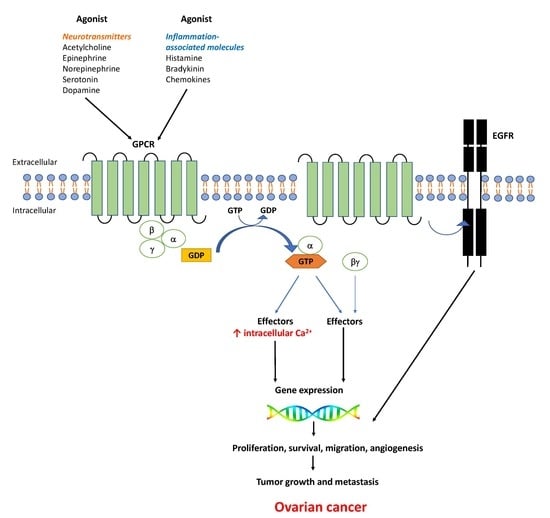
G Protein-Coupled Receptors (GPCRs)-Mediated Calcium Signaling in Ovarian Cancer: Focus on GPCRs activated by Neurotransmitters and Inflammation-Associated Molecules
Abstract
:1. Introduction
2. GPCRs Activated by Neurotransmitters in Ovarian Cancer
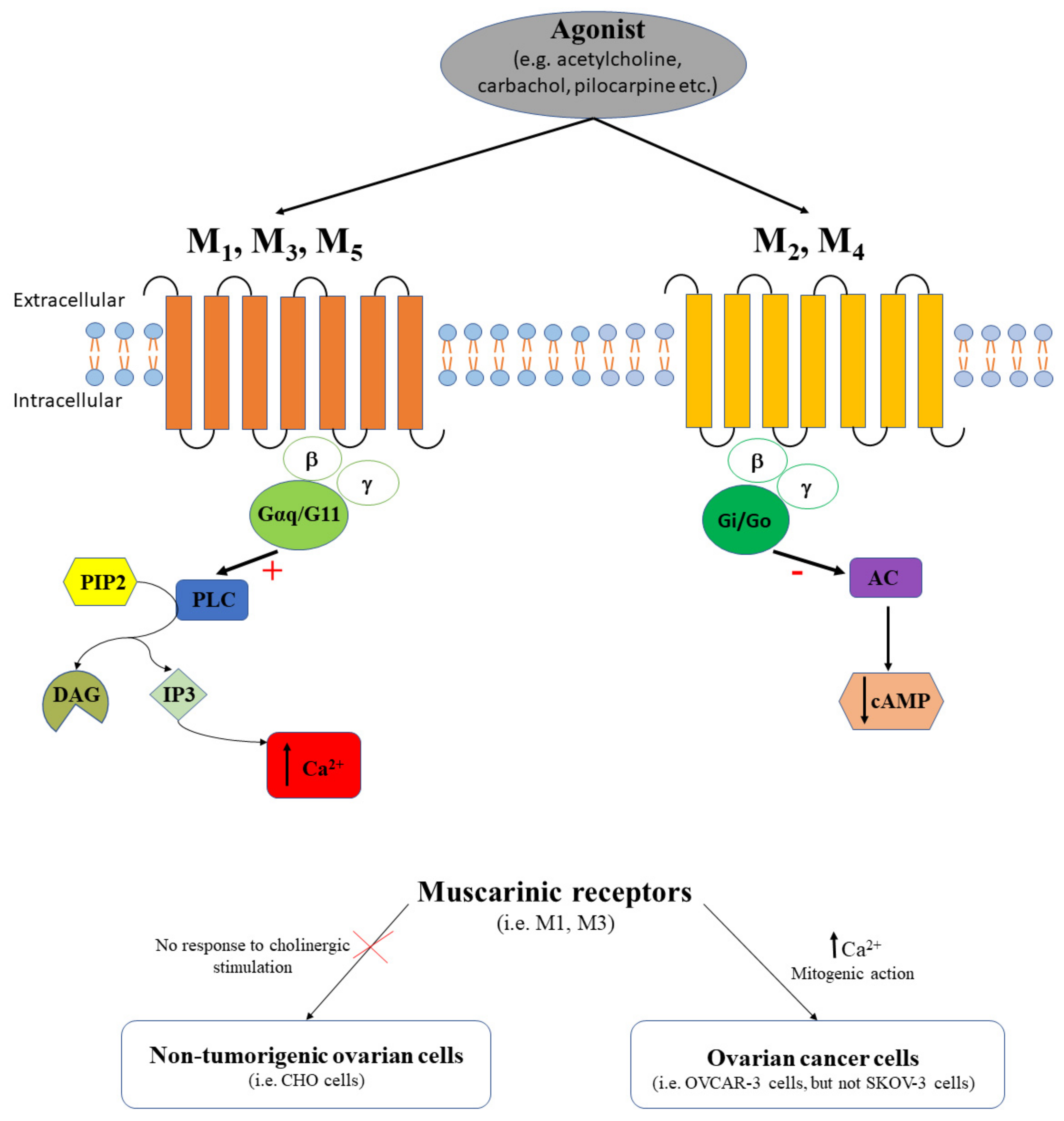
2.1. Muscarinic Receptors in Ovarian Cancer
2.2. Adrenergic Receptors in Ovarian Cancer
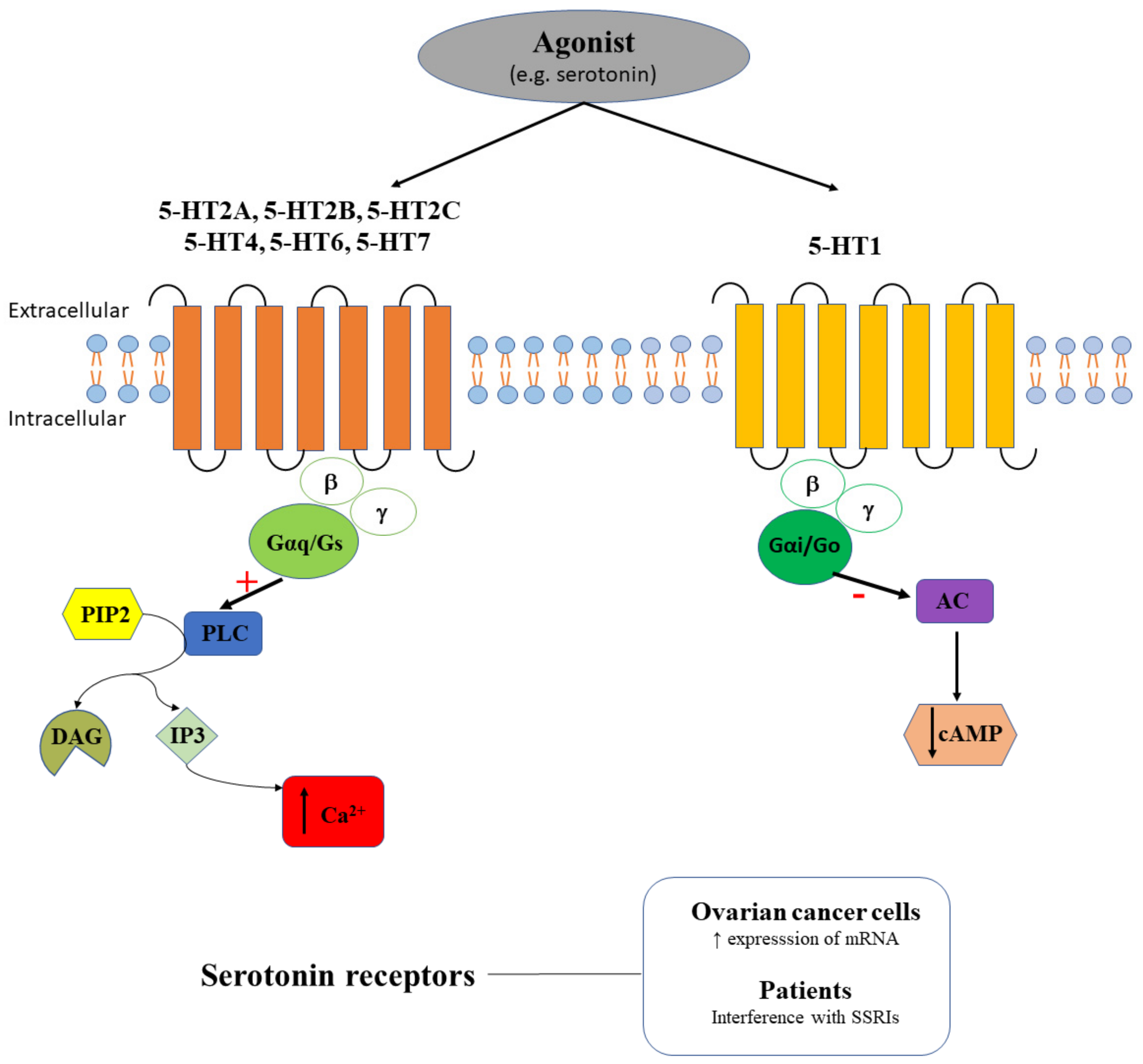
2.3. Serotonin Receptors in Ovarian Cancer
2.4. Dopamine Receptors in Ovarian Cancer
3. GPCRs Activated by Inflammation-Related Molecules in Ovarian Cancer
3.1. Bradykinin Receptors in Ovarian Cancer
3.2. Histamine Receptors in Ovarian Cancer
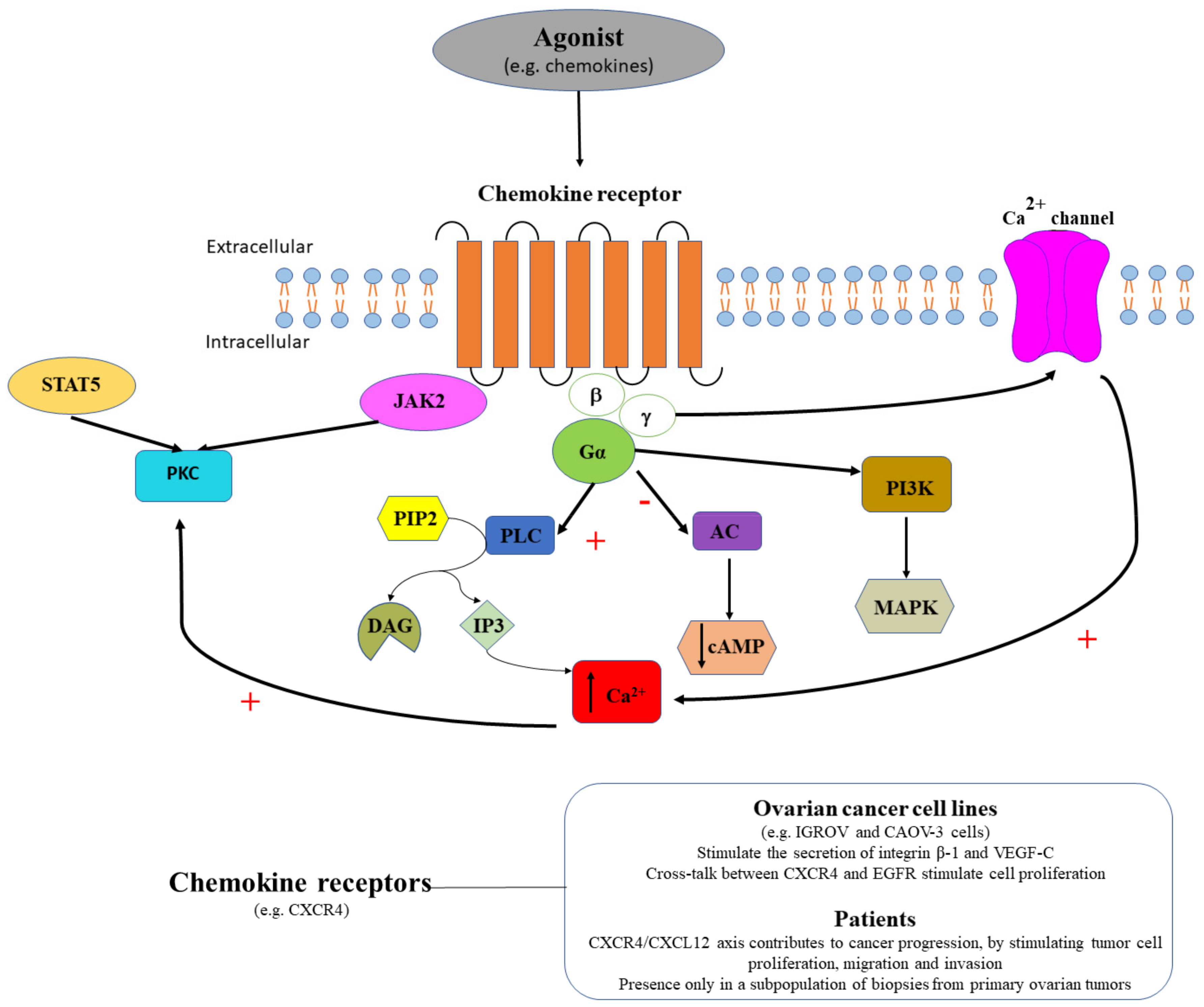
3.3. Chemokine Receptors in Ovarian Cancer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Woehler, A.; Ponimaskin, E.G. G protein--mediated signaling: Same receptor, multiple effectors. Curr. Mol. Pharmacol. 2009, 2, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Cavasotto, C.N.; Orry, A.J.; Abagyan, R.A. Structure-based identification of binding sites, native ligands and potential inhibitors for G-protein coupled receptors. Proteins 2003, 51, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, A. Modulating G-protein-coupled receptors: From traditional pharmacology to allosterics. Trends Pharmacol. Sci. 2007, 28, 431–437. [Google Scholar] [CrossRef]
- Lappano, R.; Maggiolini, M. GPCRs and cancer. Acta Pharmacol. Sin. 2012, 33, 351–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Shavit, R.; Maoz, M.; Kancharla, A.; Nag, J.K.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G Protein-Coupled Receptors in Cancer. Int. J. Mol. Sci. 2016, 17, 1320. [Google Scholar] [CrossRef] [PubMed]
- Bhola, N.E.; Grandis, J.R. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front. Biosci. 2008, 13, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Pyne, S. Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: Out of the shadow? Trends Pharmacol. Sci. 2011, 32, 443–450. [Google Scholar] [CrossRef]
- Wang, Z. Transactivation of Epidermal Growth Factor Receptor by G Protein-Coupled Receptors: Recent Progress, Challenges and Future Research. Int. J. Mol. Sci. 2016, 17, 95. [Google Scholar] [CrossRef]
- Fathy, D.B.; Kyle, D.J.; Leeb-Lundberg, L.M. High-affinity binding of peptide agonists to the human B1 bradykinin receptor depends on interaction between the peptide N-terminal L-lysine and the fourth extracellular domain of the receptor. Mol. Pharmacol. 2000, 57, 171–179. [Google Scholar]
