When Does Alzheimer′s Disease Really Start? The Role of Biomarkers
Abstract
:1. The Classical Diagnostic Criteria
2. The Inclusion of Biomarkers in the Diagnostic Criteria
3. Alzheimer’s Disease Biomarkers
3.1. Biomarkers in Cerebrospinal Fluid
Aβ42 and Tau as Biomarkers
3.2. Imaging Biomarkers in AD
3.2.1. PiB-PET
3.2.2. FDG-PET
3.2.3. Structural and Functional Magnetic Resonance Imaging (MRI)
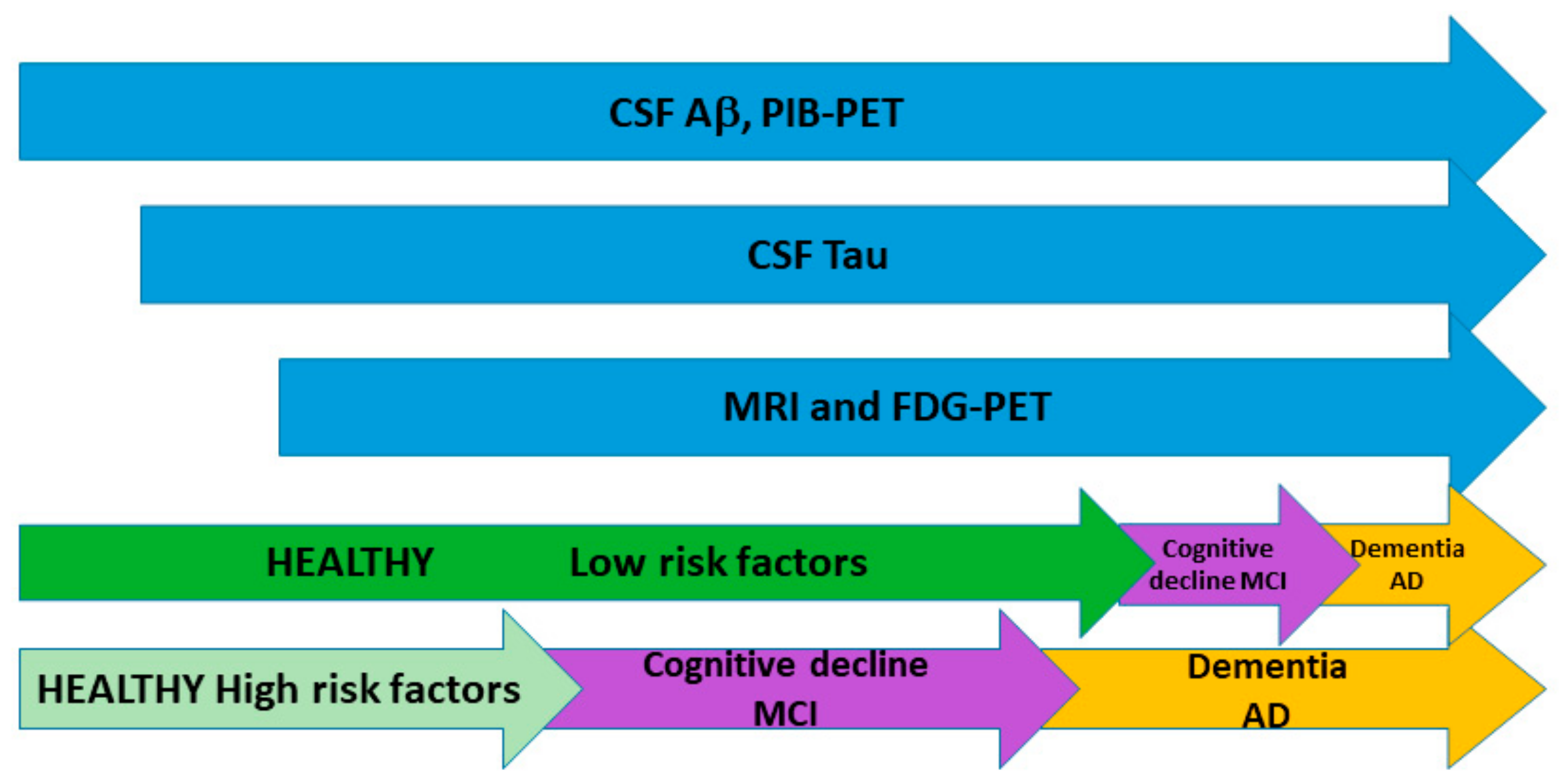
4. When Does AD Really Start?
5. Abnormalities in Biomarkers Precede Clinical Symptoms
6. Influence of Risk Factors in Biomarker Dynamics
7. New AD Classification Based on Biomarkers
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alzheimer, A. Über eigenartige Krankheitsfälle des späteren Alters. Z. Gesamte Neurol. Psychiatr. 1911, 4, 356385. [Google Scholar] [CrossRef]
- Tierney, M.C.; Fisher, R.H.; Lewis, A.J.; Zorzitto, M.L.; Snow, W.G.; Reid, D.W.; Nieuwstraten, P. The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer’s disease: A clinicopathologic study of 57 cases. Neurology 1988, 38, 359–364. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders Book, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Lim, A.; Tsuang, D.; Kukull, W.; Nochlin, D.; Leverenz, J.; McCormick, W.; Bowen, J.; Teri, L.; Thompson, J.; Peskind, E.R.; et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J. Am. Geriatr. Soc. 1999, 47, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Petrovitch, H.; White, L.R.; Ross, G.W.; Steinhorn, S.C.; Li, C.Y.; Masaki, K.H.; Davis, D.G.; Nelson, J.; Hardman, J.; Curb, J.D.; et al. Accuracy of clinical criteria for A Din the Honolulu-Asia Aging Study, a population-based study. Neurology 2001, 57, 226–234. [Google Scholar] [CrossRef]
- Varma, A.; Snowden, J.; Lloyd, J.; Talbot, P.; Mann, D.; Neary, D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer’s disease and fronto temporal dementia. J. Neurol. Neurosurg. Psychiatry 1999, 66, 184–188. [Google Scholar] [CrossRef]
- Kazee, A.M.; Eskin, T.A.; Lapham, L.W.; Gabriel, K.R.; McDaniel, K.D.; Hamill, R.W. Clinicopathologic correlates in Alzheimer disease: Assessment of clinical and pathologic diagnostic criteria. Alzheimer. Dis. Assoc. Disord. 1993, 7, 152–164. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; DeKosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.W.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Robb, M.A.; McInnes, P.M.; Califf, R.M. Biomarkers and surrogate endpoints: Developing common terminology and definitions. Jama 2016, 315, 1107–1108. [Google Scholar] [CrossRef] [PubMed]
- Humpel, C. Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol. 2011, 29, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Craig-Schapiro, R.; Fagan, A.M.; Holtzman, D.M. Biomarkers of Alzheimer’s disease. Neurobiol. Dis. 2009, 35, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Shen, Y.; Walsh, D.M.; Aisen, P.; Shaw, L.M.; Zetterberg, H.; Trojanowski, J.Q.; Blennow, K. Biological markers of amyloid β-related mechanisms in Alzheimer’s disease. Exp. Neurol. 2010, 223, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet. Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Jack, C.R.; Holtzman, D.M. Biomarker modeling of Alzheimer’s disease. Neuron 2013, 80, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Seppälä, T.T.; Nerg, O.; Koivisto, A.M.; Rummukainen, J.; Puli, L.; Zetterberg, H.; Pyykkö, O.T.; Helisalmi, S.; Alafuzoff, I.; Hiltunen, M.; et al. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 2012, 78, 1568–1575. [Google Scholar] [CrossRef]
