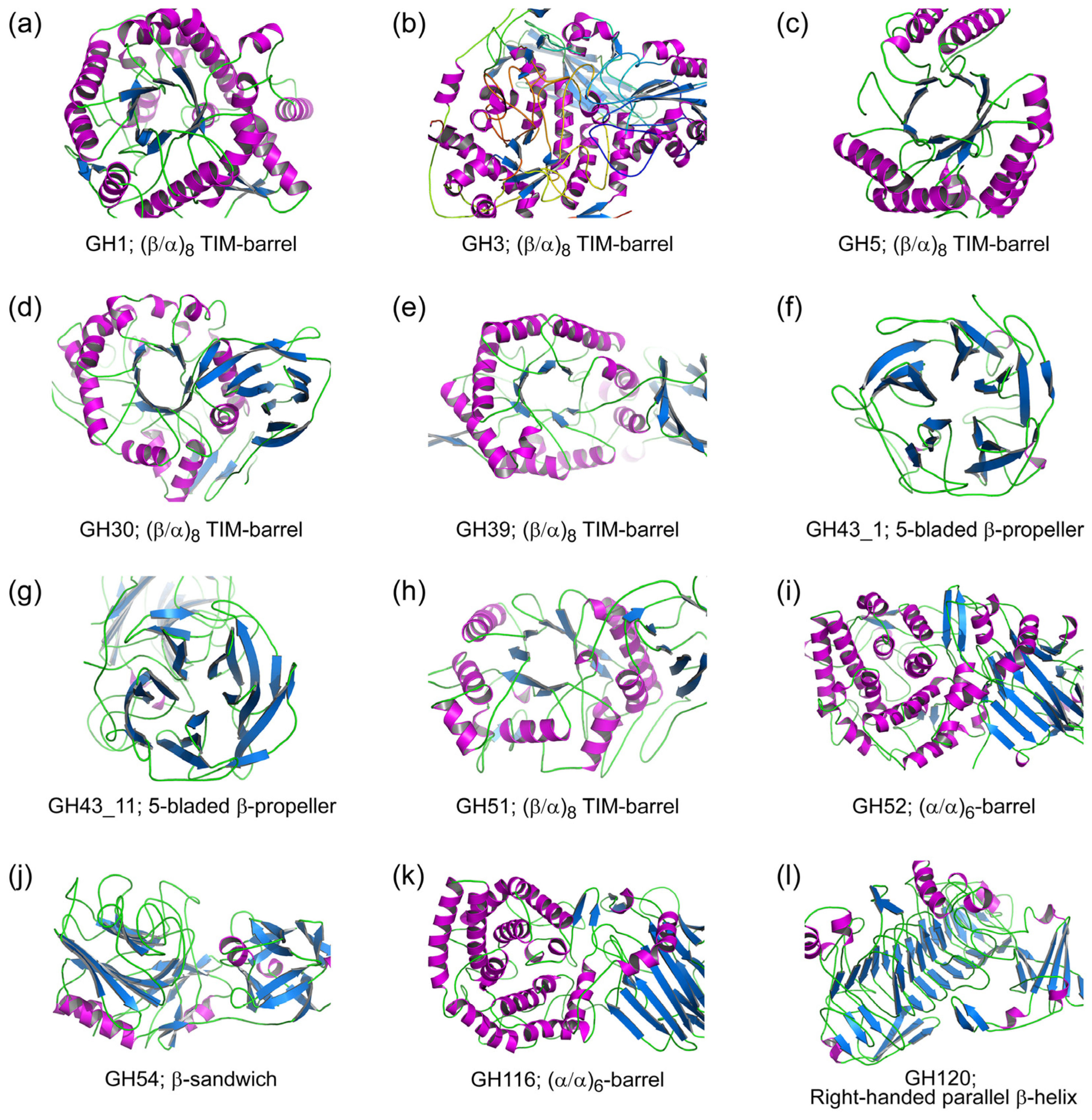
2. Structural Diversity of β-xylosidases
2.1. Glycoside Hydrolase Clan A (GH-A)
2.2. Glycoside Hydrolase Family 3 (GH3)
2.3. Glycoside Hydrolase Family 43 (GH43)
2.4. Glycoside Hydrolase Family 52 (GH52)
2.5. Glycoside Hydrolase Family 54 (GH54)
2.6. Glycoside Hydrolase Family 116 (GH116)
2.7. Glycoside Hydrolase Family 120 (GH120)
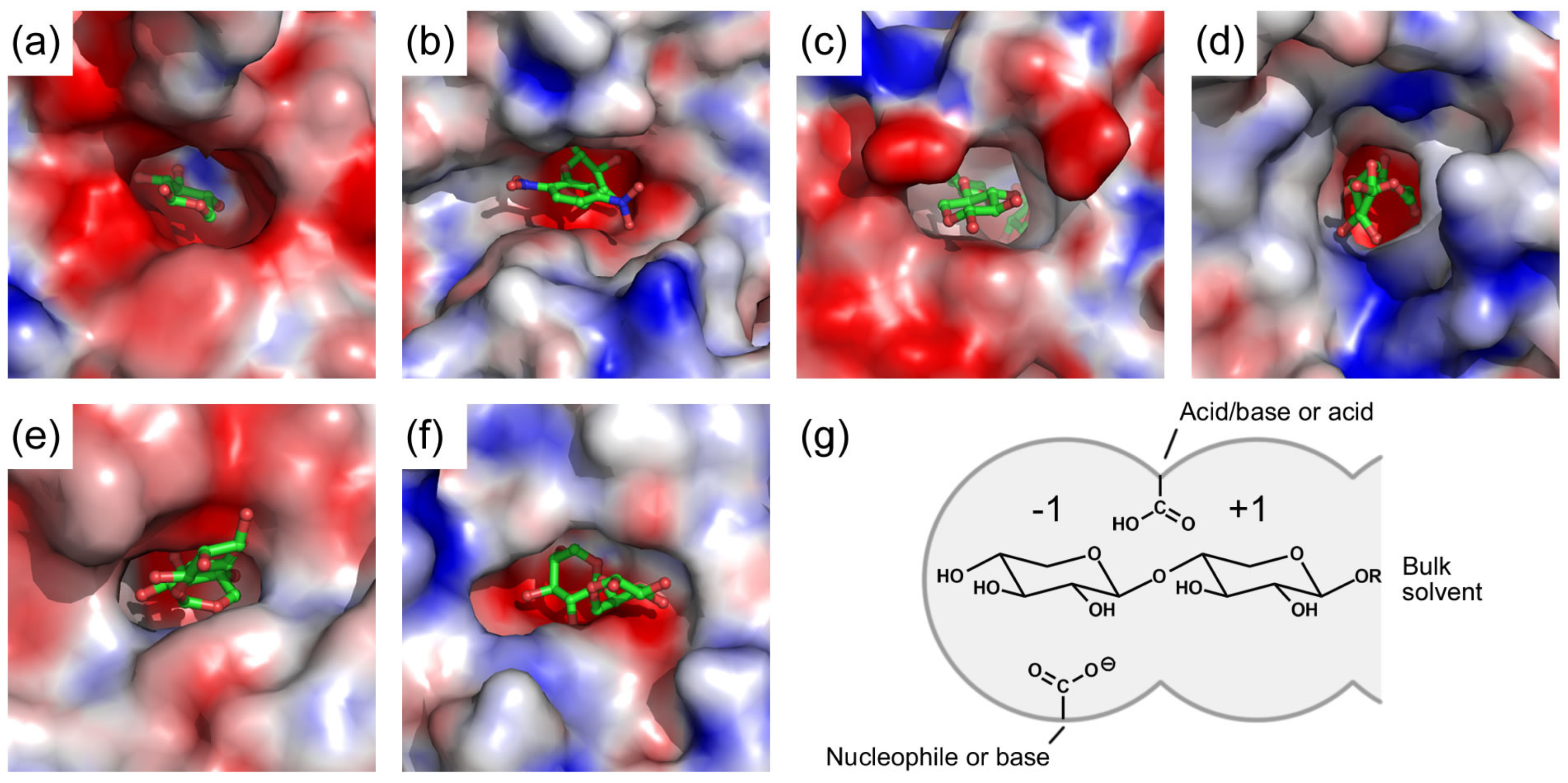
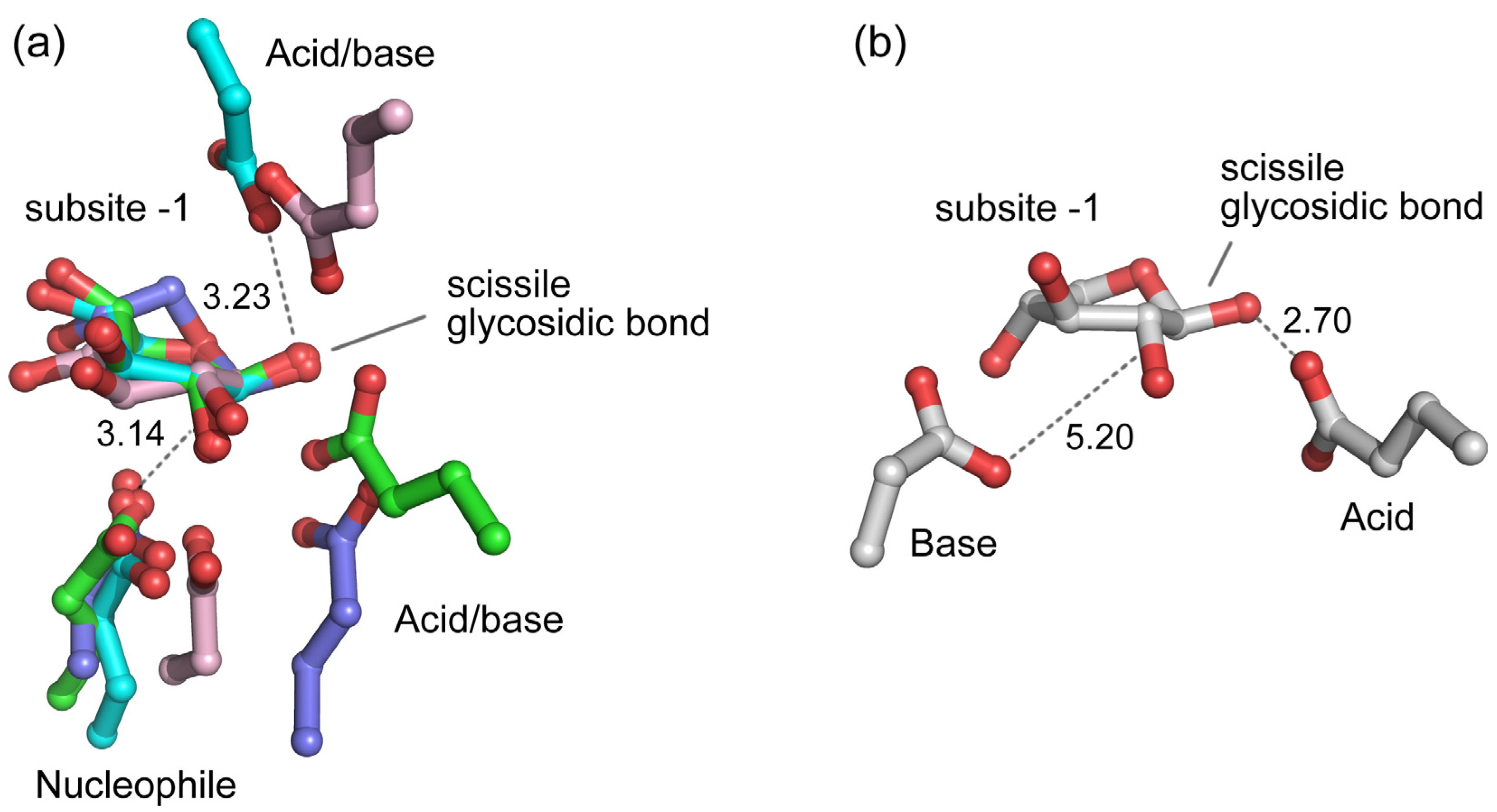
3. Active Site of β-Xylosidases
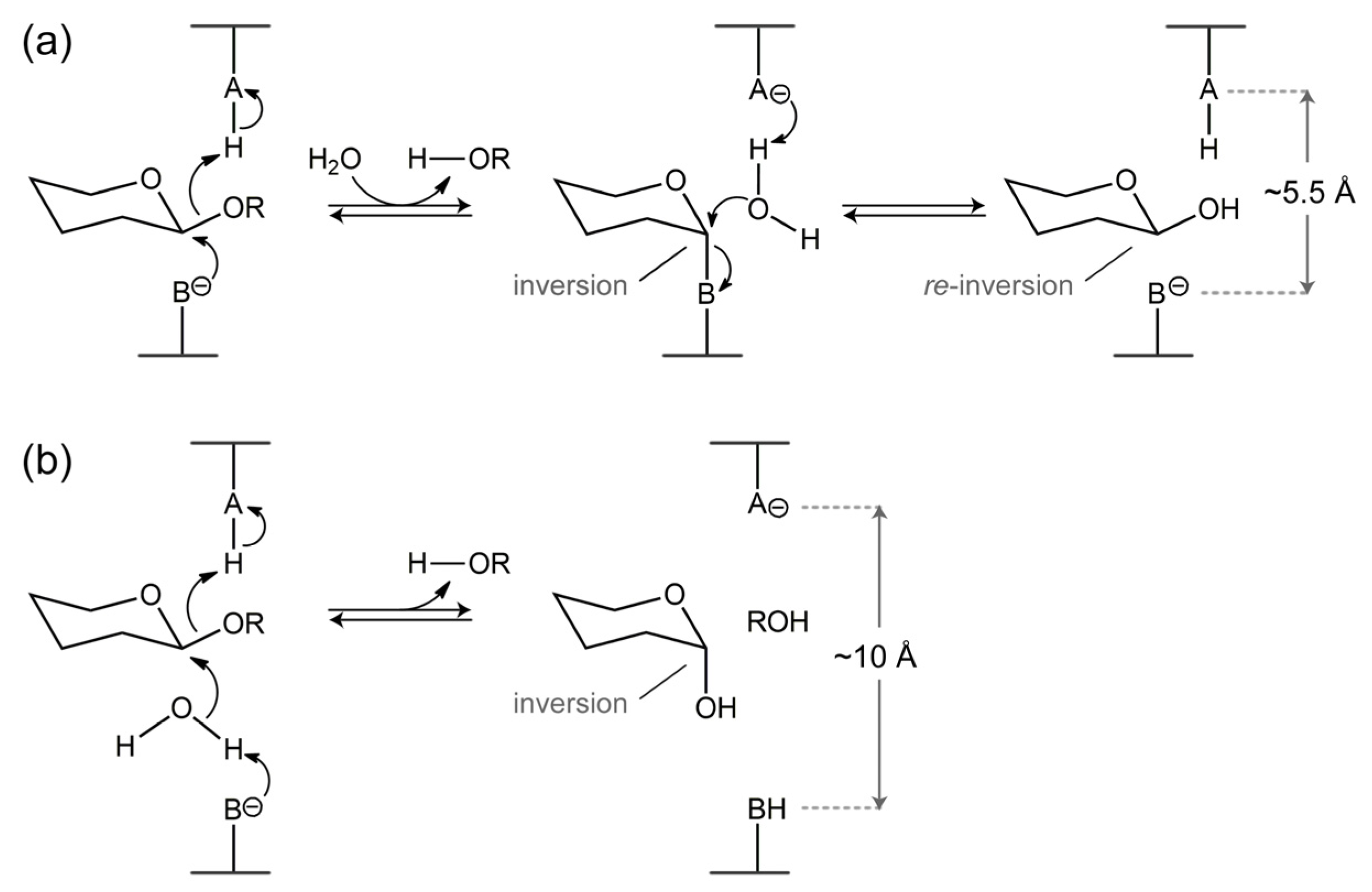
4. Catalytic Mechanism of β-Xylosidases
5. Inhibition of β-Xylosidases by Monosaccharides
5.1. Inhibition by d-xylose
5.2. Inhibition by l-arabinose
5.3. Inhibition by Other Monosaccharides
5.4. Inhibition Kinetics
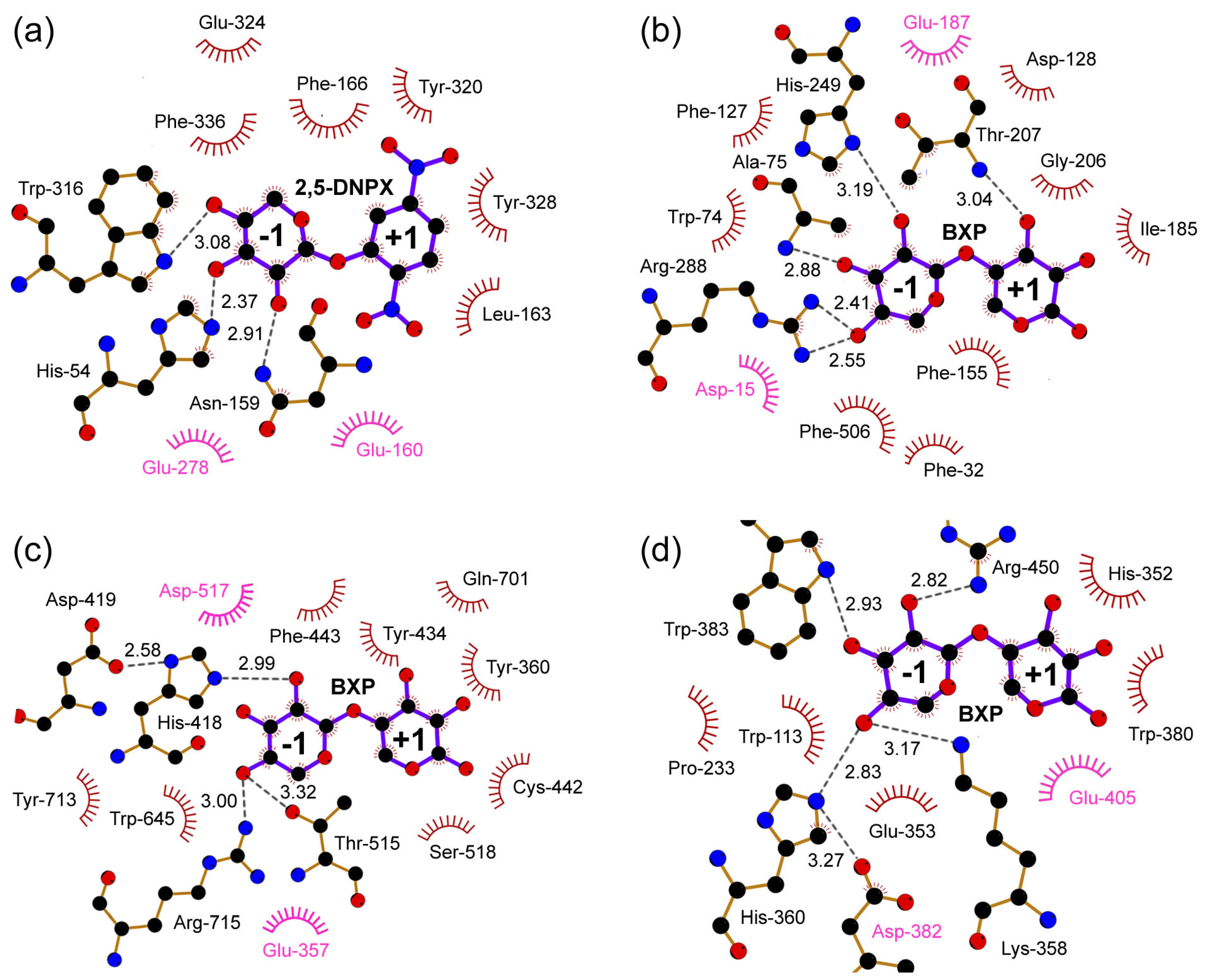
5.5. Structural Details of Inhibitor Binding in the Active Site of β-Xylosidases
5.6. Engineering to Reduce β-Xylosidase Inhibition by Monosaccharides
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Gray, K.A.; Zhao, L.; Emptage, M. Bioethanol. Curr. Opin. Chem. Biol. 2006, 10, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant. Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, R.; Dekker, R.F.H. From plant biomass to bio-based chemicals: Latest developments in xylan research. Biotechnol. Adv. 2012, 30, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hagerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Lidén, G.; Zacchi, G. Bio-ethanol—The fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Beg, Q.K.; Kapoor, M.; Mahajan, L.; Hoondal, G.S. Microbial xylanases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2001, 56, 326–338. [Google Scholar] [CrossRef]
- Biely, P. Microbial xylanolytic systems. Trends Biotechnol. 1985, 3, 286–290. [Google Scholar] [CrossRef]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23. [Google Scholar] [CrossRef]
- Polizeli, M.L.T.M.; Rizzatti, A.C.S.; Monti, R.; Terenzi, H.F.; Jorge, J.A.; Amorim, D.S. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef]
- Sunna, A.; Antranikian, G. Xylanolytic enzymes from fungi and bacteria. Crit. Rev. Biotechnol. 1997, 17, 39–67. [Google Scholar] [CrossRef] [PubMed]
- Chávez, R.; Bull, P.; Eyzaguirre, J. The xylanolytic enzyme system from the genus Penicillium. J. Biotechnol. 2006, 123, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Shendye, A.; Rao, M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol. Rev. 1999, 23, 411–456. [Google Scholar] [CrossRef] [PubMed]
- Prade, R.A. Xylanases: From biology to biotechnology. Biotechnol. Genet. Eng. Rev. 1996, 13, 101–132. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Prema, P. Biotechnology of microbial xylanases: Enzymology, molecular biology, and application. Crit. Rev. Biotechnol. 2002, 22, 33–64. [Google Scholar] [CrossRef]
- Viikari, L.; Kantelinen, A.; Sundquist, J.; Linko, M. Xylanases in bleaching: From an idea to the industry. FEMS Microbiol. Rev. 1994, 13, 335–350. [Google Scholar] [CrossRef]
- Jordan, D.B.; Wagschal, K. Properties and applications of microbial β-d-xylosidases featuring the catalytically efficient enzyme from Selenomonas ruminantium. Appl. Microbiol. Biotechnol. 2010, 86, 1647–1658. [Google Scholar] [CrossRef]
- Jordan, D.B.; Wagschal, K.; Grigorescu, A.A.; Braker, J.D. Highly active β-xylosidases of glycoside hydrolase family 43 operating on natural and artificial substrates. Appl. Microbiol. Biotechnol. 2013, 97, 4415–4428. [Google Scholar] [CrossRef]
- Lee, C.C.; Braker, J.D.; Grigorescu, A.A.; Wagschal, K.; Jordan, D.B. Divalent metal activation of a GH43 β-xylosidase. Enzym. Microb. Technol. 2013, 52, 84–90. [Google Scholar] [CrossRef]
- Qing, Q.; Wyman, C.E. Supplementation with xylanase and β-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol. Biofuels 2011, 4, 18. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624–9630. [Google Scholar] [CrossRef] [PubMed]
- Royer, J.C.; Nakas, J.P. Purification and characterization of two xylanases from Trichoderma longibrachiatum. Eur. J. Biochem. 1991, 202, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.J.; Hoos, R.; Withers, S.G. Nanomolar versus millimolar inhibition by xylobiose-derived azasugars: Significant differences between two structurally distinct xylanases. J. Am. Chem. Soc. 2000, 122, 2223–2235. [Google Scholar] [CrossRef]
- Herrmann, M.C.; Vrsanska, M.; Jurickova, M.; Hirsch, J.; Biely, P.; Kubicek, C.P. The β-d-xylosidase of Trichoderma reesei is a multifunctional β-d-xylan xylohydrolase. Biochem. J. 1997, 321, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Sun, Y.; Ko, T.P.; Chen, C.C.; Zheng, Y.; Chan, H.C.; Pang, X.; Wiegel, J.; Shao, W.; Guo, R.T. The substrate/product-binding modes of a novel GH120 β-xylosidase (XylC) from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Biochem. J. 2012, 448, 401–407. [Google Scholar] [CrossRef]
- Jordan, D.B.; Braker, J.D. Inhibition of the two-subsite β-d-xylosidase from Selenomonas ruminantium by sugars: Competitive, noncompetitive, double binding, and slow binding modes. Arch. Biochem. Biophys. 2007, 465, 231–246. [Google Scholar] [CrossRef]
- Jordan, D.B.; Wagschal, K.; Fan, Z.; Yuan, L.; Braker, J.D.; Heng, C. Engineering lower inhibitor affinities in β-d-xylosidase of Selenomonas ruminantium by site-directed mutagenesis of Trp145. J. Ind. Microbiol. Biotechnol. 2011, 38, 1821–1835. [Google Scholar] [CrossRef]
- Lagaert, S.; Pollet, A.; Courtin, C.M.; Volckaert, G. β-Xylosidases and α-l-arabinofuranosidases: Accessory enzymes for arabinoxylan degradation. Biotechnol. Adv. 2014, 32, 316–332. [Google Scholar] [CrossRef]
- Davies, G.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef]
