Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications
Abstract
:1. Introduction
2. Structural Diversity of Biocompatible and Ecologically Friendly Cationic Surfactants
2.1. Gemini Surfactants
2.2. Ionic Liquids
2.3. Cationic QA Surfactants Containing a Natural Moiety
2.3.1. Pyrimidinophanes: Macrocycles with Nucleotide/Nucleoside Moiety
2.3.2. Lipoaminoacids
2.3.3. Other QASc Containing Natural Moiety: Peptides, Diterpenoids
3. Self-Assembling Strategies for Construction of Soft Nanomaterials for Biomedical Application
3.1. Aggregates of Oppositely Charged Surfactants: Catanionic Systems
3.2. QASs in Role of Stabilizing Agents
3.2.1. Cationic Liposomes
3.2.2. Nanoemulsions
3.3. QASs in Role of Inorganic Nanomaterial Synthesis
4. Quaternary Ammonium Surfactants in Pharmaceutical Applications
4.1. Delivery of Small Drug Molecules
4.1.1. Factors Determining the Solubilization Efficacy
4.1.2. Drug-Amphiphile Interactions
4.2. Protein and Peptide Delivery and Peptides for Drug Delivery
4.2.1. Protein and Peptide Delivery by Cationic Surfactants
4.2.2. Drug Delivery by Quaternary Ammonium Containing Peptides
4.3. Gene Delivery
4.4. Antimicrobial Effects
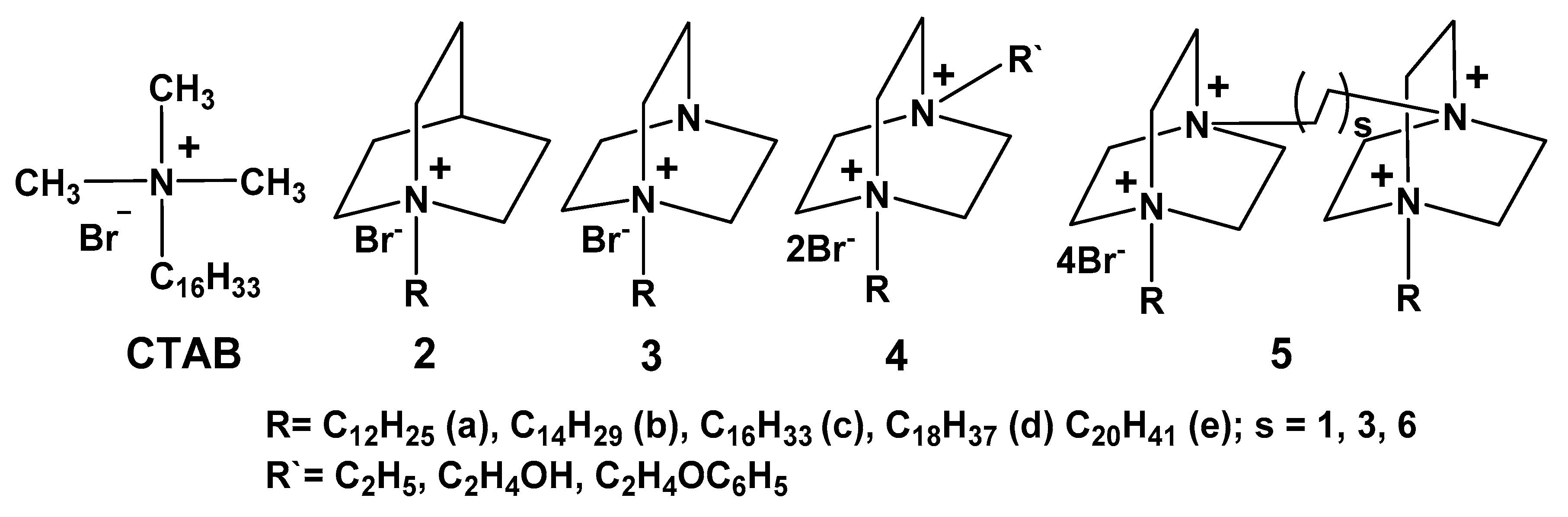
5. Self-Assembled Quaternized Derivatives of 1,4-Diazabicyclo[2.2.2]Octane and Quinuclidine
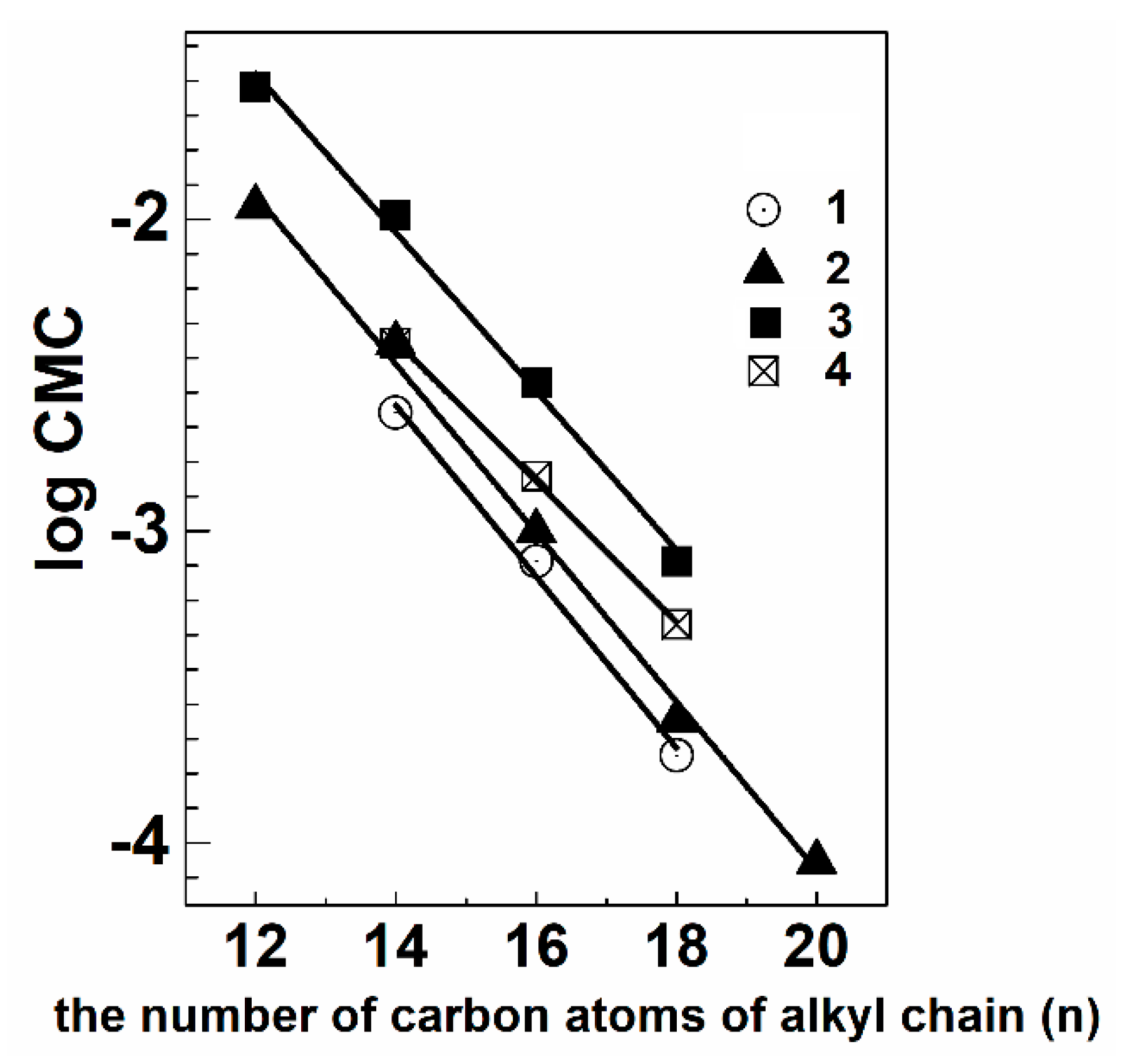
5.1. Aggregation Behavior and Morphology
5.2. Solubilization and Controlled Binding/Release of Hydrophobic Guests
5.3. Supramolecular Catalysis
5.4. Antimicrobial Activity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, W.; He, S.; Wei, M.; Evans, D.G.; Duan, W. Optical pH Sensor with Rapid Response Based on a Fluorescein-Intercalated Layered Double Hydroxide. Adv. Funct. Mater. 2010, 20, 3856–3863. [Google Scholar] [CrossRef]
- Matile, S.; Vargas Jentzsch, A.; Montenegro, J.; Fin, A. Recent synthetic transport systems. Chem. Soc. Rev. 2011, 40, 2453–2474. [Google Scholar] [CrossRef]
- Busseron, E.; Ruff, Y.; Moulin, E.; Giuseppone, N. Supramolecular self-assemblies as functional nanomaterials. Nanoscale 2013, 5, 7098–7140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharova, L.Y.; Pashirova, T.N.; Fernandes, A.R.; Doktorovova, S.; Martins-Gomes, C.; Silva, A.M.; Souto, E.B. Self-assembled quaternary ammonium surfactants for pharmaceuticals and biotechnology. In Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 601–618. [Google Scholar] [CrossRef]
- Paluch, E.; Piecuch, A.; Oblak, E.; Lamch, L.; Wilk, K.A. Antifungal activity of newly synthesized chemodegradable dicephalic-type cationic surfactants. Colloids Surf. B 2018, 164, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Q.; Zhang, Z.; Lu, Z.; Zhao, Y.; Tang, Y. Fluorescent Conjugated Polymer/Quarternary Ammonium Salt Co-assembly Nanoparticles: Applications in Highly Effective Antibacteria and Bioimaging. ACS Appl. Bio. Mater. 2018, 1, 1478–1486. [Google Scholar] [CrossRef]
- Shalabi, K.; Helmy, A.M.; El-Askalany, A.H.; Shahba, M.M. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480. [Google Scholar] [CrossRef]
- Buurma, N.J. Aggregation and reactivity in aqueous solutions of cationic surfactants and aromatic anions across concentration scales. Curr. Opin. Colloid Interface Sci. 2017, 32, 69–75. [Google Scholar] [CrossRef]
- Wang, L.; Quan, P.; Chen, S.H.; Bu, W.; Li, Y.-F.; Wu, X.; Wu, J.; Zhang, L.; Zhao, Y.; Jiang, X.; et al. Stability of Ligands on Nanoparticles Regulating the Integrity of Biological Membranes at the Nano–Lipid Interface. ACS Nano 2019, 13, 8680–8693. [Google Scholar] [CrossRef]
- Botto, C.; Mauro, N.; Amore, E.; Martorana, E.; Giammona, G.; Bondi, M.L. Surfactant effect on the physicochemical characteristics of cationic solid lipid nanoparticles. Int. J. Pharm. 2017, 516, 334–341. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Perez, L.; Pinazo, A.; Tavano, L. Pharmaceutical versatility of cationic niosomes derived from amino acid-based surfactants: Skin penetration behavior and controlled drug release. Int. J. Pharm. 2017, 529, 245–252. [Google Scholar] [CrossRef]
- Lopez-Lopez, M.; Lopez-Cornejo, P.; Martin, V.I.; Ostos, F.J.; Checa-Rodriguez, C.; Prados-Carvajal, R.; Lebron, J.A.; Huertas, P.; Moya, M.L. Importance of hydrophobic interactions in the single-chained cationic surfactant-DNA complexation. J. Colloid Interface Sci. 2018, 521, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, Z.; Song, Y.; Liu, S.; Gao, W.; Qiao, H.; Guo, L.; Wang, J. Investigation on interaction of DNA and several cationic surfactants with different head groups by spectroscopy, gel electrophoresis and viscosity technologies. Chemosphere 2017, 168, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Edeas, M.; Weissig, V. Targeting mitochondria: Strategies, innovations and challenges: The future of medicine will come through mitochondria. Mitochondrion 2013, 13, 389–390. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M.; Ouari, O.; Lopez, M.; Joseph, J.; Zielonka, J.; Dwinell, M.B. A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox Biol. 2018, 14, 316–327. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Kowalczyk, I.; Pakiet, M.; Brycki, B. Biodegradability and aquatic toxicity of new cleavable betainate cationic oligomeric surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef]
- Shahzadi, I.; Asim, M.H.; Dizdarević, A.; Wolf, J.D.; Kurpiers, M.; Matuszczak, B.; Bernkop-Schnürch, A. Arginine-based cationic surfactants: Biodegradable auxiliary agents for the formation of hydrophobic ion pairs with hydrophilic macromolecular drugs. J. Colloid Interface Sci. 2019, 552, 287–294. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Geng, T.; Ju, H.; Duan, S. Synthesis, surface/interfacial properties, and biological activity of amide-based Gemini cationic surfactants with hydroxyl in the spacer group. Colloids Surf. A 2019, 563, 1–10. [Google Scholar] [CrossRef]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef]