- Gera, L.; Roy, C.; Bawolak, M.T.; Charest-Morin, X.; Marceau, F. N-terminal extended conjugates of the agonists and antagonists of both bradykinin receptor subtypes: Structure-activity relationship, cell imaging using ligands conjugated with fluorophores and prospect for functionally active cargoes. Peptides 2012, 34, 433–446. [Google Scholar] [CrossRef]
- Rozengurt, E. Mitogenic signaling pathways induced by G protein-coupled receptors. J. Cell. Physiol. 2007, 213, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Maggiolini, M. G protein-coupled receptors: Novel targets for drug discovery in cancer. Nat. Rev. Drug Discov. 2011, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Nieto Gutierrez, A.; McDonald, P.H. GPCRs: Emerging anti-cancer drug targets. Cell. Signal. 2018, 41, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Madden, N.E.; Wong, A.S.T.; Chow, B.K.C.; Lee, L.T.O. The Role of Endocrine G Protein-Coupled Receptors in Ovarian Cancer Progression. Front. Endocrinol. (Lausanne) 2017, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Patel, A.V.; Calle, E.E.; Jacob, E.J.; Thun, M.J. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA 2001, 285, 1460–1465. [Google Scholar] [CrossRef]
- Smith, H.O.; Arias-Pulido, H.; Kuo, D.Y.; Howard, T.; Qualls, C.R.; Lee, S.J.; Verschraegen, C.F.; Hathaway, H.J.; Joste, N.E.; Prossnitz, E.R. GPR30 predicts poor survival for ovarian cancer. Gynecol. Oncol. 2009, 114, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Liu, H.; Wen, H.; Jiang, X.; Cao, X.; Zhang, G.; Liu, G. The novel estrogen receptor GPER regulates the migration and invasion of ovarian cancer cells. Mol. Cell Biochem. 2013, 378, 1–7. [Google Scholar] [CrossRef]
- Ignatov, T.; Modl, S.; Thulig, M.; Weissenborn, C.; Treeck, O.; Ortmann, O.; Zenclussen, A.; Costa, S.D.; Kalinski, T.; Ignatov, A. GPER-1 acts as a tumor suppressor in ovarian cancer. J. Ovarian Res. 2013, 6, 51. [Google Scholar] [CrossRef]
- Heublein, S.; Mayr, D.; Vrekoussis, T.; Friese, K.; Hofmann, S.S.; Jeschke, U.; Lenhard, M. The G-protein coupled estrogen receptor (GPER/GPR30) is a gonadotropin receptor dependent positive prognosticator in ovarian carcinoma patients. PLoS ONE 2013, 8, e71791. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Y.; Wen, H.; Jiang, X.; Cao, X.; Zhang, G.; Liu, G. A novel estrogen receptor GPER mediates proliferation induced by 17beta-estradiol and selective GPER agonist G-1 in estrogen receptor alpha (ERalpha)-negative ovarian cancer cells. Cell Biol. Int. 2014, 38, 631–638. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, X.; Zhao, Y.; Wen, H.; Liu, G. Role of GPER on proliferation, migration and invasion in ligand-independent manner in human ovarian cancer cell line SKOV3. Cell Biochem. Funct. 2015, 33, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.X.; Xiong, W.; Wang, M.L.; Yang, J.; Shi, H.J.; Chen, H.Q.; Niu, G. Nuclear G protein-coupled oestrogen receptor (GPR30) predicts poor survival in patients with ovarian cancer. J. Int. Med. Res. 2018, 46, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, L.; Zhang, G.; Zhang, L.; Chen, C. G protein-coupled receptor 30 in tumor development. Endocrine 2010, 38, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhu, X.; Zheng, L.; Hu, X.; Sun, L.; Zhu, X. The role of the androgen receptor in ovarian cancer carcinogenesis and its clinical implications. Oncotarget 2017, 8, 29395–29405. [Google Scholar] [CrossRef]
- Mizushima, T.; Miyamoto, H. The Role of Androgen Receptor Signaling in Ovarian Cancer. Cells 2019, 8, 176. [Google Scholar] [CrossRef]
- Sheach, L.A.; Adeney, E.M.; Kucukmetin, A.; Wilkinson, S.J.; Fisher, A.D.; Elattar, A.; Robson, C.N.; Edmondson, R.J. Androgen-related expression of G-proteins in ovarian cancer. Br. J. Cancer. 2009, 101, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Willis, R.A. The Spread of Tumors in the Human Body, 3rd ed.; Butterworths: London, UK, 1973. [Google Scholar]
- Li, S.; Sun, Y.; Gao, D. Role of the nervous system in cancer metastasis. Oncol. Lett. 2013, 5, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Arese, M.; Bussolino, F.; Pergolizzi, M.; Bizzozero, L.; Pascal, D. Tumor progression: The neuronal input. Ann. Transl Med. 2018, 6, 89. [Google Scholar] [CrossRef]
- Sarkar, C.; Chakroborty, D.; Basu, S. Neurotransmitters as regulators of tumor angiogenesis and immunity: The role of catecholamines. J. Neuroimmune Pharmacol. 2013, 8, 7–14. [Google Scholar] [CrossRef]
- Nguyen, T.; Kirsch, B.J.; Asaka, R.; Nabi, K.; Quinones, A.; Tan, J.; Antonio, M.J.; Camelo, F.; Li, T.; Nguyen, S.; et al. Uncovering the Role of N-Acetyl-Aspartyl-Glutamate as a Glutamate Reservoir in Cancer. Cell Rep. 2019, 27, 491–501. [Google Scholar] [CrossRef]
- Sood, A.K.; Bhatty, R.; Kamat, A.A.; Landen, C.N.; Han, L.; Thaker, P.H.; Li, Y.; Gershenson, D.M.; Lutgendorf, S.; Cole, S.W. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 2006, 12, 369–375. [Google Scholar]