- Henrissat, B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Claeyssens, M.; Henrissat, B. Specificity mapping of cellulolytic enzymes: Classification into families of structurally related proteins confirmed by biochemical analysis. Protein Sci. 1992, 1, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Bairoch, A. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 1996, 316, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Scharf, M.E.; Kovaleva, E.S.; Jadhao, S.; Campbell, J.H.; Buchman, G.W.; Boucias, D.G. Functional and translational analyses of a β-glucosidase gene (glycosyl hydrolase family 1) isolated from the gut of the lower termite Reticulitermes flavipes. Insect Biochem. Mol. Biol. 2010, 40, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Mattéotti, C.; Haubruge, E.; Thonart, P.; Francis, F.; De Pauw, E.; Portetelle, D.; Vandenbol, M. Characterization of a new β-glucosidase/β-xylosidase from the gut microbiota of the termite (Reticulitermes santonensis). FEMS Microbiol. Lett. 2011, 314, 147–157. [Google Scholar] [CrossRef]
- Wan, C.-F.; Chen, C.-T.; Huang, L.; Li, Y.-K. Expression, purification and characterization of a bifunctional α-l-arabinofuranosidase/β-d-xylosidase from Trichoderma koningii G-39. J. Chin. Chem. Soc. 2007, 54, 109–116. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Aurilia, V.; Riccio, G.; Henrissat, B.; Coutinho, P.M.; Strazzulli, A.; Padula, A.; Corsaro, M.M.; Pieretti, G.; Pocsfalvi, G.; et al. A new archaeal β-glycosidase from Sulfolobus solfataricus: Seeding a novel retaining β-glycan-specific glycoside hydrolase family along with the human non-lysosomal glucosylceramidase GBA2. J. Biol. Chem. 2010, 285, 20691–20703. [Google Scholar] [CrossRef]
- Miyanaga, A.; Koseki, T.; Matsuzawa, H.; Wakagi, T.; Shoun, H.; Fushinobu, S. Crystal structure of a family 54 α-l-arabinofuranosidase reveals a novel carbohydrate-binding module that can bind arabinose. J. Biol. Chem. 2004, 279, 44907–44914. [Google Scholar] [CrossRef]
- Naumoff, D.G. Hierarchical classification of glycoside hydrolases. Biochemistry 2011, 76, 764–780. [Google Scholar] [CrossRef]
- Yang, J.K.; Yoon, H.J.; Ahn, H.J.; Lee, B.I.; Pedelacq, J.-D.; Liong, E.C.; Berendzen, J.; Laivenieks, M.; Vieille, C.; Zeikus, G.J.; et al. Crystal structure of β-d-xylosidase from Thermoanaerobacterium saccharolyticum, a family 39 glycoside hydrolase. J. Mol. Biol. 2004, 335, 155–165. [Google Scholar] [CrossRef]
- Czjzek, M.; Ben-David, A.; Bravman, T.; Shoham, G.; Henrissat, B.; Shoham, Y. Enzyme-substrate complex structures of a GH39 β-xylosidase from Geobacillus stearothermophilus. J. Mol. Biol. 2005, 353, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Polo, C.C.; Corrêa, J.M.; Simão, R.C.G.; Seixas, F.A.V.; Murakami, M.T. The accessory domain changes the accessibility and molecular topography of the catalytic interface in monomeric GH39 β-xylosidases. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Huy, N.D.; Thayumanavan, P.; Kwon, T.-H.; Park, S.-M. Characterization of a recombinant bifunctional xylosidase/arabinofuranosidase from Phanerochaete chrysosporium. J. Biosci. Bioeng. 2013, 116, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Wirtz, W.; Rose, J.K.C.; Darvill, A.G.; Govers, F.; Scheel, D.; Nürnberger, T. A β-glucosidase/xylosidase from the phytopathogenic oomycete, Phytophthora infestans. Phytochemistry 2002, 59, 689–696. [Google Scholar] [CrossRef]
- Minic, Z.; Rihouey, C.; Do, C.T.; Lerouge, P.; Jouanin, L. Purification and characterization of enzymes exhibiting β-d-xylosidase activities in stem tissues of Arabidopsis. Plant. Physiol. 2004, 135, 867–878. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Suzuki, K.; Sumitani, J.-i.; Nam, Y.-W.; Nishimaki, T.; Tani, S.; Wakagi, T.; Kawaguchi, T.; Fushinobu, S. Crystal structures of glycoside hydrolase family 3 β-glucosidase 1 from Aspergillus aculeatus. Biochem. J. 2013, 452, 211–221. [Google Scholar] [CrossRef]
- Harvey, A.J.; Hrmova, M.; De Gori, R.; Varghese, J.N.; Fincher, G.B. Comparative modeling of the three-dimensional structures of family 3 glycoside hydrolases. Proteins 2000, 41, 257–269. [Google Scholar] [CrossRef]
- Pozzo, T.; Pasten, J.L.; Karlsson, E.N.; Logan, D.T. Structural and functional analyses of β-glucosidase 3B from Thermotoga neapolitana: A thermostable three-domain representative of glycoside hydrolase 3. J. Mol. Biol. 2010, 397, 724–739. [Google Scholar] [CrossRef]
- Karkehabadi, S.; Helmich, K.E.; Kaper, T.; Hansson, H.; Mikkelsen, N.-E.; Gudmundsson, M.; Piens, K.; Fujdala, M.; Banerjee, G.; Scott-Craig, J.S.; et al. Biochemical characterization and crystal structures of a fungal family 3 β-glucosidase, Cel3A from Hypocrea jecorina. J. Biol. Chem. 2014, 289, 31624–31637. [Google Scholar] [CrossRef]
- Yoshida, E.; Hidaka, M.; Fushinobu, S.; Koyanagi, T.; Minami, H.; Tamaki, H.; Kitaoka, M.; Katayama, T.; Kumagai, H. Role of a PA14 domain in determining substrate specificity of a glycoside hydrolase family 3 β-glucosidase from Kluyveromyces marxianus. Biochem. J. 2010, 431, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Litzinger, S.; Fischer, S.; Polzer, P.; Diederichs, K.; Welte, W.; Mayer, C. Structural and kinetic analysis of Bacillus subtilis N-acetylglucosaminidase reveals a unique Asp-His dyad mechanism. J. Biol. Chem. 2010, 285, 35675–35684. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Vroemen, S.; Heldens, J.; Boyd, C.; Henrissat, B.; Keen, N.T. Cloning and characterization of the bgxA gene from Erwinia chrysanthemi D1 which encodes a β-glucosidase/xylosidase enzyme. Mol. Gen. Genet. 1995, 246, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Nechitaylo, T.Y.; López-Cortés, N.; Ghazi, A.; Guazzaroni, M.-E.; Polaina, J.; Strittmatter, A.W.; Reva, O.; Waliczek, A.; Yakimov, M.M.; et al. Diversity of glycosyl hydrolases from cellulose-depleting communities enriched from casts of two earthworm species. Appl. Environ. Microbiol. 2010, 76, 5934–5946. [Google Scholar] [CrossRef]