- Tapiola, T.; Alafuzoff, I.; Herukka, S.; Parkkinen, L.; Hartikainen, P.; Soininen, H.; Pirttilä, T. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 2009, 66, 382–389. [Google Scholar] [CrossRef]
- Buerger, K.; Ewers, M.; Pirttilä, T.; Zinkowski, R.; Alafuzoff, I.; Teipel, S.J.; DeBernardis, J.; Kerkman, D.; McCulloch, C.; Soininen, H.; et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 2006, 129, 3035–3041. [Google Scholar] [CrossRef]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebro spinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Buchhave, P.; Minthon, L.; Zetterberg, H.; Wallin, A.K.; Blennow, K.; Hansson, O. Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch. Gen. Psychiatry 2012, 69, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Mintun, M.A.; Shah, A.R.; Aldea, P.; Roe, C.M.; Mach, R.H.; Marcus, D.; Morris, J.C.; Holtzman, D.M. Cerebrospinal fluid tau and ptau (181) increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol. Med. 2009, 1, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Zetterberg, H.; Hansson, O.; Andreasen, N.; Parnetti, L.; Jonsson, M.; Herukka, S.K.; van der Flier, W.M.; Blankenstein, M.A.; Ewers, M.; et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009, 302, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Head, D.; Shah, A.R.; Marcus, D.; Mintun, M.; Morris, J.C.; Holtzman, D.M. Decreased cerebrospinal fluid A beta(42) correlates with brain atrophy in cognitively normal elderly. Ann. Neurol. 2009, 65, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Roe, C.M.; Xiong, C.; Mintun, M.A.; Morris, J.C.; Holtzman, D.M. Cerebrospinal fluid tau/beta-amyloid (42) ratio as a prediction of cognitive decline in non demented older adults. Arch. Neurol. 2007, 64, 343–349. [Google Scholar] [CrossRef]
- Schott, J.M.; Bartlett, J.W.; Fox, N.C.; Barnes, J. Alzheimer’s Disease Neuroimaging Initiative Investigators. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1-42. Ann. Neurol. 2010, 68, 825–834. [Google Scholar] [CrossRef]
- Petrie, E.C.; Cross, D.J.; Galasko, D.; Schellenberg, G.D.; Raskind, M.A.; Peskind, E.R.; Minoshima, S. Preclinical evidence of Alzheimer changes: Convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch. Neurol. 2009, 66, 632–637. [Google Scholar] [CrossRef]
- Li, G.; Sokal, I.; Quinn, J.F.; Leverenz, J.B.; Brodey, M.; Schellenberg, G.D.; Kaye, J.A.; Raskind, M.A.; Zhang, J.; Peskind, E.R.; et al. CSF tau/Abeta 42 ratio for increased risk of mild cognitive impairment: A follow-upstudy. Neurology 2007, 69, 631–639. [Google Scholar] [CrossRef]
- Skoog, I.; Davidsson, P.; Aevarsson, O.; Vanderstichele, H.; Vanmechelen, E.; Blennow, K. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: A population-based study in 85-year-olds. Dement. Geriatr. Cogn. Disord. 2003, 15, 169–176. [Google Scholar] [CrossRef]
- Gustafson, D.R.; Skoog, I.; Rosengren, L.; Zetterberg, H.; Blennow, K. Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J. Neurol. Neurosurg. Psychiatry 2007, 78, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Bendlin, B.B.; Carlsson, C.M.; Johnson, S.C.; Zetterberg, H.; Blennow, K.; Willette, A.A.; Okonkwo, O.C.; Sodhi, A.; Ries, M.L.; Birdsill, A.C.; et al. CSF T-Tau/Aβ42 predicts white matter microstructure in healthy adults at risk for Alzheimer’s disease. PLoS ONE 2012, 7, e37720. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, T.; Mirza, N.; Putnam, K.T.; Linker, G.; Bhupali, D.; Durham, R.; Soares, H.; Kimmel, L.; Friedman, D.; Bergeson, J.; et al. Cerebrospinal fluid beta-amyloid 1-42 and tau in control subjects at risk for Alzheimer’s disease: The effect of APOE epsilon 4 allele. Biol. Psychiatry 2004, 56, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Younkin, S.G.; Pratico, D.; Seltzer, W.; Cole, G.M.; Geschwind, D.H.; Rodriguez-Agudelo, Y.; Schaffer, B.; Fein, J.; Sokolow, S.; et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology 2008, 71, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Schulman, H.; Becker, C.; Jones, T.; Bai, Y.; Immermann, F.; Cole, G.; Sokolow, S.; Gylys, K.; Geschwind, D.H.; et al. Proteomic changes in cerebrospinal fluid of presymptomatic and affected persons carrying familial Alzheimer disease mutations. Arch. Neurol. 2012, 69, 96–104. [Google Scholar] [CrossRef]
- Moonis, M.; Swearer, J.M.; Dayaw, M.P.E.; George-Hyslop, P.S.; Rogaeva, E.; Kawarai, T.; Pollen, D.A. Familial Alzheimer disease: Decreases in CSF A beta 42 levels precede cognitive decline. Neurology 2005, 65, 323–325. [Google Scholar] [CrossRef]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef]
- Bacskai, B.J.; Frosch, M.P.; Freeman, S.H.; Raymond, S.B.; Augustinack, J.C.; Johnson, K.A.; Irizarry, M.C.; Klunk, W.E.; Mathis, C.A.; DeKosky, S.T.; et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: A case report. Arch. Neurol. 2007, 64, 431–434. [Google Scholar] [CrossRef]
- Johnson, K.A.; Gregas, M.; Becker, J.A.; Kinnecom, C.; Salat, D.H.; Moran, E.K.; Smith, E.E.; Rosand, J.; Rentz, D.M.; Klunk, W.E.; et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann. Neurol. 2007, 62, 229–234. [Google Scholar] [CrossRef]
- Lockhart, A.; Lamb, J.R.; Osredkar, T.; Sue, L.I.; Joyce, J.N.; Ye, L.; Libri, V.; Leppert, D.; Beach, T.G. PIB is a non-specific imaging marker of amyloid-beta (A beta) peptide-related cerebral amyloidosis. Brain J. Neurol. 2007, 130, 2607–2615. [Google Scholar] [CrossRef]
- Sojkova, J.; Driscoll, I.; Iacono, D.; Zhou, Y.; Codispoti, K.E.; Kraut, M.A.; Ferrucci, L.; Pletnikova, O.; Mathis, C.A.; Klunk, W.E.; et al. In vivo fibrillar β-amyloid detected using [11c] pib positron emission tomography and neuropathologic assessment in older adults. Arch. Neurol. 2011, 68, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, I.; Troncoso, J.C.; Rudow, G.; Sojkova, J.; Pletnikova, O.; Zhou, Y.; Kraut, M.A.; Ferrucci, L.; Mathis, C.A.; Klunk, W.E.; et al. Correspondence between in vivo 11C-PiB PET amyloid imaging and post-mortem, region-matched assessment of plaques. Acta Neuropathol. 2012, 124, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, K.; Yang, C.; Schneider, J.A.; Senjem, M.L.; Reyes, D.A.; Lowe, V.J.; Barnes, L.L.; Aggarwal, N.T.; Bennett, D.A.; Smith, G.E.; et al. Antemortem amyloid imaging and β-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol. Aging 2012, 33, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Klunk, W.E.; Abrahamson, E.E.; Mathis, C.A.; Price, J.C.; Tsopelas, N.D.; Lopresti, B.J.; Ziolko, S.; Bi, W.; Paljug, W.R.; et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain J. Neurol. 2008, 131, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Mintun, M.A.; Mach, R.H.; Lee, S.Y.; Dence, C.S.; Shah, A.R.; LaRossa, G.N.; Spinner, M.L.; Klunk, W.E.; Mathis, C.A.; et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid A beta 42 in humans. Ann. Neurol. 2006, 59, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Vlassenko, A.G.; Mintun, M.A.; Xiong, C.; Sheline, Y.I.; Goate, A.M.; Benzinger, T.L.; Morris, J.C. Amyloid-beta plaque growth in cognitively normal adults: Longitudinal [11C]Pittsburgh compound B data. Ann. Neurol. 