- Schuller, H.M. Neurotransmitters and their Receptors as the Upstream Regulators of the Most Common Human Cancers and their Stem Cells. J. Neurol. Neuromed. 2018, 3, 17–26. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010, 10, 369–373. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and cancer. Environ. Health Prev Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Mayerhofer, A.; Fritz, S. Ovarian acetylcholine and muscarinic receptors: Hints of a novel intrinsic ovarian regulatory system. Microsc. Res. Tech. 2002, 59, 503–508. [Google Scholar] [CrossRef]
- Urra, J.; Blohberger, J.; Tiszavari, M.; Mayerhofer, A.; Lara, H.E. In vivo blockade of acetylcholinesterase increases intraovarian acetylcholine and enhances follicular development and fertility in the rat. Sci. Rep. 2016, 6, 30129. [Google Scholar] [CrossRef] [Green Version]
- Fritz, S.; Fohr, K.J.; Boddien, S.; Berg, U.; Brucker, C.; Mayerhofer, A. Functional and molecular characterization of a muscarinic receptor type and evidence for expression of choline-acetyltransferase and vesicular acetylcholine transporter in human granulosa–luteal cells. J. Clin. Endocrinol. Metab. 1999, 84, 1744–1750. [Google Scholar]
- Cruz, M.E.; Flores, A.; Alvarado, B.E.; Hernández, C.G.; Zárate, A.; Chavira, R.; Cárdenas, M.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R. Ovulation requires the activation on proestrus of M1 muscarinic receptors in the left ovary. Endocrine 2015, 49, 809–819. [Google Scholar] [CrossRef]
- Pronin, A.N.; Wang, Q.; Slepak, V.Z. Teaching an old drug new tricks: Agonism, antagonism, and biased signaling of pilocarpine through M3 muscarinic acetylcholine receptor. Mol. Pharmacol. 2017, 92, 601–612. [Google Scholar] [CrossRef]
- Yasuda, K.; Sumi, G.; Kanamori, C.; Nakajima, T.; Tsuzuki, T.; Cho, H.; Nishigaki, A.; Okada, H.; Kanzaki, H. Effects of ovarian hormone treatment on the gene expression of muscarinic acetylcholine receptors in the ovariectomized rat myometrium. J. Steroid Biochem. Mol. Biol. 2014, 143, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Oppitz, M.; Möbus, V.; Brock, S.; Drews, U. Muscarinic receptors in cell lines from ovarian carcinoma: Negative correlation with survival of patients. Gynecol. Oncol. 2002, 85, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Popper, L.D.; Iosif, C.S. Characterisation of muscarinic cholinergic receptors in human ovaries, ovarian tumours and tumour cell lines. Eur. J. Cancer. 1993, 29A, 1302–1306. [Google Scholar] [CrossRef]
- Spindel, E.R. Muscarinic receptor agonists and antagonists: Effects on cancer. Handb. Exp. Pharmacol. 2012, 208, 451–468. [Google Scholar]