- Lagaert, S.; Pollet, A.; Delcour, J.A.; Lavigne, R.; Courtin, C.M.; Volckaert, G. Characterization of two β-xylosidases from Bifidobacterium adolescentis and their contribution to the hydrolysis of prebiotic xylooligosaccharides. Appl. Microbiol. Biotechnol. 2011, 92, 1179–1185. [Google Scholar] [CrossRef]
- Margolles-Clark, E.; Tenkanen, M.; Nakari-Setälä, T.; Penttilä, M. Cloning of genes encoding α-l-arabinofuranosidase and β-xylosidase from Trichoderma reesei by expression in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1996, 62, 3840–3846. [Google Scholar]
- Ramírez-Escudero, M.; del Pozo, M.V.; Marín-Navarro, J.; González, B.; Golyshin, P.N.; Polaina, J.; Ferrer, M.; Sanz-Aparicio, J. Structural and functional characterization of a ruminal β-glycosidase defines a novel subfamily of glycoside hydrolase family 3 with permuted domain topology. J. Biol. Chem. 2016, 291, 24200–24214. [Google Scholar] [CrossRef]
- Nurizzo, D.; Turkenburg, J.P.; Charnock, S.J.; Roberts, S.M.; Dodson, E.J.; McKie, V.A.; Taylor, E.J.; Gilbert, H.J.; Davies, G.J. Cellvibrio japonicus α-l-arabinanase 43A has a novel five-blade β-propeller fold. Nat. Struct. Biol. 2002, 9, 665–668. [Google Scholar] [CrossRef]
- Yoshida, S.; Hespen, C.W.; Beverly, R.L.; Mackie, R.I.; Cann, I.K.O. Domain analysis of a modular α-l-arabinofuranosidase with a unique carbohydrate binding strategy from the fiber-degrading bacterium Fibrobacter succinogenes S85. J. Bacteriol. 2010, 192, 5424–5436. [Google Scholar] [CrossRef]
- Mewis, K.; Lenfant, N.; Lombard, V.; Henrissat, B. Dividing the large glycoside hydrolase family 43 into subfamilies: A Motivation for detailed enzyme characterization. Appl. Environ. Microbiol. 2016, 82, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Ghazi, A.; Beloqui, A.; Vieites, J.M.; López-Cortés, N.; Marín-Navarro, J.; Nechitaylo, T.Y.; Guazzaroni, M.-E.; Polaina, J.; Waliczek, A.; et al. Functional metagenomics unveils a multifunctional glycosyl hydrolase from the family 43 catalysing the breakdown of plant polymers in the calf rumen. PLoS ONE 2012, 7, e38134. [Google Scholar] [CrossRef] [PubMed]
- Ratnadewi, A.A.I.; Fanani, M.; Kurniasih, S.D.; Sakka, M.; Wasito, E.B.; Sakka, K.; Nurachman, Z.; Puspaningsih, N.N.T. β-d-Xylosidase from Geobacillus thermoleovorans IT-08: Biochemical characterization and bioinformatics of the enzyme. Appl. Biochem. Biotechnol. 2013, 170, 1950–1964. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.B.; Braker, J.D.; Wagschal, K.; Lee, C.C.; Chan, V.J.; Dubrovska, I.; Anderson, S.; Wawrzak, Z. X-ray crystal structure of divalent metal-activated β-xylosidase, RS223BX. Appl. Biochem. Biotechnol. 2015, 177, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Kaneko, S.; Kishine, N.; Fujimoto, Z.; Yaoi, K. Crystal structure of metagenomic β-xylosidase/α-l-arabinofuranosidase activated by calcium. J. Biochem. 2017, 162, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Brüx, C.; Ben-David, A.; Shallom-Shezifi, D.; Leon, M.; Niefind, K.; Shoham, G.; Shoham, Y.; Schomburg, D. The structure of an inverting GH43 β-xylosidase from Geobacillus stearothermophilus with its substrate reveals the role of the three catalytic residues. J. Mol. Biol. 2006, 359, 97–109. [Google Scholar] [CrossRef]
- Brunzelle, J.S.; Jordan, D.B.; McCaslin, D.R.; Olczak, A.; Wawrzak, Z. Structure of the two-subsite β-d-xylosidase from Selenomonas ruminantium in complex with 1,3-bis[tris(hydroxymethyl)methylamino]propane. Arch. Biochem. Biophys. 2008, 474, 157–166. [Google Scholar] [CrossRef]
- Hong, S.; Kyung, M.; Jo, I.; Kim, Y.-R.; Ha, N.-C. Structure-based protein engineering of bacterial β-xylosidase to increase the production yield of xylobiose from xylose. Biochem. Biophys. Res. Commun. 2018, 501, 703–710. [Google Scholar] [CrossRef]
- Zhang, R.; Li, N.; Liu, Y.; Han, X.; Tu, T.; Shen, J.; Xu, S.; Wu, Q.; Zhou, J.; Huang, Z. Biochemical and structural properties of a low-temperature-active glycoside hydrolase family 43 β-xylosidase: Activity and instability at high neutral salt concentrations. Food Chem. 2019, 301, 125266. [Google Scholar] [CrossRef]
- Rohman, A.; van Oosterwijk, N.; Puspaningsih, N.N.T.; Dijkstra, B.W. Structural basis of product inhibition by arabinose and xylose of the thermostable GH43 β-1,4-xylosidase from Geobacillus thermoleovorans IT-08. PLoS ONE 2018, 13, e0196358. [Google Scholar] [CrossRef]
- Morais, S.; Salama-Alber, O.; Barak, Y.; Hadar, Y.; Wilson, D.B.; Lamed, R.; Shoham, Y.; Bayer, E.A. Functional association of catalytic and ancillary modules dictates enzymatic activity in glycoside hydrolase family 43 β-xylosidase. J. Biol. Chem. 2012, 287, 9213–9221. [Google Scholar] [CrossRef] [PubMed]
- Ontañon, O.M.; Ghio, S.; Marrero Díaz de Villegas, R.; Piccinni, F.E.; Talia, P.M.; Cerutti, M.L.; Campos, E. EcXyl43 β-xylosidase: Molecular modeling, activity on natural and artificial substrates, and synergism with endoxylanases for lignocellulose deconstruction. Appl. Microbiol. Biotechnol. 2018, 102, 6959–6971. [Google Scholar] [CrossRef] [PubMed]
- Espina, G.; Eley, K.; Pompidor, G.; Schneider, T.R.; Crennell, S.J.; Danson, M.J. A novel β-xylosidase structure from Geobacillus thermoglucosidasius: The first crystal structure of a glycoside hydrolase family GH52 enzyme reveals unpredicted similarity to other glycoside hydrolase folds. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Charoenwattanasatien, R.; Pengthaisong, S.; Breen, I.; Mutoh, R.; Sansenya, S.; Hua, Y.; Tankrathok, A.; Wu, L.; Songsiriritthigul, C.; Tanaka, H.; et al. Bacterial β-glucosidase reveals the structural and functional basis of genetic defects in human glucocerebrosidase 2 (GBA2). ACS Chem. Biol. 2016, 11, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Xue, Y.; Wu, A.; Kataeva, I.; Pei, J.; Wu, H.; Wiegel, J. Characterization of a novel β-xylosidase, XylC, from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl. Environ. Microbiol. 2011, 77, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot{+}: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- McCarter, J.D.; Withers, S.G. Mechanisms of enzymatic glycoside hydrolysis. Curr. Opin. Struct. Biol. 1994, 4, 885–892. [Google Scholar] [CrossRef]