2011, 70, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Price, J.C.; Mathis, C.A.; Tsopelas, N.D.; Lopresti, B.J.; Ziolko, S.K.; Bi, W.; Hoge, J.A.; Cohen, A.D.; Ikonomovic, M.D.; et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 6174–6184. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Ataka, S.; Mizuno, T.; Brooks, W.S.; Wada, Y.; Kondo, M.; Jones, G.; Watanabe, Y.; Mulligan, R.; Nakagawa, M.; et al. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch. Neurol. 2009, 66, 1537–1544. [Google Scholar] [CrossRef]
- Schöll, M.; Almkvist, O.; Axelman, K.; Stefanova, E.; Wall, A.; Westman, E.; Långström, B.; Lannfelt, L.; Graff, C.; Nordberg, A.; et al. Glucose metabolism and PIB binding in carriers of a His163Tyr presenilin 1 mutation. Neurobiol. Aging 2011, 32, 1388–1399. [Google Scholar] [CrossRef]
- Rowe, C.C.; Ng, S.; Ackermann, U.; Gong, S.J.; Pike, K.; Savage, G.; Cowie, T.F.; Dickinson, K.L.; Maruff, P.; Darby, D.; et al. Imaging β-amyloid burden in aging and dementia. Neurology 2007, 68, 1718–1725. [Google Scholar] [CrossRef]
- Maruyama, M.; Shimada, H.; Suhara, T.; Shinotoh, H.; Ji, B.; Maeda, J.; Zhang, M.R.; Trojanowski, J.Q.; Lee, V.M.Y.; Ono, M.; et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 2013, 79, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Walji, A.M.; Hostetler, E.D.; Selnick, H.; Zeng, Z.; Miller, P.; Bennacef, I.; Salinas, C.; Connolly, B.; Gantert, L.; Holahan, M.; et al. Discovery of 6-(Fluoro-(18)F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([(18)F]-MK-6240): A positron emission tomography (PET) imaging agent for quantification of neurofibrillary tangles (NFTs). J. Med. Chem. 2016, 59, 4778–4789. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies—Still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef] [PubMed]
- Firouzian, A.; Whittington, A.; Searle, G.E.; Koychev, I.; Zamboni, G.; Lovestone, S. Imaging Aβ and tau in early stage Alzheimer’s disease with [18 F] AV45 and [18 F] AV1451. EJNMMI Res. 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Firouzian, A.; Whittington, A.; Searle, G.E.; Koychev, I.; Zamboni, G.; Lovestone, S.; Gunn, R.N. Deep and Frequent Phenotyping study team. PET tau and amyloid-β burden in mild Alzheimer’s disease: Divergent relationship with age, cognition, and cerebrospinal fluid biomarkers. J. Alzheimer’s Dis. 2017, 60, 283–293. [Google Scholar]
- Jagust, W.; Reed, B.; Mungas, D.; Ellis, W.; DeCarli, C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 2007, 69, 871–877. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Smith, C.B.; Davidsen, L.; Savaki, H.; Sokoloff, L.; Mata, M.; Fink, D.J.; Gainer, H. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science 1979, 205, 723–725. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An Energy Budget for Signaling in the Grey Matter of the Brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Minoshima, S.; Giordani, B.; Berent, S.; Frey, K.A.; Foster, N.L.; Kuhl, D.E. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann. Neurol. 1997, 42, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Xie, S.X.; Trojanowski, J.Q.; Shaw, L.M. Longitudinal change in CSF Tau and Aβ biomarkers for up to 48 months in ADNI. Acta Neuropathol. 2013, 126, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Ewers, M.; Brendel, M.; Rizk-Jackson, A.; Rominger, A.; Bartenstein, P.; Schuff, N.; Weiner, M.W. Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. NeuroImage Clin. 2013, 4, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Reiman, E.M.; Caselli, R.J.; Yun, L.S.; Chen, K.; Bandy, D.; Minoshima, S.; Thibodeau, S.N.; Osborne, D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N. Engl. J. Med. 1996, 334, 752–758. [Google Scholar] [CrossRef]