- Popper, L.; Batra, S. Muscarinic acetylcholine and histamine-receptor mediated calcium mobilization and cell-growth in human ovarian-cancer cells. Int. J. Oncol. 1994, 4, 453–459. [Google Scholar] [CrossRef]
- Huang, T.; Tworoger, S.S.; Hecht, J.L.; Rice, M.S.; Sood, A.K.; Kubzansky, L.D.; Poole, E.M. Association of Ovarian Tumor β2-Adrenergic Receptor Status with Ovarian Cancer Risk Factors and Survival. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1587–1594. [Google Scholar] [CrossRef]
- Nagaraja, A.S.; Dorniak, P.L.; Sadaoui, N.C.; Kang, Y.; Lin, T.; Armaiz-Pena, G.; Wu, S.Y.; Rupaimoole, R.; Allen, J.K.; Gharpure, K.M.; et al. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene 2016, 35, 2390–2397. [Google Scholar] [CrossRef]
- Modzelewska, B.; Jóźwik, M.; Jóźwik, M.; Sulkowski, S.; Pędzińska-Betiuk, A.; Kleszczewski, T.; Kostrzewska, A. Altered uterine contractility in response to β-adrenoceptor agonists in ovarian cancer. J. Physiol. Sci. 2017, 67, 711–722. [Google Scholar] [CrossRef]
- Moreno-Smith, M.; Lu, C.; Shahzad, M.M.; Pena, G.N.; Allen, J.K.; Stone, R.L.; Mangala, L.S.; Han, H.D.; Kim, H.S.; Farley, D.; et al. Dopamine blocks stress-mediated ovarian carcinoma growth. Clin. Cancer Res. 2011, 17, 3649–3659. [Google Scholar] [CrossRef]
- Kang, Y.; Nagaraja, A.S.; Armaiz-Pena, G.N.; Dorniak, P.L.; Hu, W.; Rupaimoole, R.; Liu, T.; Gharpure, K.M.; Previs, R.A.; Hansen, J.M.; et al. Adrenergic Stimulation of DUSP1 Impairs Chemotherapy Response in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 1713–1724. [Google Scholar]
- Nilsson, M.B.; Armaiz-Pena, G.; Takahashi, R.; Lin, Y.G.; Trevino, J.; Li, Y.; Jennings, N.; Arevalo, J.; Lutgendorf, S.K. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J. Biol. Chem. 2007, 282, 29919–29926. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.; Deen, S.; Entschladen, F.; Coveney, C.; Rees, R.; Zänker, K.S.; Powe, D.G. Alpha1B adrenoceptor expression is a marker of reduced survival and increased tumor recurrence in patients with endometrioid ovarian cancer. World J. Obstet. Gynecol. 2016, 5, 118–126. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Young, S.N.; Leyton, M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol. Biochem. Behav. 2002, 71, 857–865. [Google Scholar] [CrossRef]
- Lavoie, B.; Lian, J.B.; Mawe, G.M. Regulation of Bone Metabolism by Serotonin. Adv. Exp. Med. Biol. 2017, 1033, 35–46. [Google Scholar] [PubMed]