- Braun, C.; Meinke, A.; Ziser, L.; Withers, S.G. Simultaneous high-performance liquid chromatographic determination of both the cleavage pattern and the stereochemical outcome of the hydrolysis reactions catalyzed by various glycosidases. Anal. Biochem. 1993, 212, 259–262. [Google Scholar] [CrossRef]
- Jordan, D.B.; Li, X.-L.; Dunlap, C.A.; Whitehead, T.R.; Cotta, M.A. Structure-function relationships of a catalytically efficient β-d-xylosidase. Appl. Biochem. Biotechnol. 2007, 141, 51–76. [Google Scholar] [CrossRef]
- Shallom, D.; Leon, M.; Bravman, T.; Ben-David, A.; Zaide, G.; Belakhov, V.; Shoham, G.; Schomburg, D.; Baasov, T.; Shoham, Y. Biochemical characterization and identification of the catalytic residues of a family 43 β-d-xylosidase from Geobacillus stearothermophilus T-6. Biochemistry 2005, 44, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Rye, C.S.; Withers, S.G. Glycosidase mechanisms. Curr. Opin. Chem. Biol. 2000, 4, 573–580. [Google Scholar] [CrossRef]
- Ximenes, F.D.A.; de Paula Silveira, F.Q.; FFilho, E.X. Production of β-xylosidase activity by Trichoderma harzianum strains. Curr. Microbiol. 1996, 33, 71–77. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Qi, Z.; Zhao, L.; Pei, J.; Tang, F. Characterization of a novel thermostable and xylose-tolerant GH 39 β-xylosidase from Dictyoglomus thermophilum. BMC Biotechnol. 2018, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Wagschal, K.; Franqui-Espiet, D.; Lee, C.C.; Robertson, G.H.; Wong, D.W.S. Cloning, expression and characterization of a glycoside hydrolase family 39 xylosidase from Bacillus halodurans C-125. Appl. Biochem. Biotechnol. 2008, 146, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Banka, A.L.; Guralp, S.A.; Gulari, E. Secretory expression and characterization of two hemicellulases, xylanase, and β-xylosidase, isolated from Bacillus subtilis M015. Appl. Biochem. Biotechnol. 2014, 174, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Hiraga, K.; Suda, M.; Yukawa, H.; Inui, M. Functional characterization of Corynebacterium alkanolyticum β-xylosidase and xyloside ABC transporter in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2015, 81, 4173–4183. [Google Scholar] [CrossRef]
- Bhalla, A.; Bischoff, K.M.; Sani, R.K. Highly thermostable GH39 β-xylosidase from a Geobacillus sp. strain WSUCF1. BMC Biotechnol. 2014, 14, 963. [Google Scholar] [CrossRef]
- Huang, D.; Liu, J.; Qi, Y.; Yang, K.; Xu, Y.; Feng, L. Synergistic hydrolysis of xylan using novel xylanases, β-xylosidases, and an α-l-arabinofuranosidase from Geobacillus thermodenitrificans NG80-2. Appl. Microbiol. Biotechnol. 2017, 101, 6023–6037. [Google Scholar] [CrossRef]
- Michlmayr, H.; Hell, J.; Lorenz, C.; Böhmdorfer, S.; Rosenau, T.; Kneifel, W. Arabinoxylan oligosaccharide hydrolysis by family 43 and 51 glycosidases from Lactobacillus brevis DSM 20054. Appl. Environ. Microbiol. 2013, 79, 6747–6754. [Google Scholar] [CrossRef]
- Xu, B.; Dai, L.; Zhang, W.; Yang, Y.; Wu, Q.; Li, J.; Tang, X.; Zhou, J.; Ding, J.; Han, N.; et al. Characterization of a novel salt-, xylose- and alkali-tolerant GH43 bifunctional β-xylosidase/α-l-arabinofuranosidase from the gut bacterial genome. J. Biosci. Bioeng. 2019, 128, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Yoon, K.-H. Characterization of a Paenibacillus woosongensis β-xylosidase/α-arabinofuranosidase produced by recombinant Escherichia coli. J. Microbiol. Biotechnol. 2010, 20, 1711–1716. [Google Scholar] [PubMed]
- Whitehead, T.R.; Cotta, M.A. Identification of a broad-specificity xylosidase/arabinosidase important for xylooligosaccharide fermentation by the ruminal anaerobe Selenomonas ruminantium GA192. Curr. Microbiol. 2001, 43, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.; Xu, J.; Saccone, G.; Li, K.; Zhang, H. Discovery and characterization of endo-xylanase and β-xylosidase from a highly xylanolytic bacterium in the hindgut of Holotrichia parallela larvae. J. Mol. Catal. B Enzym. 2014, 105, 33–40. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, J.; Zhao, L.; Pei, J.; Su, E.; Xiao, W.; Wang, Z. Cloning, overexpression and characterization of a thermostable β-xylosidase from Thermotoga petrophila and cooperated transformation of ginsenoside extract to ginsenoside 20(S)-Rg3 with a β-glucosidase. Bioorg. Chem. 2019, 85, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, X.; Gu, H.; Zhang, Y.; Huang, Y.; Wang, L.; Wang, F. Biochemical properties of a novel thermostable and highly xylose-tolerant β-xylosidase/α-arabinosidase from Thermotoga. thermarum. Biotechnol. Biofuels 2013, 6, 27. [Google Scholar] [CrossRef]
- Kumar, S.; Ramón, D. Purification and regulation of the synthesis of a β-xylosidase from Aspergillus nidulans. FEMS Microbiol. Lett. 1996, 135, 287–293. [Google Scholar] [CrossRef]
- John, M.; Schmidt, B.; Schmidt, J. Purification and some properties of five endo-1,4-β-d-xylanases and a β-d-xylosidase produced by a strain of Aspergillus niger. Can. J. Biochem. 1979, 57, 125–134. [Google Scholar] [CrossRef]
- Patel, H.; Kumar, A.K.; Shah, A. Purification and characterization of novel bi-functional GH3 family β-xylosidase/β-glucosidase from Aspergillus niger ADH-11. Int. J. Biol. Macromol. 2018, 109, 1260–1269. [Google Scholar] [CrossRef]