- Small, G.W.; Mazziotta, J.C.; Collins, M.T.; Baxter, L.R.; Phelps, M.E.; Mandelkern, M.A.; Kaplan, A.; La Rue, A.; Adamson, C.F.; Chang, L.; et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 1995, 273, 942–947. [Google Scholar] [CrossRef]
- Reiman, E.M.; Chen, K.; Alexander, G.E.; Caselli, R.J.; Bandy, D.; Osborne, D.; Saunders, A.M.; Hardy, J. Correlations between apolipoprotein E epsilon 4 gene dose and brain-imaging measurements of regional hypometabolism. Proc. Natl. Acad. Sci. USA 2005, 102, 8299–8302. [Google Scholar] [CrossRef]
- Reiman, E.M.; Chen, K.; Alexander, G.E.; Caselli, R.J.; Bandy, D.; Osborne, D.; Saunders, A.M.; Hardy, J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc. Natl. Acad. Sci. USA 2004, 101, 284–289. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Smith, R.; Ohlsson, T.; Strandberg, O.; Mattsson, N.; Insel, P.S.; Palmqvist, S.; Hansson, O. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology 2019, 92, e601–e612. [Google Scholar] [CrossRef]
- Bobinski, M.; De Leon, M.J.; Wegiel, J.; Desanti, S.; Convit, A.; Saint Louis, L.A.; Rusinek, H.; Wisniewski, H.M. The histological validation of postmortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience 2000, 95, 721–725. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Fiandaca, M.S.; Mapstone, M.E.; Cheema, A.K.; Federoff, H.J. The critical need for defining preclinical biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, S196–S212. [Google Scholar] [CrossRef] [PubMed]
- Den Heijer, T.; Geerlings, M.I.; Hoebeek, F.E.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch. Gen. Psychiatry 2006, 63, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Csernansky, J.G.; Wang, L.; Swank, J.; Miller, J.P.; Gado, M.; McKeel, D.; Miller, M.I.; Morris, J.C. Preclinical detection of Alzheimer’s disease: Hippocampal shape and volume predict dementia onset in the elderly. Neuroimage 2005, 25, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.B.; Smith, C.D.; Collins, H.R.; Schmitt, F.A.; Gold, B.T. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol. Aging 2010, 31, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Rusinek, H.; De Santi, S.; Frid, D.; Tsui, W.H.; Tarshish, C.Y.; Convit, A.; de Leon, M.J. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology 2003, 229, 691–696. [Google Scholar] [CrossRef]
- Jack, C.R.; Petersen, R.C.; Xu, Y.; O’brien, P.C.; Smith, G.E.; Ivnik, R.J.; Boeve, B.F.; Tangalos, E.G.; Kokmen, E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 2000, 55, 484–489. [Google Scholar] [CrossRef]
- Apostolova, L.G.; Mosconi, L.; Thompson, P.M.; Green, A.E.; Hwang, K.S.; Ramirez, A.; Mistur, R.; Tsui, W.H.; de Leon, M.J. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol. Aging 2010, 31, 1077–1088. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Stoub, T.R.; Shah, R.C.; Sperling, R.A.; Killiany, R.J.; Albert, M.S.; Hyman, B.T.; Blacker, D.; Detoledo-Morrell, L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011, 76, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, B.C.; Bakkour, A.; Salat, D.H.; Feczko, E.; Pacheco, J.; Greve, D.N.; Grodstein, F.; Wright, C.I.; Blacker, D.; Rosas, H.D.; et al. The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 2009, 19, 497–510. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Wolk, D.A. Alzheimer’s Disease Neuroimaging Initiative. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology 2012, 78, 84–90. [Google Scholar] [CrossRef]