- Marshall, A.M.; Hernandez, L.L.; Horseman, N.D. Serotonin and serotonin transport in the regulation of lactation. J. Mammary Gland Biol. Neoplasia. 2014, 19, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.P.; Fink, H. Serotonin controlling feeding and satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef]
- Uphouse, L. Pharmacology of serotonin and female sexual behavior. Pharmacol. Biochem. Behav. 2014, 121, 31–42. [Google Scholar] [CrossRef]
- Melancon, M.O.; Lorrain, D.; Dionne, I.J. Exercise and sleep in aging: Emphasis on serotonin. Pathol. Biol. (Paris) 2014, 62, 276–283. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Seyedabadi, M.; Fakhfouri, G.; Ramezani, V.; Mehr, S.E.; Rahimian, R. The role of serotonin in memory: Interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 2014, 232, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and cancer: What is the link? Curr. Mol. Med. 2015, 15, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Sarrouilhe, D.; Mesnil, M. Serotonin and human cancer: A critical view. Biochimie 2019, 161, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Jasim, R.H.; Matlab, N.S. Serotonin as a Marker to the Response of Patients with Advanced Stages of Cancer during Treatment with Chemotherapy and Radiotherapy. Clin. Med. Biochem. 2017, 3, 132. [Google Scholar] [CrossRef]
- Holck, A.; Wolkowitz, O.M.; Mellon, S.H.; Reus, V.I.; Nelson, J.C.; Westrin, Å.; Lindqvist, D. Plasma serotonin levels are associated with antidepressant response to SSRIs. J. Affect. Disord. 2019, 250, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.K.; Armaiz-Pena, G.N.; Ramirez, E.; Matsuo, K.; Zimmerman, B.; Zand, B.; Shinn, E.; Goodheart, M.J.; Bender, D.; Thaker, P.H.; et al. SSRI use and clinical outcomes in epithelial ovarian cancer. Oncotarget 2016, 7, 33179–33191. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, R.; Dizeyi, N.; Abrahamsson, P.A. Expression of serotonin receptors 5-HT1A, 5-HT1B, 5-HT2B and 5-HT4 in ovary and in ovarian tumours. Anticancer Res. 2012, 32, 1361–1366. [Google Scholar]
- Arias-Carrión, O.; Pŏppel, E. Dopamine, learning, and reward-seeking behavior. Acta Neurobiol. Exp. (Wars) 2007, 67, 481–488. [Google Scholar]
- Badgaiyan, R.D.; Fischman, A.J.; Alpert, N.M. Dopamine release during human emotional processing. Neuroimage 2009, 47, 2041–2045. [Google Scholar] [CrossRef] [Green Version]
- Groenewegen, H.J. The basal ganglia and motor control. Neural Plast. 2003, 10, 107–120. [Google Scholar] [CrossRef]