- Dobberstein, J.; Emeis, C.C. Purification and characterization of β-xylosidase from Aureobasidium pullulans. Appl. Microbiol. Biotechnol. 1991, 35, 210–215. [Google Scholar] [CrossRef]
- Yanai, T.; Sato, M. Purification and characterization of an β-d-xylosidase from Candida utilis IFO 0639. Biosci. Biotechnol. Biochem. 2001, 65, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Cintra, L.C.; Fernandes, A.G.; Oliveira, I.C.M.d.; Siqueira, S.J.L.; Costa, I.G.O.; Colussi, F.; Jesuíno, R.S.A.; Ulhoa, C.J.; Faria, F.P.d. Characterization of a recombinant xylose tolerant β-xylosidase from Humicola grisea var. thermoidea and its use in sugarcane bagasse hydrolysis. Int. J. Biol. Macromol. 2017, 105, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, P.; Huang, H.; Luo, H.; Wang, Y.; Zhang, W.; Yao, B. Two xylose-tolerant GH43 bifunctional β-xylosidase/α-arabinosidases and one GH11 xylanase from Humicola insolens and their synergy in the degradation of xylan. Food Chem. 2014, 148, 381–387. [Google Scholar] [CrossRef]
- Yan, Q.J.; Wang, L.; Jiang, Z.Q.; Yang, S.Q.; Zhu, H.F.; Li, L.T. A xylose-tolerant β-xylosidase from Paecilomyces thermophila: Characterization and its co-action with the endogenous xylanase. Bioresour. Technol. 2008, 99, 5402–5410. [Google Scholar] [CrossRef] [PubMed]
- Mhetras, N.; Liddell, S.; Gokhale, D. Purification and characterization of an extracellular β-xylosidase from Pseudozyma hubeiensis NCIM 3574 (PhXyl), an unexplored yeast. AMB Express 2016, 6, 73. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, X.; Pilgaard, B.; Holck, J.; Muschiol, J.; Li, S.; Lange, L. Identification and characterization of GH11 xylanase and GH43 xylosidase from the chytridiomycetous fungus, Rhizophlyctis rosea. Appl. Microbiol. Biotechnol. 2019, 103, 777–791. [Google Scholar] [CrossRef]
- Zanoelo, F.F.; Polizeli Md, M.d.L.T.d.M.; Terenzi, H.F.; Jorge, J.A. Purification and biochemical properties of a thermostable xylose-tolerant β-d-xylosidase from Scytalidium thermophilum. J. Ind. Microbiol. Biotechnol. 2004, 31, 170–176. [Google Scholar] [CrossRef]
- Fujii, T.; Yu, G.; Matsushika, A.; Kurita, A.; Yano, S.; Murakami, K.; Sawayama, S. Ethanol production from xylo-oligosaccharides by xylose-fermenting Saccharomyces cerevisiae expressing β-xylosidase. Biosci. Biotechnol. Biochem. 2011, 75, 1140–1146. [Google Scholar] [CrossRef]
- Maruthamuthu, M.; Jiménez, D.J.; van Elsas, J.D. Characterization of a furan aldehyde-tolerant β-xylosidase/α-arabinosidase obtained through a synthetic metagenomics approach. J. Appl. Microbiol. 2017, 123, 145–158. [Google Scholar] [CrossRef]
- Gruninger, R.J.; Gong, X.; Forster, R.J.; McAllister, T.A. Biochemical and kinetic characterization of the multifunctional β-glucosidase/β-xylosidase/α-arabinosidase, Bgxa1. Appl. Microbiol. Biotechnol. 2014, 98, 3003–3012. [Google Scholar] [CrossRef]
- Bao, L.; Huang, Q.; Chang, L.; Sun, Q.; Zhou, J.; Lu, H. Cloning and characterization of two β-glucosidase/xylosidase enzymes from yak rumen metagenome. Appl. Biochem. Biotechnol. 2012, 166, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.E.; Sorensen, H.R.; Vind, J.; Viksø-Nielsen, A. Mode of action and properties of the β-xylosidases from Talaromyces emersonii and Trichoderma reesei. Biotechnol. Bioeng. 2006, 94, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Kersters-Hilderson, H.; Loontiens, F.G.; Claeyssens, M.; De Bruyne, C.K. Partial purification and properties of an induced β-d-xylosidase of Bacillus pumilus 12. Eur. J. Biochem. 1969, 7, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Wagschal, K.; Heng, C.; Lee, C.C.; Robertson, G.H.; Orts, W.J.; Wong, D.W.S. Purification and characterization of a glycoside hydrolase family 43 β-xylosidase from Geobacillus thermoleovorans IT-08. Appl. Biochem. Biotechnol. 2009, 155, 304–313. [Google Scholar] [CrossRef]
- Wagschal, K.; Heng, C.; Lee, C.C.; Wong, D.W.S. Biochemical characterization of a novel dual-function arabinofuranosidase/xylosidase isolated from a compost starter mixture. Appl. Microbiol. Biotechnol. 2009, 81, 855–863. [Google Scholar] [CrossRef]
- Rapp, P.; Wagner, F. Production and properties of xylan-degrading enzymes from Cellulomonas uda. Appl. Environ. Microbiol. 1986, 51, 746–752. [Google Scholar]
- Marcolongo, L.; La Cara, F.; Del Monaco, G.; Paixão, S.M.; Alves, L.; Marques, I.P.; Ionata, E. A novel β-xylosidase from Anoxybacillus sp. 3M towards an improved agro-industrial residues saccharification. Int. J. Biol. Macromol. 2019, 122, 1224–1234. [Google Scholar] [CrossRef]
- Wagschal, K.; Jordan, D.B.; Braker, J.D. Catalytic properties of β-d-xylosidase XylBH43 from Bacillus halodurans C-125 and mutant XylBH43-W147G. Process. Biochem. 2012, 47, 366–372. [Google Scholar] [CrossRef]
- Jordan, D.B.; Stoller, J.R.; Lee, C.C.; Chan, V.J.; Wagschal, K. Biochemical characterization of a GH43 β-Xylosidase from Bacteroides ovatus. Appl. Biochem. Biotechnol. 2017, 182, 250–260. [Google Scholar] [CrossRef]
- Hudson, R.C.; Schofield, L.R.; Coolbear, T.; Daniel, R.M.; Morgan, H.W. Purification and properties of an aryl β-xylosidase from a cellulolytic extreme thermophile expressed in Escherichia coli. Biochem. J. 1991, 273, 645–650. [Google Scholar] [CrossRef]
- Fan, Z.; Yuan, L.; Jordan, D.B.; Wagschal, K.; Heng, C.; Braker, J.D. Engineering lower inhibitor affinities in β-d-xylosidase. Appl. Microbiol. Biotechnol. 2010, 86, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Pinphanichakarn, P.; Tangsakul, T.; Thongnumwon, T.; Talawanich, Y.; Thamchaipenet, A. Purification and characterization of β-xylosidase from Streptomyces sp. CH7 and its gene sequence analysis. World J. Microbiol. Biotechnol. 2004, 20, 727–733. [Google Scholar] [CrossRef]