- Campbell, R.B.; Ying, B.; Kuesters, G.M.; Hemphill, R. Fighting Cancer: From the Bench to Bedside Using Second Generation Cationic Liposomal Therapeutics. J. Pharm. Sci. 2009, 98, 411–429. [Google Scholar] [CrossRef]
- Schuch, G. EndoTAG-1. MediGene. Curr. Opin. Investig. Drugs 2005, 6, 1259–1265. [Google Scholar]
- Ignatiadis, M.; Zardavas, D.; Lemort, M.; Wilke, C.; Vanderbeeken, M.-C.; D’Hondt, V.; De Azambuja, E.; Gombos, A.; Lebrun, F.; Dal Lago, L.; et al. Feasibility Study of EndoTAG-1, a Tumor Endothelial Targeting Agent, in Combination with Paclitaxel followed by FEC as Induction Therapy in HER2-Negative Breast Cancer. PLoS ONE 2016, 11, e0154009. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.; Dias, K.; Pereira, T.A.; Bernardi, D.S.; Lopez, R.F. Topical delivery of ocular therapeutics: Carrier systems and physical methods. J. Pharm. Pharm. 2014, 66, 507–530. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Silva, A.M.; Garcia, M.L.; Souto, E.B. Current nanotechnology approaches for the treatment and management of diabetic retinopathy. Eur J. Pharm. Biopharm. 2014, 95, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Silva, A.M.; Souto, E.B. Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity. Int. J. Pharm. 2014, 461, 64–73. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Zakharova, L.Y. Self-assembly of mixed systems based on nonionic and carbamate-bearing cationic surfactants as a tool for fabrication of biocompatible nanocontainers. J. Mol. Liq. 2019, 292, 111407. [Google Scholar] [CrossRef]
- Dhawan, V.V.; Nagarsenker, M.S. Catanionic systems in nanotherapeutics – Biophysical aspects and novel trends in drug delivery applications. J. Control. Release 2017, 266, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.; Grossen, P.; Cullis, P.R.; Huwyler, J.; Witzigmann, D. Lipid-Based DNA Therapeutics: Hallmarks of Non-Viral Gene Delivery. ACS Nano 2019, 13, 3754–3782. [Google Scholar] [CrossRef]
- Zhi, D.; Bai, Y.; Yang, J.; Cui, S.; Zhao, Y.; Chen, H.; Zhang, S. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 2018, 253, 117–140. [Google Scholar] [CrossRef]
- Grijalvo, S.; Puras, G.; Zárate, J.; Sainz-Ramos, M.; Qtaish, N.A.L.; López, T.; Mashal, M.; Attia, N.; Díaz, D.; Pons, R.; et al. Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics 2019, 11, 50. [Google Scholar] [CrossRef]
- Damen, M.; Groenen, A.J.J.; van Dongen, S.F.M.; Nolte, R.J.M.; Scholte, B.J.; Feiters, M.C. Transfection by cationic gemini lipids and surfactants. Med. Chem. Comm. 2018, 9, 1404–1425. [Google Scholar] [CrossRef]
- Silva, L.L.; Zapelini, I.W.; Cardoso, D. Catalytic transesterification by hybrid silicas containing CnTA+ surfactants. Catal. Today 2019. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E.J.A.M. Biotechnology. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef]
- Fait, M.E.; Bakas, L.; Garrote, G.L.; Morcelle, S.R.; Saparrat, M.C.N.J.A.M. Biotechnology. Cationic surfactants as antifungal agents. Appl. Microbiol. Biotechnol. 2019, 103, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Le Guenic, S.; Chaveriat, L.; Lequart, V.; Joly, N.; Martin, P. Renewable Surfactants for Biochemical Applications and Nanotechnology. J. Surfactants Deterg. 2019, 22, 5–21. [Google Scholar] [CrossRef]
- Kapitanov, I.V.; Jordan, A.; Karpichev, Y.; Spulak, M.; Perez, L.; Kellett, A.; Kümmerer, K.; Gathergood, N. Synthesis, self-assembly, bacterial and fungal toxicity, and preliminary biodegradation studies of a series of l-phenylalanine-derived surface-active ionic liquids. Green Chem. 2019, 21, 1777–1794. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable Cationic Antibacterial Amphiphiles: Synthesis, Mechanism of Action, and Cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Kirby, A.J.; Camilleri, P.; Engberts, J.B.F.N.; Feiters, M.C.; Nolte, R.J.M.; Söderman, O.; Bergsma, M.; Bell, P.C.; Fielden, M.L.; García Rodríguez, C.L.; et al. Gemini Surfactants: New Synthetic Vectors for Gene Transfection. Angew. Chem. Int. Ed. 2003, 42, 1448–1457. [Google Scholar] [CrossRef]
- Castro, M.; Griffiths, D.; Patel, A.; Pattrick, N.; Kitson, C.; Ladlow, M. Effect of chain length on transfection properties of spermine-based gemini surfactants. Org. Biomol. Chem. 2004, 2, 2814–2820. [Google Scholar] [CrossRef]
- Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Pérez, L. Cationic Surfactants Derived from Lysine: Effects of Their Structure and Charge Type on Antimicrobial and Hemolytic Activities. J. Med. Chem. 2011, 54, 989–1002. [Google Scholar] [CrossRef]
- Al-Dulaymi, M.; Mohammed-Saeid, W.; El-Aneed, A.; Badea, I. Peptide-Modified Gemini Surfactants: Preparation and Characterization for Gene Delivery. Methods Mol. Biol. 2019, 2000, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Pisárčik, M.; Polakovičová, M.; Markuliak, M.; Lukáč, M.; Devínsky, F. Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer. Molecules 2019, 24, 1481. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Biswas, J.; Bhattacharya, S. How does spacer length of imidazolium gemini surfactants control the fabrication of 2D-Langmuir films of silver-nanoparticles at the air-water interface? J. Colloid Interface Sci. 2014, 430, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Adak, S.; Datta, S.; Bhattacharya, S.; Banerjee, R. Role of spacer length in interaction between novel gemini imidazolium surfactants and Rhizopus oryzae lipase. Int. J. Biol. Macromol. 2015, 81, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.; Marron, E.; Martin, V.I.; Moya, M.L.; Lopez-Cornejo, P. Conformational changes of DNA in the presence of 12-s-12 gemini surfactants (s = 2 and 10). Role of the spacer’s length in the interaction surfactant-polynucleotide. Colloids Surf. B 2014, 118, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Alama, T.; Kusamori, K.; Morishita, M. Mechanistic Studies on the Absorption-Enhancing Effects of Gemini Surfactant on the Intestinal Absorption of Poorly Absorbed Hydrophilic Drugs in Rats. Pharmaceutics 2019, 11, 170. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Morais, C.M.; Cruz, A.R.; Silva, S.G.; do Vale, M.L.; Marques, E.F.; de Lima, M.C.; Jurado, A.S. New serine-derived gemini surfactants as gene delivery systems. Eur. J. Pharm. Biopharm. 2015, 89, 347–356. [Google Scholar] [CrossRef]