- Fox, N.C.; Crum, W.R.; Scahill, R.I.; Stevens, J.M.; Janssen, J.C.; Rossor, M.N. Imaging of onset and progression of Alzheimer’s disease with voxel-compression mapping of serial magnetic resonance images. Lancet. Lond. Engl. 2001, 358, 201–205. [Google Scholar] [CrossRef]
- Flier, J.S.; Underhill, L.H.; Yankner, B.A.; Mesulam, M.-M. β-Amyloid and the Pathogenesis of Alzheimer’s Disease. N. Engl. J. Med. 1991, 325, 1849–1857. [Google Scholar] [CrossRef]
- Arendt, T.; Bigl, V. Alzheimer’s diseaseas a presumptive threshold phenomenon. Neurobiol. Aging 1987, 8, 552–554. [Google Scholar] [CrossRef]
- Mann, D.M. The Pathogenesis and Progression of the Pathological Changes of Alzheimer’s disease. Ann. Med. 1989, 21, 133–136. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Lowe, V.J.; Weigand, S.D.; Wiste, H.J.; Senjem, M.L.; Knopman, D.S.; Shiung, M.M.; Gunter, J.L.; Boeve, B.F.; Kemp, B.J.; et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain J. Neurol. 2009, 132, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E.; Schneider, L.S.; Koo, E.H. Anti-Aβ Therapeutics in Alzheimer’s disease: The Need for a Paradigm Shift. Neuron 2011, 69, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Petrella, J.R.; Hao, W.; Rao, A.; Doraiswamy, P.M. Computational Causal Modeling of the Dynamic Biomarker Cascade in Alzheimer’s disease. Comput. Math. Methods Med. 2019. [Google Scholar] [CrossRef]
- Davatzikos, C.; Bhatt, P.; Shaw, L.M.; Batmanghelich, K.N.; Trojanowski, J.Q. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol. Aging 2011, 32, 2322-e19. [Google Scholar] [CrossRef]
- Okello, A.; Koivunen, J.; Edison, P.; Archer, H.A.; Turkheimer, F.E.; Någren, K.U.; Bullock, R.; Walker, Z.; Kennedy, A.; Fox, N.C.; et al. Conversion of amyloid positive and negative MCI to AD over 3 years: An 11C-PIB PET study. Neurology 2009, 73, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Anastasio, T.J. Data driven modelling of Alzheimer’s disease pathogenesis. J. Theor. Biol. 2011, 290, 60–72. [Google Scholar] [CrossRef]
- Horn, D.; Ruppin, E.; Usher, M.; Hermann, M. Neural network modeling of memory deterioration in Alzheimer’s disease. Neural Comput. 1993, 5, 736–749. [Google Scholar] [CrossRef]
- Ding, X.; Bucholc, M.; Wang, H.; Glass, D.H.; Wang, H.; Clarke, D.H.; Bjourson, A.J.; Dowey, L.R.C.; O’Kane, M.; Prasad, G.; et al. A hybrid computational approach for efficient Alzheimer’s disease classification based on heterogeneous data. Sci. Rep. 2018, 8, 9774. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, T.J. Exploring the contribution of estrogen to amyloid-beta regulation: A novel multifactorial computational modelling approach. Front. Pharmacol. 2013, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Diem, A.K.; Tan, M.; Bressloff, N.W.; Hawkes, C.; Morris, A.W.; Weller, R.O.; Carare, R.O. A simulation model of periarterial clearance of amyloid-β from the brain. Front. Aging Neurosci. 2016, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.J.; Boche, D.; Gray, D.A.; Nicoll, J.A. Investigating interventions in alzheimer’s disease with computer simulation models. PLoS ONE 2013, 8, e73631. [Google Scholar] [CrossRef] [PubMed]