- Vocadlo, D.J.; Wicki, J.; Rupitz, K.; Withers, S.G. A case for reverse protonation: Identification of Glu160 as an acid/base catalyst in Thermoanaerobacterium saccharolyticum β-xylosidase and detailed kinetic analysis of a site-directed mutant. Biochemistry 2002, 41, 9736–9746. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.A.; Kiss, L. Purification and characterization of a recombinant β-d-xylosidase from Thermobifida fusca TM51. Protein J. 2012, 31, 641–650. [Google Scholar] [CrossRef]
- Yin, Y.-R.; Xian, W.-D.; Han, M.-X.; Zhou, E.-M.; Liu, L.; Alkhalifah, D.H.M.; Hozzein, W.N.; Xiao, M.; Li, W.-J. Expression and characterisation of a pH and salt tolerant, thermostable and xylose tolerant recombinant GH43 β-xylosidase from Thermobifida halotolerans YIM 90462T for promoting hemicellulose degradation. Antonie Leeuwenhoek 2019, 112, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Ristroph, D.L.; Humphrey, A.E. The β-xylosidase of Thermomonospora. Biotechnol. Bioeng. 1985, 27, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.L.; McCarthy, A.J. Purification and characterization of a thermostable β-xylosidase from Thermomonospora fusca. J. Gen. Microbiol. 1989, 135, 293–299. [Google Scholar] [CrossRef][Green Version]
- Büttner, R.; Bode, R. Purification and characterization of β-xylosidase activities from the yeast Arxula adeninivorans. J. Basic Microbiol. 1992, 32, 159–166. [Google Scholar] [CrossRef]
- Eneyskaya, E.V.; Ivanen, D.R.; Bobrov, K.S.; Isaeva-Ivanova, L.S.; Shabalin, K.A.; Savel'ev, A.N.; Golubev, A.M.; Kulminskaya, A.A. Biochemical and kinetic analysis of the GH3 family β-xylosidase from Aspergillus awamori X-100. Arch. Biochem. Biophys. 2007, 457, 225–234. [Google Scholar] [CrossRef]
- Kiss, T.; Kiss, L. Purification and characterization of an extracellular β-d-xylosidase from Aspergillus carbonarius. World J. Microbiol. Biotechnol. 2000, 16, 465–470. [Google Scholar] [CrossRef]
- Kitpreechavanich, V.; Hayashi, M.; Nagai, S. Purification and characterization of extracellular β-xylosidase and β-glucosidase from Aspergillus fumigatus. Agric. Biol. Chem. 1986, 50, 1703–1711. [Google Scholar] [CrossRef]
- Semenova, M.V.; Drachevskaya, M.I.; Sinitsyna, O.A.; Gusakov, A.V.; Sinitsyn, A.P. Isolation and properties of extracellular β-xylosidases from fungi Aspergillus japonicus and Trichoderma reesei. Biochemistry 2009, 74, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, N.A.; Tavobilov, I.M.; Bezborodov, A.M. β-Xylosidase from Aspergillus niger 15: Purification and properties. J. Appl. Biochem. 1983, 5, 300–312. [Google Scholar] [PubMed]
- La Grange, D.C.; Pretorius, I.S.; Claeyssens, M.; Van Zyl, W.H. Degradation of xylan to d-xylose by recombinant Saccharomyces cerevisiae coexpressing the Aspergillus niger β-xylosidase (xlnD) and the Trichoderma reesei xylanase II (xyn2) genes. Appl. Environ. Microbiol. 2001, 67, 5512–5519. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.J.; Knoshaug, E.P.; Decker, S.R.; Baker, J.O.; Himmel, M.E.; Adney, W.S. Heterologous expression of Aspergillus niger β-d-xylosidase (XlnD): Characterization on lignocellulosic substrates. Appl. Biochem. Biotechnol. 2008, 146, 57–68. [Google Scholar] [CrossRef]
- van Peij, N.N.; Brinkmann, J.; Vrsanska, M.; Visser, J.; de Graaff, L.H. β-Xylosidase activity, encoded by xlnD, is essential for complete hydrolysis of xylan by Aspergillus niger but not for induction of the xylanolytic enzyme spectrum. Eur. J. Biochem. 1997, 245, 164–173. [Google Scholar] [CrossRef]
- Boyce, A.; Walsh, G. Purification and characterisation of a thermostable β-xylosidase from Aspergillus niger van Tieghem of potential application in lignocellulosic bioethanol production. Appl. Biochem. Biotechnol. 2018, 186, 712–730. [Google Scholar] [CrossRef]
- Kirikyali, N.; Wood, J.; Connerton, I.F. Characterisation of a recombinant β-xylosidase (xylA) from Aspergillus oryzae expressed in Pichia pastoris. AMB Express 2014, 4, 68. [Google Scholar] [CrossRef]
- Chakrabarti, S.K.; Ranu, R.S. Characterization of a β-xylosidase from Aspergillus terreus (IJIRA 6.2). J. Plant Biochem. Biotechnol. 1995, 4, 117–120. [Google Scholar] [CrossRef]
- Andrade, S.d.V.; Polizeli, M.d.L.T.d.M.; Terenzi, H.F.; Jorge, J.A.l. Effect of carbon source on the biochemical properties of β-xylosidases produced by Aspergillus versicolor. Process Biochem. 2004, 39, 1931–1938. [Google Scholar] [CrossRef]
- Bankeeree, W.; Akada, R.; Lotrakul, P.; Punnapayak, H.; Prasongsuk, S. Enzymatic hydrolysis of black liquor xylan by a novel xylose-tolerant, thermostable β-xylosidase from a tropical strain of Aureobasidium pullulans CBS 135684. Appl. Biochem. Biotechnol. 2018, 184, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.R.d.; Carli, S.; Meleiro, L.P.; Rosa, J.C.; Oliveira, A.H.C.d.; Jorge, J.A.; Furriel, R.P.M. A halotolerant bifunctional β-xylosidase/α-l-arabinofuranosidase from Colletotrichum graminicola: Purification and biochemical characterization. Int. J. Biol. Macromol. 2018, 114, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Purification and properties of an extracellular β-xylosidase from a newly isolated Fusarium proliferatum. Bioresour. Technol. 2003, 90, 33–38. [Google Scholar] [CrossRef]
- Saha, B.C. Purification and characterization of an extracellular β-xylosidase from a newly isolated Fusarium verticillioides. J. Ind. Microbiol. Biotechnol. 2001, 27, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Shi, P.; Xu, X.; Qian, L.; Cui, Y.; Xia, M.; Yao, B. High level expression of a novel family 3 neutral β-xylosidase from Humicola insolens Y1 with high tolerance to d-xylose. PLoS ONE 2015, 10, e0117578. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Campayo, V.; Wood, T.M. Purification and characterisation of a β-d-xylosidase from the anaerobic rumen fungus Neocallimastix frontalis. Carbohydr. Res. 1993, 242, 229–245. [Google Scholar] [CrossRef]