- Emara, M.M.; Abdel-Salam, F.H.; Ali, R.A.; Turky, A.S.; Elghayish, M.M. Synthesis and Evaluation of Surface Activity of Gemini Borate Surfactants Based on Glucose Moiety. J. Dispers. Sci. Technol. 2016, 37, 733–742. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A.; Pankratov, V.A.; Lukashenko, S.S.; Rizvanova, L.Z.; Konovalov, A.I. Geminal alkylammonium surfactants: Aggregation properties and catalytic activity. Russ. J. Gen. Chem. 2006, 76, 1625–1631. [Google Scholar] [CrossRef]
- Zana, R. Dimeric (gemini) surfactants: Effect of the spacer group on the association behavior in aqueous solution. J. Colloid Interface Sci. 2002, 248, 203–220. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Yackevich, E.I.; Lukashenko, S.S.; Zakharova, L.Y.; Konovalov, A.I. Solubilization and catalytic behavior of micellar system based on gemini surfactant with hydroxyalkylated head group. J. Mol. Liq. 2012, 169, 106–109. [Google Scholar] [CrossRef]
- Łuczyński, J.; Frąckowiak, R.; Włoch, A.; Kleszczyńska, H.; Witek, S. Gemini ester quat surfactants and their biological activity. Cell. Mol. Biol. Lett. 2013, 18, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Tehrani-Bagha, A.R.; Holmberg, K.; van Ginkel, C.G.; Kean, M. Cationic gemini surfactants with cleavable spacer: Chemical hydrolysis, biodegradation, and toxicity. J. Colloid Interface Sci. 2015, 449, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Casal-Dujat, L.; Griffiths, P.C.; Rodríguez-Abreu, C.; Solans, C.; Rogers, S.; Pérez-García, L. Nanocarriers from dicationic bis-imidazolium amphiphiles and their interaction with anionic drugs. J. Mater. Chem. B 2013, 1, 4963–4971. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Ge, L.; Yu, L.; Liu, Z.; Guo, R. Micellization behavior of the ionic liquid lauryl isoquinolinium bromide in aqueous solution. Colloid Polym. Sci. 2014, 292, 1111–1120. [Google Scholar] [CrossRef]
- Ping, A.; Geng, P.; Zhang, J.; Liu, J.; Sun, D.; Zhang, X.; Li, Q.; Liu, J.; Wei, X. Rheological Behavior of Aqueous Solutions of An Ionic Liquid As A Surfactant. Soft Matter 2014, 12, 326–333. [Google Scholar] [CrossRef]
- Tourne-Peteilh, C.; Coasne, B.; In, M.; Brevet, D.; Devoisselle, J.M.; Vioux, A.; Viau, L. Surfactant behavior of ionic liquids involving a drug: From molecular interactions to self-assembly. Langmuir 2014, 30, 1229–1238. [Google Scholar] [CrossRef]
- Jiao, J.; Han, B.; Lin, M.; Cheng, N.; Yu, L.; Liu, M. Salt-free catanionic surface active ionic liquids 1-alkyl-3-methylimidazolium alkylsulfate: Aggregation behavior in aqueous solution. J. Colloid Interface Sci. 2013, 412, 24–30. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Wang, J.; Li, Z.; Zhang, S. The first evidence for unilamellar vesicle formation of ionic liquids in aqueous solutions. Chem. Commun. 2013, 49, 5222–5224. [Google Scholar] [CrossRef] [Green Version]
- Mester, P.; Wagner, M.; Rossmanith, P. Antimicrobial effects of short chained imidazolium-based ionic liquids-influence of anion chaotropicity. Ecotoxicol. Env. Saf. 2015, 111, 96–101. [Google Scholar] [CrossRef]
- Rodrigues, M.; Calpena, A.C.; Amabilino, D.B.; Ramos-López, D.; Lapuentee, J.; Pérez-García, L. Water-soluble gold nanoparticles based on imidazolium gemini amphiphiles incorporating piroxicam. RSC. Adv. 2014, 4, 9279–9287. [Google Scholar] [CrossRef]
- Doktorovova, S.; Silva, A.M.; Gaivao, I.; Souto, E.B.; Teixeira, J.P.; Martins-Lopes, P. Comet assay reveals no genotoxicity risk of cationic solid lipid nanoparticles. J. Appl. Toxicol. 2014, 34, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V. Chemistry of pyrimidinophanes: Synthesis and applications: A review from 1990 until recently. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 1–22. [Google Scholar] [CrossRef]
- Kharlamov, S.V.; Voronin, M.A.; Semenov, V.E.; Gabdrakhmanov, D.R.; Strobykina, A.S.; Nikolaev, A.E.; Reznik, V.S.; Zakharova, L.Y.; Konovalov, A.I. Tunable biomimetic systems based on a novel amphiphilic pyrimidinophane and a helper nonionic surfactant. Colloids Surf. B 2013, 111, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.; Syakaev, V.; Voronin, M.; Semenov, V.; Valeeva, F.; Ibragimova, A.; Bilalov, A.; Giniyatullin, R.; Latypov, S.; Reznik, V.; et al. New self-assembling systems based on bola-type pyrimidinic surfactants. J. Colloid Interface Sci. 2010, 342, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.Y.; Semenov, V.E.; Syakaev, V.V.; Voronin, M.A.; Gabdrakhmanov, D.R.; Valeeva, F.G.; Mikhailov, A.S.; Voloshina, A.D.; Reznik, V.S.; Latypov, S.K.; et al. Amphiphilic macrocycles bearing biofragment: Molecular design as factor controlling self-assembly. Mater. Biol. Appl. 2014, 38, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Voronin, M.A.; Gabdrakhmanov, D.R.; Semenov, V.E.; Valeeva, F.G.; Mikhailov, A.S.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y.; Reznik, V.S.; Konovalov, A.I. Novel bolaamphiphilic pyrimidinophane as building block for design of nanosized supramolecular systems with concentration-dependent structural behavior. ACS Appl. Mater. Interfaces 2011, 3, 402–409. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Valeeva, F.G.; Nikolaev, A.E.; Giniyatullin, R.K.; Semenov, V.E.; Reznik, V.S.; Zakharova, L.Y. Self-organization of oligomeric amphiphiles with pyrimidine moieties: The role of the structural factor. J. Struct. Chem. 2014, 55, 1548–1555. [Google Scholar] [CrossRef]
- Tavano, L.; Pinazo, A.; Abo-Riya, M.; Infante, M.R.; Manresa, M.A.; Muzzalupo, R.; Pérez, L. Cationic vesicles based on biocompatible diacyl glycerol-arginine surfactants: Physicochemical properties, antimicrobial activity, encapsulation efficiency and drug release. Colloids Surf. B 2014, 120, 160–167. [Google Scholar] [CrossRef]