- Liu, X.; Herbison, A.E. Dopamine regulation of gonadotropin-releasing hormone neuron excitability in male and female mice. Endocrinology 2013, 154, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Pinoli, M.; Marino, F.; Cosentino, M. Dopaminergic Regulation of Innate Immunity: A Review. J. Neuroimmune Pharmacol. 2017, 12, 602–623. [Google Scholar] [CrossRef] [PubMed]
- Warren, N.; O’Gorman, C.; Lehn, A.; Siskind, D. Dopamine dysregulation syndrome in Parkinson’s disease: A systematic review of published cases. J. Neurol. Neurosurg. Psychiatry 2017, 88, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Perez-Costas, E.; Melendez-Ferro, M.; Rice, M.W.; Conley, R.R.; Roberts, R.C. Dopamine pathology in schizophrenia: Analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front. Psychiatry 2012, 3, 31. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Khakpai, F. The Modulatory Role of Dopamine in Anxiety-like Behavior. Arch. Iran. Med. 2015, 18, 591–603. [Google Scholar]
- Basu, S.; Dasgupta, P.S. Role of dopamine in malignant tumor growth. Endocrine 2000, 12, 237–241. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.B.; Luo, C.; Mao, X.Y.; Li, X.; Yin, J.Y.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. The Prospective Value of Dopamine Receptors on Bio-Behavior of Tumor. J. Cancer 2019, 10, 1622–1632. [Google Scholar] [CrossRef]
- Yong, M.; Yu, T.; Tian, S.; Liu, S.; Xu, J.; Hu, J.; Hu, L. DR2 blocker thioridazine: A promising drug for ovarian cancer therapy. Oncol. Lett. 2017, 14, 8171–8177. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, W.; Li, H.; Niu, C.; Ou, Y.; Song, L.; Zhang, Y. Stonin 2 Overexpression is Correlated with Unfavorable Prognosis and Tumor Invasion in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 1653. [Google Scholar] [CrossRef]
- Moreno-Smith, M.; Lee, S.J.; Lu, C.; Nagaraja, A.S.; He, G.; Rupaimoole, R.; Han, H.D.; Jennings, N.B.; Roh, J.W.; Nishimura, M.; et al. Biologic effects of dopamine on tumor vasculature in ovarian carcinoma. Neoplasia 2013, 15, 502–510. [Google Scholar] [CrossRef]
- Kang, S.K.; Kim, D.K.; Damron, D.S.; Baek, K.J.; Im, M.J. Modulation of intracellular Ca(2+) via alpha(1B)-adrenoreceptor signaling molecules, G alpha(h) (transglutaminase II) and phospholipase C-delta 1. Biochem. Biophys. Res. Commun. 2002, 293, 383–390. [Google Scholar] [CrossRef]
- Niewiarowska-Sendo, A.; Polit, A.; Piwowar, M.; Tworzydło, M.; Kozik, A.; Guevara-Lora, I. Bradykinin B2 and dopamine D2 receptors form a functional dimer. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Webb, C.; Holford, J.; Burgess, G.M. Signal transduction pathways for B1 and B2 bradykinin receptors in bovine pulmonary artery endothelial cells. Mol. Pharmacol. 1995, 47, 525–534. [Google Scholar] [PubMed]