- Kirikyali, N.; Connerton, I.F. Heterologous expression and kinetic characterisation of Neurospora crassa β-xylosidase in Pichia pastoris. Enzym. Microb. Technol. 2014, 57, 63–68. [Google Scholar] [CrossRef]
- Terrasan, C.R.F.; Guisan, J.M.; Carmona, E.C. Xylanase and β-xylosidase from Penicillium janczewskii: Purification, characterization and hydrolysis of substrates. Electron. J. Biotechnol. 2016, 23, 54–62. [Google Scholar] [CrossRef]
- Ye, Y.; Li, X.; Zhao, J. Production and characteristics of a novel xylose- and alkali-tolerant GH 43 β-xylosidase from Penicillium oxalicum for promoting hemicellulose degradation. Sci. Rep. 2017, 7, 11600. [Google Scholar] [CrossRef]
- Knob, A.; Carmona, E.C. Cell-associated acid β-xylosidase production by Penicillium sclerotiorum. N. Biotechnol. 2009, 26, 60–67. [Google Scholar] [CrossRef]
- Nieto-Domínguez, M.; de Eugenio, L.I.; Barriuso, J.; Prieto, A.; Fernández de Toro, B.; Canales-Mayordomo, Á.; Martínez, M.J. Novel pH-stable glycoside hydrolase family 3 β-xylosidase from Talaromyces amestolkiae: An Enzyme displaying regioselective transxylosylation. Appl. Environ. Microbiol. 2015, 81, 6380–6392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Z.; Jia, H.; Yang, Y.; Yan, Q.; Jiang, Z.; Teng, C. Secretory expression of a β-xylosidase gene from Thermomyces lanuginosus in Escherichia coli and characterization of its recombinant enzyme. Lett. Appl. Microbiol. 2012, 55, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Yao, H.J.; Cho, Y.t. Effective induction, purification and characterization of Trichoderma koningii G-39 β-xylosidase with high transferase activity. Biotechnol. Appl. Biochem. 2000, 31, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Dekker, R.F. Bioconversion of hemicellulose: Aspects of hemicellulase production by Trichoderma reesei QM 9414 and enzymic saccharification of hemicellulose. Biotechnol. Bioeng. 1983, 25, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, K.; Puls, J. Characteristics of Trichoderma reesei β-xylosidase and its use in the hydrolysis of solubilized xylans. Appl. Microbiol. Biotechnol. 1988, 28, 425–432. [Google Scholar] [CrossRef]
- Chinen, I.; Oouchi, K.; Tamaki, H.; Fukuda, N. Purification and properties of thermostable β-xylosidase from immature stalks of Saccharum officinarum L. (sugar cane). J. Biochem. 1982, 92, 1873–1881. [Google Scholar] [CrossRef]
- Zhou, J.; Bao, L.; Chang, L.; Zhou, Y.; Lu, H. Biochemical and kinetic characterization of GH43 β-d-xylosidase/α-l-arabinofuranosidase and GH30 α-l-arabinofuranosidase/β-d-xylosidase from rumen metagenome. J. Ind. Microbiol. Biotechnol. 2012, 39, 143–152. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Zhao, L.; Pei, J.; Wang, Z.; Xiao, W. Highly efficient biotransformation of Astragaloside IV to Cycloastragenol by sugar-stimulated β-glucosidase and β-xylosidase from Dictyoglomus thermophilum. J. Microbiol. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Bachmann, S.L.; McCarthy, A.J. Purification and cooperative activity of enzymes constituting the xylan-degrading system of Thermomonospora fusca. Appl. Environ. Microbiol. 1991, 57, 2121–2130. [Google Scholar]
| Family (GH) | Total Number of β-xylosidase Sequences | Clan | Overall Fold of the Catalytic Domain | Catalytic Mechanism † | Nucleophile | General Acid/Base |
|---|---|---|---|---|---|---|
| ‡ 1 | 2 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 3 | 103 | n.a. # | (β/α)8 TIM-barrel | Retention | Asp | Glu |
| 5 | 1 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 30 | 4 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 39 | 24 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 43 | 96 | F | 5-bladed β-propeller | Inversion | Asp § | Glu |
| 51 | 2 | A | (β/α)8 TIM-barrel | Retention | Glu | Glu |
| 52 | 11 | O | (α/α)6-barrel | Retention | Glu | Asp |
| ‡ 54 | 2 | n.a. # | β-sandwich % | Retention | Glu % | Asp % |
| ‡ 116 | 1 | O | (α/α)6-barrel | Retention | Glu | Asp |
| 120 | 2 | n.a. # | right-handed parallel β-helix | Retention | Asp | Glu |
| Organism | GH Family | d-xylose Concentration (mM) | Inhibition (%) | Reference |
|---|---|---|---|---|
| Bacteria: | ||||
| Bacillus halodurans C-125 | GH39 | 200 | 0 | [85] |
| Bacillus subtilis M015 | GH43_11 | 20 | 45 | [86] |
| Corynebacterium alkanolyticum ATCC 21511 | GH3 | 200 | 70 | [87] |
| Dictyoglomus thermophilum DSM 3960 | GH39 | 3000 | 40 | [84] |
| Geobacillus sp. WSUCF1 | GH39 | 300 | 50 | [88] |
| Geobacillus thermodenitrificans NG80-2 | GH39 | 400 | 50 | [89] |
| Geobacillus thermodenitrificans NG80-2 | GH43 | 300 | 50 | [89] |
| Geobacillus thermodenitrificans NG80-2 | GH52 | 600 | 50 | [89] |
| Lactobacillus brevis ATCC 14869 | GH43_11 | 100 | 20 | [90] |
| Lactobacillus brevis ATCC 14869 | GH43_12 | 100 | 66 | [90] |
| Massilia sp. RBM26 | GH43_11 | 500 | 50 | [91] |
| Paenibacillus woosongensis KCTC 3953 | GH43_35 | 100 | 25 | [92] |
| Selenomonas ruminantium GA192 | GH43_11 | 40 | 57 | [93] |
| Sphingobacterium sp. HP455 | GH43_1 | 247 | 50 | [94] |
| Thermoanaerobacterium saccharolyticum JW/SL-YS485 | GH120 | 200 | 30 | [75] |
| Thermotoga petrophila DSM 13995 | GH3 | 150 | 50 | [95] |
| Thermotoga thermarum DSM 5069 | GH3 | 1000 | 50 | [96] |
| Fungi: | ||||
| Aspergillus nidulans CECT2544 | n.a. # | 25 | 44 | [97] |
| Aspergillus niger 11 | n.a. # | 10 | 50 | [98] |
| Aspergillus niger ADH-11 | GH3 | 12 | 50 | [99] |
| Aureobasidium pullulans CBS 58475 | n.a. # | 6,6 | 42 | [100] |
| Candida utilis IFO 0639 | n.a. # | 300 | 0 | [101] |
| Humicola grisea var. thermoidea | GH43_1 | 603 | 50 | [102] |
| Humicola insolens Y1 | GH43_1 | 79 | 50 | [103] |
| Humicola insolens Y1 | GH43_11 | 292 | 50 | [103] |
| Paecilomyces thermophila J18 | n.a. # | 139 | 50 | [104] |
| Phanerochaete chrysosporium BKM-F-1767 | GH43_14 | 50 | 70 | [43] |