- Perez, L.; Pinazo, A.; Teresa Garcia, M.; Lozano, M.; Manresa, A.; Angelet, M.; Pilar Vinardell, M.; Mitjans, M.; Pons, R.; Rosa Infante, M. Cationic surfactants from lysine: Synthesis, micellization and biological evaluation. Eur. J. Med. Chem. 2009, 44, 1884–1892. [Google Scholar] [CrossRef]
- Tavano, L.; Infante, M.R.; Abo Riya, M.; Pinazo, A.; Vinardell, M.P.; Mitjans, M.; Manresa, M.A.; Perez, L. Role of aggregate size in the hemolytic and antimicrobial activity of colloidal solutions based on single and gemini surfactants from arginine. Soft Matter 2013, 9, 306–319. [Google Scholar] [CrossRef]
- Teixeira, R.S.; Cova, T.F.; Silva, S.M.; Oliveira, R.; do Vale, M.L.; Marques, E.F.; Pais, A.A.; Veiga, F.J. Novel serine-based gemini surfactants as chemical permeation enhancers of local anesthetics: A comprehensive study on structure-activity relationships, molecular dynamics and dermal delivery. Eur. J. Pharm. Biopharm. 2015, 93, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bordes, R.; Holmberg, K. Amino acid-based surfactants - do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.R.; del Carmen Moran, M.; Mitjans, M.; Perez, L.; Ramos, D.; de Lapuente, J.; Pilar Vinardell, M. Lysine-based surfactants in nanovesicle formulations: The role of cationic charge position and hydrophobicity in in vitro cytotoxicity and intracellular delivery. Nanotoxicology 2014, 8, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.R.; Moran, M.C.; Mitjans, M.; Martinez, V.; Perez, L.; Vinardell, M.P. New cationic nanovesicular systems containing lysine-based surfactants for topical administration: Toxicity assessment using representative skin cell lines. Eur. J. Pharm. Biopharm. 2013, 83, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Calejo, M.T.; Cardoso, A.M.; Marques, E.F.; Araujo, M.J.; Kjoniksen, A.L.; Sande, S.A.; de Lima, M.C.; Jurado, A.S.; Nystrom, B. In vitro cytotoxicity of a thermoresponsive gel system combining ethyl(hydroxyethyl) cellulose and lysine-based surfactants. Colloids Surf. B 2013, 102, 682–686. [Google Scholar] [CrossRef]
- Teixeira, R.S.; Cova, T.F.; Silva, S.M.; Oliveira, R.; Araujo, M.J.; Marques, E.F.; Pais, A.A.; Veiga, F.J. Lysine-based surfactants as chemical permeation enhancers for dermal delivery of local anesthetics. Int. J. Pharm. 2014, 474, 212–222. [Google Scholar] [CrossRef]
- Akong, F.O.; Pasc, A.; Emo, M.; Gérardin-Charbonnier, C. A supramolecular hydrogel based on an original pseudopeptidic catanionic surfactant. New J. Chem. 2013, 37, 559–562. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Voronin, M.A.; Zakharova, L.Y.; Konovalov, A.I.; Khaybullin, R.N.; Strobykina, I.Y.; Kataev, V.E.; Faizullin, D.A.; Gogoleva, N.E.; Konnova, T.A.; et al. Supramolecular design of biocompatible nanocontainers based on amphiphilic derivatives of a natural compound isosteviol. Phys. Chem. Chem. Phys. 2013, 15, 16725–16735. [Google Scholar] [CrossRef]
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef]
- Gonçalves Lopes, R.C.F.; Silvestre, O.F.; Faria, A.R.; do Vale, M.L.C.; Marques, E.F.; Nieder, J.B. Surface charge tunable catanionic vesicles based on serine-derived surfactants as efficient nanocarriers for the delivery of the anticancer drug doxorubicin. Nanoscale 2019, 11, 5932–5941. [Google Scholar] [CrossRef]
- Kumar, A.; Chen, F.; Mozhi, A.; Zhang, X.; Zhao, Y.; Xue, X.; Hao, Y.; Wang, P.C.; Liang, X.J. Innovative pharmaceutical development based on unique properties of nanoscale delivery formulation. Nanoscale 2013, 5, 8307–8325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, H.; Holm, R.; Mullertz, A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int. J. Pharm. 2013, 453, 215–224. [Google Scholar] [CrossRef]
- Lépori, C.M.O.; Correa, N.M.; Silber, J.J.; Falcone, R.D.; López-López, M.; Moyá, M.L. Use of Ionic Liquids-like Surfactants for the Generation of Unilamellar Vesicles with Potential Applications in Biomedicine. Langmuir 2019. [Google Scholar] [CrossRef]
- Teixeira, H.F.; Bruxel, F.; Fraga, M.; Schuh, R.S.; Zorzi, G.K.; Matte, U.; Fattal, E. Cationic nanoemulsions as nucleic acids delivery systems. Int. J. Pharm. 2017, 534, 356–367. [Google Scholar] [CrossRef]
- Shaban, S.M.; Abd-Elaal, A.A. Studying the silver nanoparticles influence on thermodynamic behavior and antimicrobial activities of novel amide Gemini cationic surfactants. Mater. Sci. Eng. 2017, 76, 871–885. [Google Scholar] [CrossRef]
- Fameau, A.L.; Zemb, T. Self-assembly of fatty acids in the presence of amines and cationic components. Adv. Colloid Interface Sci. 2014, 207, 43–64. [Google Scholar] [CrossRef]
- Oh, H.; Lu, A.X.; Javvaji, V.; DeVoe, D.L.; Raghavan, S.R. Light-Directed Self-Assembly of Robust Alginate Gels at Precise Locations in Microfluidic Channels. ACS Appl. Mater. Interfaces 2016, 8, 17529–17538. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, J. A light-responsive organofluid based on reverse worm-like micelles formed from an equi-charged, mixed, anionic gemini surfactant with an azobenzene spacer and a cationic conventional surfactant. Soft Matter 2016, 12, 4044–4051. [Google Scholar] [CrossRef]
- Pucci, C.; Perez, L.; La Mesa, C.; Pons, R. Characterization and stability of catanionic vesicles formed by pseudo-tetraalkyl surfactant mixtures. Soft Matter 2014, 10, 9657–9667. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ou, K.; Yang, Z.; Lin, M.; Dong, Z. Thermodynamic insights and molecular environments into catanionic surfactant systems: Influence of chain length and molar ratio. J. Colloid Interface Sci. 2019, 548, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Tah, B.; Pal, P.; Talapatra, G.B. Interaction of insulin with SDS/CTAB catanionic vesicles. J. Lumin. 2014, 145, 81–87. [Google Scholar] [CrossRef]
- Castagnos, P.; Siqueira-Moura, M.P.; Leme Goto, P.; Perez, E.; Franceschi, S.; Rico-Lattes, I.; Tedesco, A.C.; Blanzat, M. Catanionic vesicles charged with chloroaluminium phthalocyanine for topical photodynamic therapy. In vitro phototoxicity towards human carcinoma and melanoma cell lines. RSC Adv. 2014, 4, 39372–39377. [Google Scholar] [CrossRef]