- Geerts, H.; Spiros, A.; Roberts, P. Impact of amyloid-beta changes on cognitive outcomes in Alzheimer’s disease: Analysis of clinical trials using a quantitative systems pharmacology model. Alzheimers Res. Ther. 2018, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.D.; Spiros, A.; Geerts, H. Simulations of symptomatic treatments for Alzheimer’s disease: Computational analysis of pathology and mechanisms of drug action. Alzheimers Res. Ther. 2012, 4, 50. [Google Scholar] [CrossRef]
- Stefanovski, L.; Triebkorn, P.; Spiegler, A.; Diaz-Cortes, M.A.; Solodkin, A.; Jirsa, V.; McIntosh, A.R.; Ritter, P. Alzheimer’s Disease Neuroimaging Initiative. Linking Molecular Pathways and Large-Scale Computational Modeling to Assess Candidate Disease Mechanisms and Pharmacodynamics in Alzheimer’s Disease. Front. Comput. Neurosci. 2019, 13, 54. [Google Scholar] [CrossRef]
- Mintun, M.A.; Larossa, G.N.; Sheline, Y.I.; Dence, C.S.; Lee, S.Y.; Mach, R.H.; Klunk, W.E.; Mathis, C.A.; DeKosky, S.T.; Morris, J.C. 11C]PIB in a non-demented population: Potential antecedent marker of Alzheimer disease. Neurology 2006, 67, 446–452. [Google Scholar] [CrossRef]
- Aizenstein, H.J.; Nebes, R.D.; Saxton, J.A.; Price, J.C.; Mathis, C.A.; Tsopelas, N.D.; Ziolko, S.K.; James, J.A.; Snitz, B.E.; Houck, P.R.; et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008, 65, 1509–1517. [Google Scholar] [CrossRef]
- Savva, G.M.; Wharton, S.B.; Ince, P.G.; Forster, G.; Matthews, F.E.; Brayne, C. Age, neuropathology, and dementia. N. Engl. J. Med. 2009, 360, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Parisi, J.E.; Salviati, A.; Floriach-Robert, M.; Boeve, B.F.; Ivnik, R.J.; Smith, G.E.; Dickson, D.W.; Johnson, K.A.; Petersen, L.E.; et al. Neuropathology of cognitively normal elderly. Journal of Neuropathology and Experimental. Neurology 2003, 62, 1087–1095. [Google Scholar]
- Lazarczyk, M.J.; Hof, P.R.; Bouras, C.; Giannakopoulos, P. Preclinical Alzheimer’s disease: Identification of cases at risk among cognitively intact older individuals. BMC Med. 2012, 10, 1–13. [Google Scholar] [CrossRef]
- Hanseeuw, B.J.; Betensky, R.A.; Jacobs, H.I.; Schultz, A.P.; Sepulcre, J.; Becker, J.A.; Cosio, D.M.O.; Farrell, M.; Quiroz, Y.T.; Mormino, E.C.; et al. Association of Amyloid and Tau With Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol. 2019, 76, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug. Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.N. The molecular and geneticsbasis of AD: The end of the beginning. The 2000 Watenberg lecture. Neurology 2000, 54, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.; Sala-Llonch, R.; Bartrés-Faz, D.; Lladó, A.; Solés Padullés, C.; Bosch, B.; Antonell, A.; Olives, J.; Sanchez-Valle, R.; Molinuevo, J.L.; et al. Cognitively preserved subjects with transitional cerebrospinal fluid ß-amyloid 1-42 values have thicker cortex in Alzheimer’s disease vulnerable áreas. Biol. Psychiatry 2011, 70, 183–190. [Google Scholar] [CrossRef]
- Defrancesco, M.; Egger, K.; Marksteiner, J.; Esterhammer, R.; Hinterhuber, H.; Deisenhammer, E.A.; Schocke, M. Changes in White matter integrity before conversión from mild cognitive impairment to alzheimer’s disease. PLoS ONE 2014, 9, e106062. [Google Scholar] [CrossRef]
- van Loenhoud, A.C.; van der Flier, W.M.; Wink, A.M.; Dicks, E.; Groot, C.; Twisk, J.; Barkhof, F.; Scheltens, P.; Ossenkoppele, R. Alzheimer’s Disease Neuroimaging Initiative. Cognitive reserve and clinical progression in Alzheimer disease: A paradoxical relationship. Neurology 2019, 93, e334–e346. [Google Scholar] [CrossRef]