| Pseudozyma hubeiensis NCIM 3574 | n.a. # | 75 | 50 | [105] |
| Rhizophlyctis rosea Fischer NBRC 105426 | GH43_1 | 100 | 49 | [106] |
| Scytalidium thermophilum 77.7.8 | n.a. # | 200 | 0 | [107] |
| Trichoderma harzianum C | n.a. # | 2 | 100 | [83] |
| Trichoderma reesei QM 9414 | GH3 | 53 | 80 | [108] |
| Metagenomes: | ||||
| Synthetic metagenome | GH43_1 | 20 | 44 | [109] |
| Uncultured rumen metagenome | GH3 | 5 | 27 | [110] |
| Yak rumen metagenome (RuBg3A §) | GH3 | 5 | 18 | [111] |
| Yak rumen metagenome (RuBg3B §) | GH3 | 5 | 3 | [111] |
| Organism | GH Family | Inhibition Constant (Ki, mM) | Reference |
|---|---|---|---|
| Bacteria: | |||
| Alkaliphilus metalliredigens QYMF | GH43_11 | 16.2 | [18] |
| Anoxybacillus sp. 3M | GH52 | 21.3 | [117] |
| Bacillus halodurans C-125 | GH43_11 | 62.3 | [118] |
| Bacillus pumilus 12 | n.a. # | 26.2 | [113] |
| Bacillus pumilus IPO | GH43_11 | 70 | [18] |
| Bacillus subtilis subsp. subtilis str. 168 | GH43_11 | 15.6 | [18] |
| Bacteroides ovatus V975 | GH43_1 | 6.6 | [119] |
| Caldocellum saccharolyticum Tp8T6.3.3.1 | n.a. # | 40.0 | [120] |
| Cellulomonas uda | n.a. # | 650.0 | [116] |
| Enterobacter sp. | GH43_11 | 79.9 | [72] |
| Geobacillus thermoleovorans IT-08 | GH43_12 | 76.0 | [114] |
| Lactobacillus brevis ATCC 367 | GH43_11 | 30.1 | [18] |
| Selenomonas ruminantium GA192 | GH43_11 | 6.24 | [121] |
| Streptomyces sp. CH7 | GH3 | 40.0 | [122] |
| Thermoanaerobacterium saccharolyticum B6A-RI | GH39 | 20 | [123] |
| Thermobifida fusca TM51 | GH43_11 | 67.0 | [124] |
| Thermobifida halotolerans YIM 90462T | GH43_11 | 43.8 | [125] |
| Thermomonospora | n.a. # | 35-100 | [126] |
| Thermomonospora fusca BD21 | n.a. # | 19 | [127] |
| Fungi: | |||
| Arxula adeninivorans SBUG 724 | n.a. # | 5.8 | [128] |
| Aspergillus awamori X-100 | GH3 | 7.7 | [129] |
| Aspergillus carbonarius KLU-93 | n.a. # | 1.9 | [130] |
| Aspergillus fumigatus | n.a. # | 4.5 | [131] |
| Aspergillus japonicus | GH3 | 2.9 | [132] |
| Aspergillus niger 15 | n.a. # | 2.9 | [133] |
| Aspergillus niger 90196 | GH3 | 8.3 | [134] |
| Aspergillus niger ATCC 10864 | GH3 | 3.3 | [135] |
| Aspergillus niger NW147 (xlnD I §) | GH3 | 9.8 | [136] |
| Aspergillus niger NW147 (xlnD II §) | GH3 | 13.2 | [136] |
| Aspergillus niger van Tieghem (DSM 22593) | GH3 | 7.5 | [137] |
| Aspergillus oryzae KBN616 | GH3 | 2.7 | [138] |
| Aspergillus terreus IJIRA 6.2 | n.a. # | 10.5 | [139] |
| Aspergillus versicolor (xylose-induced) | n.a. # | 5.3 | [140] |
| Aspergillus versicolor (xylan-induced) | n.a. # | 2.0 | [140] |
| Aureobasidium pullulans CBS 135684 | n.a. # | 18.0 | [141] |
| Colletotrichum graminicola | GH3 | 3.3 | [142] |
| Fusarium proliferatum NRRL 26517 | n.a. # | 5.0 | [143] |
| Fusarium verticillioides NRRL 26518 | n.a. # | 6.0 | [144] |
| Humicola insolens Y1 | GH3 | 29.0 | [145] |
| Neocallimastix frontalis RK 21 | n.a. # | 4.0 | [146] |
| Neurospora crassa ST A (74 A) | GH3 | 1.7 | [147] |
| Penicillium janczewskii CRM 1348 | n.a. # | 6 | [148] |
| Penicillium oxalicum 114-2 | GH43 | 28.1 | [149] |
| Penicillium sclerotiorum | n.a. # | 28.7 | [150] |
| Talaromyces amestolkiae | GH3 | 1.7 | [151] |
| Talaromyces emersonii | GH3 | 1.3 | [112] |
| Thermomyces lanuginosus CAU44 | GH43_1 | 63.0 | [152] |
| Trichoderma koningii G-39 | n.a. # | 5.0 | [153] |
| Trichoderma reesei (βXTR §) | GH3 | 2.4 | [112] |
| Trichoderma reesei | GH3 | 1.4 | [132] |
| Trichoderma reesei QM 9414 | n.a. # | 11.0 | [154] |
| Trichoderma reesei RUT C30 | n.a. # | 2.3 | [155] |
| Trichoderma reesei RUT C30 | n.a. # | 2.4 | [24] |
| Plant: | |||
| Saccharum officinarum L. | n.a. # | 8.0 | [156] |
| Metagenomes: | |||
| Compost starter | GH43 | 145.0 | [115] |
| Mixed microorganism (RS223-BX §) | GH43_1 | 3.4 | [19] |
| Uncultured rumen bacterium | GH30_2 | 10.6 | [157] |
| Uncultured rumen bacterium | GH43_1 | 76.0 | [157] |
| Organism | GH Family | l-arabinose Concentration (mM) | Inhibition (%) | Reference |
|---|---|---|---|---|
| Bacteria: | ||||
| Bacillus pumilus 12 | n.a. # | 50 | 21 | [113] |
| Caldocellum saccharolyticum Tp8T6.3.3.1 | n.a. # | 50 | 15 | [120] |
| Corynebacterium alkanolyticum ATCC 21511 | GH3 | 200 | 40 | [87] |
| Lactobacillus brevis ATCC 14869 | GH43_11 | 100 | 39 | [90] |
| Lactobacillus brevis ATCC 14869 | GH43_12 | 100 | 38 | [90] |
| Paenibacillus woosongensis KCTC 3953 | GH43_35 | 100 | 40 | [92] |
| Selenomonas ruminantium GA192 | GH43_11 | 80 | 61 | [93] |
| Fungi: | ||||
| Aspergillus niger 11 | n.a. # | 25 | 10 | [98] |
| Aspergillus niger van Tieghem (DSM 22593) | GH3 | 200 | 30 | [137] |
| Colletotrichum graminicola | GH3 | 50 | 15 | [142] |
| Penicillium oxalicum 114-2 | GH43 | 20 | 11 | [149] |
| Phanerochaete chrysosporium BKM-F-1767 | GH43_14 | 50 | 70 | [43] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohman, A.; Dijkstra, B.W.; Puspaningsih, N.N.T. β-Xylosidases: Structural Diversity, Catalytic Mechanism, and Inhibition by Monosaccharides. Int. J. Mol. Sci. 2019, 20, 5524. https://doi.org/10.3390/ijms20225524
Rohman A, Dijkstra BW, Puspaningsih NNT. β-Xylosidases: Structural Diversity, Catalytic Mechanism, and Inhibition by Monosaccharides. International Journal of Molecular Sciences. 2019; 20(22):5524. https://doi.org/10.3390/ijms20225524
Chicago/Turabian StyleRohman, Ali, Bauke W. Dijkstra, and Ni Nyoman Tri Puspaningsih. 2019. "β-Xylosidases: Structural Diversity, Catalytic Mechanism, and Inhibition by Monosaccharides" International Journal of Molecular Sciences 20, no. 22: 5524. https://doi.org/10.3390/ijms20225524
APA StyleRohman, A., Dijkstra, B. W., & Puspaningsih, N. N. T. (2019). β-Xylosidases: Structural Diversity, Catalytic Mechanism, and Inhibition by Monosaccharides. International Journal of Molecular Sciences, 20(22), 5524. https://doi.org/10.3390/ijms20225524