- Oh, H.; Javvaji, V.; Yaraghi, N.A.; Abezgauz, L.; Danino, D.; Raghavan, S.R. Light-induced transformation of vesicles to micelles and vesicle-gels to sols. Soft Matter 2013, 9, 11576–11584. [Google Scholar] [CrossRef]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; Martinez-Navarrete, G.; Soto-Sánchez, C.; Diaz-Tahoces, A.; Aviles-Trigueros, M.; et al. The influence of the polar head-group of synthetic cationic lipids on the transfection efficiency mediated by niosomes in rat retina and brain. Biomaterials 2016, 77, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Shi, J.; Zhang, C.; Li, M.; Gan, L.; Xu, H.; Yang, X. Co-delivery of thioredoxin 1 shRNA and doxorubicin by folate-targeted gemini surfactant-based cationic liposomes to sensitize hepatocellular carcinoma cells. J. Mater. Chem. B 2014, 2, 4901. [Google Scholar] [CrossRef]
- Saengkrit, N.; Saesoo, S.; Srinuanchai, W.; Phunpee, S.; Ruktanonchai, U.R. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf. B 2014, 114, 349–356. [Google Scholar] [CrossRef]
- Duangjit, S.; Pamornpathomkul, B.; Opanasopit, P.; Rojanarata, T.; Obata, Y.; Takayama, K.; Ngawhirunpat, T. Role of the charge, carbon chain length, and content of surfactant on the skin penetration of meloxicam-loaded liposomes. Int. J. Nanomed. 2014, 9, 2005–2017. [Google Scholar] [CrossRef]
- Pinazo, A.; Petrizelli, V.; Bustelo, M.; Pons, R.; Vinardell, M.P.; Mitjans, M.; Manresa, A.; Perez, L. New cationic vesicles prepared with double chain surfactants from arginine: Role of the hydrophobic group on the antimicrobial activity and cytotoxicity. Colloids Surf. B 2016, 141, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Bazylińska, U.; Zieliński, W.; Kulbacka, J.; Samoć, M.; Wilk, K.A. New diamidequat-type surfactants in fabrication of long-sustained theranostic nanocapsules: Colloidal stability, drug delivery and bioimaging. Colloids Surf. B 2016, 137, 121–132. [Google Scholar] [CrossRef]
- Malik, P.; Singh, M. Study of curcumin antioxidant activities in robust oil–water nanoemulsions. New J. Chem. 2017, 41, 12506–12519. [Google Scholar] [CrossRef]
- Kamimura, M.; Kim, J.O.; Kabanov, A.V.; Bronich, T.K.; Nagasaki, Y. Block ionomer complexes of PEG-block-poly(4-vinylbenzylphosphonate) and cationic surfactants as highly stable, pH responsive drug delivery system. J. Control. Release 2012, 160, 486–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengani, L.C.; Chauhan, A. Extended delivery of an anionic drug by contact lens loaded with a cationic surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Santos, D.L.; Costa, I.; Andreani, T.; Souto, E.B.; Silva, A.M. Cationic solid lipid nanoparticles interfere with the activity of antioxidant enzymes in hepatocellular carcinoma cells. Int. J. Pharm. 2014, 471, 18–27. [Google Scholar] [CrossRef]
- Pisárčik, M.; Lukáč, M.; Jampílek, J.; Bilka, F.; Bilková, A.; Pašková, Ľ.; Devínsky, F.; Horáková, R.; Opravil, T. Silver nanoparticles stabilised with cationic single-chain surfactants. Structure-physical properties-biological activity relationship study. J. Mol. Liq. 2018, 272, 60–72. [Google Scholar] [CrossRef]
- Alea-Reyes, M.E.; González, A.; Calpena, A.C.; Ramos-López, D.; de Lapuente, J.; Pérez-García, L. Gemini pyridinium amphiphiles for the synthesis and stabilization of gold nanoparticles for drug delivery. J. Colloid Interface Sci. 2017, 502, 172–183. [Google Scholar] [CrossRef]
- Andreani, T.; Kiill, C.P.; Souza, A.L.R.d.; Fangueiro, J.F.; Fernandes, L.; Doktorovová, S.; Santos, D.L.; Garcia, M.L.; Gremião, M.P.D.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf. B 2014, 123, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Andreani, T.; Silva, A.M.; Souto, E.B. Silica-based matrices: State of the art and new perspectives for therapeutic drug delivery. Biotechnol. Appl. Biochem. 2015. [Google Scholar] [CrossRef]
- Cesaretti, A.; Carlotti, B.; Gentili, P.L.; Clementi, C.; Germani, R.; Elisei, F. Spectroscopic investigation of the pH controlled inclusion of doxycycline and oxytetracycline antibiotics in cationic micelles and their magnesium driven release. J. Phys. Chem. B 2014, 118, 8601–8613. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of hydrophobic dyes in surfactant solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef]
- Mahajan, S.; Sharma, R.; Mahajan, R.K. An investigation of drug binding ability of a surface active ionic liquid: Micellization, electrochemical, and spectroscopic studies. Langmuir 2012, 28, 17238–17246. [Google Scholar] [CrossRef]
- Masrat, R.; Maswal, M.; Dar, A.A. Competitive solubilization of naphthalene and pyrene in various micellar systems. J. Hazard. Mater. 2013, 244–245, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhadoria, A.; Parikh, K.; Yadav, S.K.; Kumar, S.; Aswal, V.K.; Kumar, S. Self-Assembly in Aqueous Oppositely Charged Gemini Surfactants: A Correlation between Morphology and Solubilization Efficacy. J. Phys. Chem. B 2017, 121, 8756–8766. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Takahashi, N.; Yada, S.; Yoshimura, T. Solubilization ability of star-shaped trimeric quaternary ammonium bromide surfactant. J. Mol. Liq. 2019, 291, 111254. [Google Scholar] [CrossRef]
- Choudhary, S.; Talele, P.; Kishore, N. Thermodynamic insights into drug–surfactant interactions: Study of the interactions of naporxen, diclofenac sodium, neomycin, and lincomycin with hexadecytrimethylammonium bromide by using isothermal titration calorimetry. Colloids Surf. B 2015, 132, 313–321. [Google Scholar] [CrossRef]
- Hoque, M.A.; Hossain, M.D.; Khan, M.A. Interaction of cephalosporin drugs with dodecyltrimethylammonium bromide. J. Chem. Thermodyn. 2013, 63, 135–141. [Google Scholar] [CrossRef]