- McDade, E.; Wang, G.; Gordon, B.A.; Hassenstab, J.; Benzinger, T.L.; Buckles, V.; Fagan, A.M.; Holtzman, D.M.; Cairns, N.J.; Goate, A.M.; et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 2018, 91, e1295–e1306. [Google Scholar] [CrossRef]
- Lafirdeen, A.S.M.; Cognat, E.; Sabia, S.; Hourregue, C.; Lilamand, M.; Dugravot, A.; Bouaziz-Amar, E.; Laplanche, J.-L.; Hugon, J.; Singh-Manoux, A.; et al. Biomarker profiles of Alzheimer’s disease and dynamic of the association between cerebrospinal fluid levels of β-amyloid peptide and tau. PLoS ONE 2019, 14, e0217026. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Therneau, T.M.; Weigand, S.D.; Wiste, H.J.; Knopman, D.S.; Vemuri, P.; Lowe, V.J.; Mielke, M.M.; Roberts, R.O.; Machulda, M.M.; et al. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol. 2019, e191971. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.; Elliot, C.; Ryan, L.; Masliah, E.; Hodes, R. NIA commentary on the NIA-AA Research Framework: Towards a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 576–578. [Google Scholar] [CrossRef]
| Criteria | Options |
|---|---|
| Main Criteria for AD Diagnosis (Obligatory) | (A) Early episodic memory failure represented by a gradual or progressive memory dysfunction at the beginning of the disease, informed by the patient or family, lasting more than six months. Associated with objective evidence of significant decline in episodic memory through tests (deferred memory). |
| Support Criteria for AD Diagnosis (At least one present) | (B) Loss of volume of the hippocampus, entorhinal cortex, amygdala or other mesial-temporal structures, evidenced by magnetic resonance imaging (MRI). |
| (C) Abnormality in CSF biomarkers such as - Low concentrations of Aβ; - Increased t-tau or p-tau concentrations; or - A combination of the three. | |
| (D) Specific metabolic pattern evidenced by PET such as hypometabolism of glucose in bilateral temporal parietal regions. | |
| (E) Autosomal dominant family genetic mutations On chromosomes 21 (APP), 14 (PS1), or 1 (PS2). |
| A/T/N Profiles | Biomarker Outcome | Diagnosis | ||
|---|---|---|---|---|
| A+ | T+ | N+ | AD | AD SPECTRUM |
| N− | AD | |||
| T− | N+ | AD and suspected non-AD pathologic change | ||
| N− | Alzheimer’s pathological change | |||
| A− | T+ | N+ | Non AD pathological change | No AD |
| N− | Non AD pathological change | |||
| T− | N+ | Non AD pathological change | ||
| N− | Normal BIOMARKERS | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloret, A.; Esteve, D.; Lloret, M.-A.; Cervera-Ferri, A.; Lopez, B.; Nepomuceno, M.; Monllor, P. When Does Alzheimer′s Disease Really Start? The Role of Biomarkers. Int. J. Mol. Sci. 2019, 20, 5536. https://doi.org/10.3390/ijms20225536
Lloret A, Esteve D, Lloret M-A, Cervera-Ferri A, Lopez B, Nepomuceno M, Monllor P. When Does Alzheimer′s Disease Really Start? The Role of Biomarkers. International Journal of Molecular Sciences. 2019; 20(22):5536. https://doi.org/10.3390/ijms20225536
Chicago/Turabian StyleLloret, Ana, Daniel Esteve, Maria-Angeles Lloret, Ana Cervera-Ferri, Begoña Lopez, Mariana Nepomuceno, and Paloma Monllor. 2019. "When Does Alzheimer′s Disease Really Start? The Role of Biomarkers" International Journal of Molecular Sciences 20, no. 22: 5536. https://doi.org/10.3390/ijms20225536
APA StyleLloret, A., Esteve, D., Lloret, M.-A., Cervera-Ferri, A., Lopez, B., Nepomuceno, M., & Monllor, P. (2019). When Does Alzheimer′s Disease Really Start? The Role of Biomarkers. International Journal of Molecular Sciences, 20(22), 5536. https://doi.org/10.3390/ijms20225536