- Grujić, M.; Popović, M.; Popović, G.; Nikolic, K.; Agbaba, D. Protolytic Equilibria of Sartans in Micellar Solutions of Differently Charged Surfactants. J. Pharm. Sci. 2016, 105, 2444–2452. [Google Scholar] [CrossRef]
- Sanan, R.; Kaur, R.; Mahajan, R.K. Micellar transitions in catanionic ionic liquid–ibuprofen aqueous mixtures; effects of composition and dilution. RSC Adv. 2014, 4, 64877–64889. [Google Scholar] [CrossRef]
- Lin, Y.A.; Cheetham, A.G.; Zhang, P.; Ou, Y.C.; Li, Y.; Liu, G.; Hermida-Merino, D.; Hamley, I.W.; Cui, H. Multiwalled nanotubes formed by catanionic mixtures of drug amphiphiles. ACS Nano 2014, 8, 12690–12700. [Google Scholar] [CrossRef]
- Nnyigide, O.S.; Lee, S.-G.; Hyun, K. In Silico Characterization of the Binding Modes of Surfactants with Bovine Serum Albumin. Sci. Rep. 2019, 9, 10643. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Zhou, L.; Yang, L.; Xia, G.; Chen, Z.; Duan, M. Synthesis and binding with BSA of a new gemini surfactant. Colloids Surf. A 2013, 436, 1159–1169. [Google Scholar] [CrossRef]
- Choudhary, S.; Kishore, N. Drug-protein interactions in micellar media: Thermodynamic aspects. J. Colloid Interface Sci. 2014, 413, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Hirose, H.; Tanaka, G.; Pujals, S.; Katayama, S.; Nakase, I.; Futaki, S. Effect of the attachment of a penetration accelerating sequence and the influence of hydrophobicity on octaarginine-mediated intracellular delivery. Mol. Pharm. 2012, 9, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Nam, N.H.; Kumar, A.; Saleh, A.; Shenoy, D.B.; Amiji, M.M.; Lin, X.; Sun, G.; Parang, K. Synthesis and evaluation of tripodal peptide analogues for cellular delivery of phosphopeptides. J. Med. Chem. 2007, 50, 3604–3617. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.G.; Zhang, P.; Lin, Y.A.; Lock, L.L.; Cui, H. Supramolecular nanostructures formed by anticancer drug assembly. J. Am. Chem. Soc. 2013, 135, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhang, P.; Cheetham, A.G.; Walston, J.; Abadir, P.; Cui, H. Dual peptide conjugation strategy for improved cellular uptake and mitochondria targeting. Bioconjug. Chem. 2015, 26, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Szymanski, M.; Favaro, M.; Azzoni, A.R.; Chaud, M.V.; Santana, M.H.; Silva, A.M.; Souto, E.B. Development and characterization of a cationic lipid nanocarrier as non-viral vector for gene therapy. Eur. J. Pharm. Sci. 2015, 66, 78–82. [Google Scholar] [CrossRef]
- Andrzejewska, W.; Wilkowska, M.; Chrabąszczewska, M.; Kozak, M. The study of complexation between dicationic surfactants and the DNA duplex using structural and spectroscopic methods. RSC Adv. 2017, 7, 26006–26018. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.M.; Morais, C.M.; Silva, S.G.; Marques, E.F.; de Lima, M.C.; Jurado, M.A. Bis-quaternary gemini surfactants as components of nonviral gene delivery systems: A comprehensive study from physicochemical properties to membrane interactions. Int. J. Pharm. 2014, 474, 57–69. [Google Scholar] [CrossRef]
- Zakharova, L.; Voronin, M.; Semenov, V.; Gabdrakhmanov, D.; Syakaev, V.; Gogolev, Y.; Giniyatullin, R.; Lukashenko, S.; Reznik, V.; Latypov, S.; et al. Supramolecular Systems Based on Novel Mono- and Dicationic Pyrimidinic Amphiphiles and Oligonucleotides: A Self-Organization and Complexation Study. Chem. Phys. Chem. 2012, 13, 788–796. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Kashapov, R.R.; Vagapova, G.I.; Gabdrakhmanov, D.R.; Vasilieva, E.A. Comparative Study of Aqueous Solutions of Cationic Surfactants: Structure/Activity Relation in Their Aggregation and Solubilization Behavior and Complexation with an Oligonucleotide. Chem. Lett. 2012, 41, 1226–1228. [Google Scholar] [CrossRef]
- Sharma, V.D.; Lees, J.; Hoffman, N.E.; Brailoiu, E.; Madesh, M.; Wunder, S.L.; Ilies, M.A. Modulation of pyridinium cationic lipid-DNA complex properties by pyridinium gemini surfactants and its impact on lipoplex transfection properties. Mol. Pharm. 2014, 11, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.Y.; Gabdrakhmanov, D.R.; Ibragimova, A.R.; Vasilieva, E.A.; Nizameev, I.R.; Kadirov, M.K.; Ermakova, E.A.; Gogoleva, N.E.; Faizullin, D.A.; Pokrovsky, A.G.; et al. Structural, biocomplexation and gene delivery properties of hydroxyethylated gemini surfactants with varied spacer length. Colloids Surf. B 2016, 140, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lukac, M.; Mojzis, J.; Mojzisova, G.; Mrva, M.; Ondriska, F.; Valentova, J.; Lacko, I.; Bukovsky, M.; Devinsky, F.; Karlovska, J. Dialkylamino and nitrogen heterocyclic analogues of hexadecylphosphocholine and cetyltrimetylammonium bromide: Effect of phosphate group and environment of the ammonium cation on their biological activity. Eur. J. Med. Chem. 2009, 44, 4970–4977. [Google Scholar] [CrossRef]
- Sauerová, P.; Pilgrová, T.; Pekař, M.; Hubálek Kalbáčová, M. Hyaluronic acid in complexes with surfactants: The efficient tool for reduction of the cytotoxic effect of surfactants on human cell types. Int. J. Biol. Macromol. 2017, 103, 1276–1284. [Google Scholar] [CrossRef]
- Zupancic, S.; Kocbek, P.; Zariwala, M.G.; Renshaw, D.; Gul, M.O.; Elsaid, Z.; Taylor, K.M.; Somavarapu, S. Design and development of novel mitochondrial targeted nanocarriers, DQAsomes for curcumin inhalation. Mol. Pharm. 2014, 11, 2334–2345. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Ziganshina, A.Y.; Sultanova, E.D.; Lukashenko, S.S.; Kudryashova, Y.R.; Zhiltsova, E.P.; Zakharova, L.Y.; Konovalov, A.I. Supramolecular systems based on calix[4]resorcine with mono-, di-, and tetracationic surfactants: Synergetic structural and solubilization behavior. Colloids Surf. A 2014, 448, 67–72. [Google Scholar] [CrossRef]
- Engel, R.; Ghani, I.; Montenegro, D.; Thomas, M.; Klaritch-Vrana, B.; Castano, A.; Friedman, L.; Leb, J.; Rothman, L.; Lee, H.; et al. Polycationic glycosides. Molecules 2011, 16, 1508–1518. [Google Scholar] [CrossRef]
- Burakova, E.; Kovalev, N.; Zenkova, M.; Vlassov, V.; Silnikov, V. Structure–activity relationships in new polycationic molecules based on two 1,4-diazabicyclo[2.2.2]octanes as artificial ribonucleases. Bioorganic Chem. 2014, 57, 127–131. [Google Scholar] [CrossRef]
- Zhiltsova, E.P.; Lukashenko, S.S.; Pashirova, T.N.; Valeeva, F.G.; Zakharova, L.Y. Self-assembling systems based on diquaternized derivatives of 1,4-diazabicyclo[2.2.2]octane. J. Mol. Liq. 2015. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Lukashenko, S.S.; Zakharov, S.V.; Voloshina, A.D.; Zhiltsova, E.P.; Zobov, V.V.; Souto, E.B.; Zakharova, L.Y. Self-assembling systems based on quaternized derivatives of 1,4-diazabicyclo[2.2.2]octane in nutrient broth as antimicrobial agents and carriers for hydrophobic drugs. Colloids Surf. B 2015, 127, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Burilova, E.A.; Pashirova, T.N.; Lukashenko, S.S.; Sapunova, A.S.; Voloshina, A.D.; Zhiltsova, E.P.; Campos, J.R.; Souto, E.B.; Zakharova, L.Y. Synthesis, biological evaluation and structure-activity relationships of self-assembled and solubilization properties of amphiphilic quaternary ammonium derivatives of quinuclidine. J. Mol. Liq. 2018, 272, 722–730. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zhil’tsova, E.P.; Kashapov, R.R.; Lukashenko, S.S.; Litvinov, A.I.; Kadirov, M.K.; Zakharova, L.Y.; Konovalov, A.I. Supramolecular systems based on 1-alkyl-4-aza-1-azoniabicyclo[2.2.2]octane bromides. Russ. Chem. Bull. 2010, 1745–1752. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Pashirova, T.N.; Kharlamov, S.V.; Ziganshina, A.Y.; Ziltsova, E.P.; Lukashenko, S.S.; Zakharova, L.Y.; Habicher, W.D.; Latypov, S.K.; Konovalov, A.I. Novel self-assembling system based on resorcinarene and cationic surfactant. Phys. Chem. Chem. Phys. 2011, 13, 15891–15898. [Google Scholar] [CrossRef] [Green Version]
- Kharlamov, S.V.; Kashapov, R.R.; Pashirova, T.N.; Zhiltsova, E.P.; Lukashenko, S.S.; Ziganshina, A.Y.; Gubaidullin, A.T.; Zakharova, L.Y.; Gruner, M.; Habicher, W.D.; et al. A Supramolecular Amphiphile Based on Calix[4]resorcinarene and Cationic Surfactant for Controlled Self-Assembly. J. Phys. Chem. C 2013, 117, 20280–20288. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Pashirova, T.N.; Zhiltsova, E.P.; Lukashenko, S.S.; Ziganshina, A.Y.; Zakharova, L.Y. Supramolecular systems based on aminomethylated calix[4]resorcinarene and a cationic surfactant: Catalysts of the hydrolysis of esters of phosphorus acids. Russ. J. Phys. Chem. A 2012, 86, 200–204. [Google Scholar] [CrossRef]
- Zhiltsova, E.P.; Pashirova, T.N.; Kashapov, R.R.; Gaisin, N.K.; Gnezdilov, O.I.; Lukashenko, S.S.; Voloshina, A.D.; Kulik, N.V.; Zobov, V.V.; Zakharova, L.Y.; et al. Alkylated 1,4- diazabicyclo[2.2.2]octanes: Self-association, catalytic properties, and biological activity. Russ. Chem. Bull. 2012, 61, 113–120. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Pashirova, T.N.; Kashapov, R.R.; Zhil’tsova, E.P.; Gaisin, N.K.; Gnezdilov, O.I.; Konov, A.B.; Lukashenko, S.S.; Magdeev, I.M. Catalytic properties of micellar systems based on 4-aza-1-alkyl-1-azoniabicyclo[2.2.2]octane bromides. Kinet. Catal. 2011, 52, 179–185. [Google Scholar] [CrossRef]
- Zhil’tsova, E.P.; Gimranova, R.F.; Lukashenko, S.S.; Pashirova, T.N.; Kharlampidi, K.E.; Zakharova, L.Y. Supramolecular catalytic systems based on alkylated diquaternary 1,4-diazabicyclo[2.2.2]octane derivatives. Kinet. Catal. 2013, 54, 552–558. [Google Scholar] [CrossRef]
- Zhil’tsova, E.P.; Kashapov, R.R.; Zakharova, L.Y.; Lukashenko, S.S.; Timosheva, A.P.; Kasymova, E.M.; Kayupov, A.R.; Burilov, A.R. Alkylated polyethyleneimine-cationic surfactant-calix[4]resorcinarene-chloroform catalytic system. Kinet. Catal. 2012, 53, 231–238. [Google Scholar] [CrossRef]
- Valeeva, F.G.; Kuryashov, D.A.; Zakharov, S.V.; Vagapova, G.I.; Vasilieva, E.A.; Bashkirtseva, N.Y.; Zakharova, L.Y.; Konovalov, A.I. Supramolecular system 4-aza-1-hexadecyl-1-azoniabicyclo[2.2.2]octane bromide-sodium salicylate. Russ. Chem. Bull. 2013, 62, 989–993. [Google Scholar] [CrossRef]
- Gaisin, N.K.; Gnezdilov, O.I.; Pashirova, T.N.; Zhil’tsova, E.P.; Lukashenko, S.S.; Zakharova, L.Y.; Osipova, V.V.; Dzhabarov, V.I.; Galyametdinov, Y.G. Micellar and liquid-crystalline properties of bicyclic fragment-containing cationic surfactant. Colloid J. 2010, 72, 764–770. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Gaysin, N.K.; Gnezdilov, O.I.; Bashirov, F.I.; Kashapov, R.R.; Zhiltsova, E.P.; Pashirova, T.N.; Lukashenko, S.S. Micellization of alkylated 1.4-diazabicyclo[2.2.2]octane by nuclear magnetic resonance technique using pulsed gradient of static magnetic field. J. Mol. Liq. 2012, 167, 89–93. [Google Scholar] [CrossRef]
- Gaynanova, G.A.; Vagapova, G.I.; Valeeva, F.G.; Vasilieva, E.A.; Galkina, I.V.; Zakharova, L.Y.; Sinyashin, O.G. A novel supramolecular catalytic system based on amphiphilic triphenylphosphonium bromide for the hydrolysis of phosphorus acid esters. Colloids Surf. A 2016, 489, 95–102. [Google Scholar] [CrossRef]
- Pashirova, T.; Burilova, E.; Lukashenko, S.; Gaysin, N.; Gnezdilov, O.; Sapunova, A.; Fernandes, A.; Voloshina, A.; Souto, E.B.; Zhiltsova, E.; et al. Nontoxic antimicrobial micellar systems based on mono- and di-cationic Dabco-surfactants and furazolidone: Structure-solubilization properties relationships. J. Mol. Liq. 2019. accepted. [Google Scholar] [CrossRef]
- Zhiltsova, E.P.; Lukashenko, S.S.; Pashirova, T.N.; Zakharova, L.Y.; Konovalov, A.I. Supramolecular catalytic systems based on 1,4-vdiazabicyclo[2.2.2]octane, its alkylated quaternary derivatives and lanthanum nitrate. Russ. Chem. Bull. Int. Ed. 2015, 64, 2690–2696. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zhiltsova, E.P.; Lukashenko, S.S.; Gabdrakhmanov, D.R.; Zakharova, L.Y.; Konovalov, A.I. Mono-, bis- and tetra-quaternary derivatives of 1,4-diazabicyclo[2.2.2]octane: Self-assemble behavior and properties. In Proceedings of the SIS, Coimbra, Portugal; p. 266. Available online: http://www.uc.pt/fctuc/dquimica/sis2014/programme/Poster1 (accessed on 6 November 2019).
- Sun, T.; Shen, J.; Yan, H.; Hao, J.; Hao, A. Stable vesicles assembled by “supramolecular amphiphiles” with double hydrophobic chains. Colloids Surf. A 2012, 414, 41–49. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C. Supramolecular amphiphiles. Chem Soc. Rev. 2011, 40, 94–101. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Hu, W.; Zou, G.; Zhang, Q. Morphology modulation in an azobenzene based supramolecular amphiphiles system. J. Photochem. Photobiol. A. 2012, 245, 28–32. [Google Scholar] [CrossRef]
- Wang, K.; Guo, D.S.; Liu, Y. Temperature-controlled supramolecular vesicles modulated by p-sulfonatocalix[5]arene with pyrene. Chemistry 2010, 16, 8006–8011. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Uhlenheuer, D.A.; Petkau, K.; Brunsveld, L. Combining supramolecular chemistry with biology. Chem. Soc. Rev. 2010, 39, 2817–2826. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X.; Guo, X. Assembly behaviors of calixarene-based amphiphile and supra-amphiphile and the applications in drug delivery and protein recognition. Adv. Colloid Interface Sci. 2019, 269, 187–202. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Gibadullina, E.M.; Burilov, A.R.; Kashapov, R.R.; Zhiltsova, E.P.; Syakaev, V.V.; Habicher, W.D.; Rummeli, M.H.; Latypov, S.K.; Konovalov, A.I.; et al. Amphiphilic O-functionalized calix[4]resocinarenes with tunable structural behavior. RSC Adv. 2014, 4, 9912–9919. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Kharlamov, S.V.; Sultanova, E.D.; Mukhitova, R.K.; Kudryashova, Y.R.; Zakharova, L.Y.; Ziganshina, A.Y.; Konovalov, A.I. Controlling the size and morphology of supramolecular assemblies of viologen-resorcin[4]arene cavitands. Chemistry 2014, 20, 14018–14025. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Reactivity and Catalysis. In Supramolecular Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 55–67. [Google Scholar] [CrossRef]
- Rico-Lattes, I.; Perez, E.; Franceschi-Messant, S.; Lattes, A. Organized molecular systems as reaction media. Comptes Rendus Chim. 2011, 14, 700–715. [Google Scholar] [CrossRef]
- Gilbert, P.; Al-taae, A. Antimicrobial activity of some alkyltrimethylammonium bromides. Lett. Appl. Microbiol. 1985, 1, 101–104. [Google Scholar] [CrossRef]
- Daoud, N.N.D.; Dickinson, N.A.; Gilbert, P. Antibacterial activity and physico-chemical properties of some alkyl-dimethylbenzyl ammonium chlorides. Microbios 1983, 37, 75–85. [Google Scholar]





| Amino Acid | Type of QAS | Type of Aggregates | References |
|---|---|---|---|
| Alanine | Gemini ester quat surfactants | / | [53] |
| Arginine | Alkylated Arg (LAM), gemini alkylated Arg (C6(LA2); C9(LA2), C12(LA2)) | LAM, C6(LA2) -Micelles, C9(LA2), C12(LA2)-vesicles | [72] |
| Diacylglycero Arg | Vesicles | [70] | |
| Lysine | / | Lysine-surfactants in liposomes | [75,76] |
| Lysine-gel based systems | [77,78] | ||
| Serine | / | Serine-gene delivery systems | [48,73] |
| Surfactant | Additives | CMC × 103 (M) (Based on Different Techniques) | Refs | |||||
|---|---|---|---|---|---|---|---|---|
| Tensiometry | Conductometry | Potentiometry | NMR | Fluorimetry | Spectrophotometry | |||
| 2b | 2.5 | 2.85 | 3.0 | 1.5 | ||||
| 2c | 0.85 | 0.6 | 0.94 | 0.8 | ||||
| 2d | 0.2 | 0.24 | 0.3 | 0.3 | ||||
| 3a | 11 | 14 | 16 | 15 | - | [144,149] | ||
| 3b | 4.0 | 3.0 | 3.7 | 3.4 | 4.3 | [144] | ||
| 3c | 1.0 | 1.0 | 1.9 | 0.85 | [144,153] | |||
| 3d | 0.24 | 0.11 | 0.22 | 0.11 | - | [144,149] | ||
| 4a-Et | 26.5 | 28.4 | 29.7 | 28 | [157] | |||
| 4b-Et | 10.3 | 8.5 | 8.4 | 8.1 | 9.5 | [157] | ||
| 4c-Et | 3.0 | 3.1 | 2.0 | - | - | 2.3 | [157] | |
| 4c-EtOH | 2.0 | 2.5 | 3.0 | - | - | - | [138,157] | |
| 4d-Et | 0.80 | 0.83 | 0.98 | 1.1 | [157] | |||
| 5b | 4.0 | 3.0 | 2.0 | 4.2 | 3.6 | [158] | ||
| 5c | 1.5 | 1.7 | 2.3 | 0.8 | 1.7 | [158] | ||
| 5d | 0.5 | 0.5 | 0.15 | 0.29 | 0.2 | [158] | ||
| 3c | CR-1 | 2 | 0.1 | - | - | - | - | [138] |
| 4c-Et | CR-1 | 1.5 | 1.5 | - | - | - | 0.8 | [138] |
| 5c | CR-1 | 1 | 0.4 | - | - | - | - | [138] |
| 3c | CR-1 | 0.4 | 0.1 | 4.9 | 0.45 | - | 5.0 | [145] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, L.Y.; Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. https://doi.org/10.3390/ijms20225534
Zakharova LY, Pashirova TN, Doktorovova S, Fernandes AR, Sanchez-Lopez E, Silva AM, Souto SB, Souto EB. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. International Journal of Molecular Sciences. 2019; 20(22):5534. https://doi.org/10.3390/ijms20225534
Chicago/Turabian StyleZakharova, Lucia Ya., Tatiana N. Pashirova, Slavomira Doktorovova, Ana R. Fernandes, Elena Sanchez-Lopez, Amélia M. Silva, Selma B. Souto, and Eliana B. Souto. 2019. "Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications" International Journal of Molecular Sciences 20, no. 22: 5534. https://doi.org/10.3390/ijms20225534
APA StyleZakharova, L. Y., Pashirova, T. N., Doktorovova, S., Fernandes, A. R., Sanchez-Lopez, E., Silva, A. M., Souto, S. B., & Souto, E. B. (2019). Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. International Journal of Molecular Sciences, 20(22), 5534. https://doi.org/10.3390/ijms20225534