- Mathis, S.A.; Criscimagna, N.L.; Leeb-Lundberg, L.M. B1 and B2 kinin receptors mediate distinct patterns of intracellular Ca2+ signaling in single cultured vascular smooth muscle cells. Mol. Pharmacol. 1996, 50, 128–139. [Google Scholar]
- Yoshimura, Y.; Espey, L.; Hosoi, Y.; Adachi, T.; Atlas, S.J.; Ghodgaonkar, R.B.; Dubin, N.H.; Wallach, E.E. The effects of bradykinin on ovulation and prostaglandin production by the perfused rabbit ovary. Endocrinology 1988, 122, 2540–2546. [Google Scholar] [CrossRef]
- Hornig, B.; Drexler, H. Endothelial function and bradykinin in humans. Drugs 1997, 54 (Suppl. 5), 42–47. [Google Scholar] [CrossRef]
- Barry, E.F.; Johns, E.J. Intrarenal bradykinin elicits reno-renal reflex sympatho-excitation and renal nerve-dependent fluid retention. Acta Physiol. (Oxf.) 2015, 213, 731–739. [Google Scholar] [CrossRef]
- Wang, G.; Sun, J.; Liu, G.; Fu, Y.; Zhang, X. Bradykinin Promotes Cell Proliferation, Migration, Invasion, and Tumor Growth of Gastric Cancer Through ERK Signaling Pathway. J. Cell Biochem. 2017, 118, 4444–4453. [Google Scholar] [CrossRef]
- Stewart, J.M. Bradykinin antagonists as anti-cancer agents. Curr. Pharm. Des. 2003, 9, 2036–2042. [Google Scholar] [CrossRef]
- Wunderer, G.; Walter, I. T-kinin in human ovarian carcinoma ascites. Adv. Exp. Med. Biol. 1989, 247B, 109–114. [Google Scholar]
- Ahmed, N.; Stenvers, K.L. Getting to know ovarian cancer ascites: Opportunities for targeted therapy-based translational research. Front. Oncol. 2013, 3, 256. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; MacKinnon, A.C.; Cummings, J.; Tufail-Hanif, U.; Jodrell, D.; Haslett, C.; Sethi, T. Increased gastrin-releasing peptide (GRP) receptor expression in tumour cells confers sensitivity to [Arg6,D-Trp7,9,NmePhe8]-substance P (6-11)-induced growth inhibition. Br. J. Cancer 2003, 88, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Jutras, S.; Bachvarova, M.; Keita, M.; Bascands, J.L.; Mes-Masson, A.M.; Stewart, J.M.; Gera, L.; Bachvarov, D. Strong cytotoxic effect of the bradykinin antagonist BKM-570 in ovarian cancer cells—Analysis of the molecular mechanisms of its antiproliferative action. FEBS J. 2010, 277, 5146–5160. [Google Scholar] [CrossRef] [PubMed]
- Orchel, J.; Witek, L.; Kimsa, M.; Strzalka-Mrozik, B.; Kimsa, M.; Olejek, A.; Mazurek, U. Expression patterns of kinin-dependent genes in endometrial cancer. Int. J. Gynecol. Cancer 2012, 22, 937–944. [Google Scholar] [CrossRef]
- Dębska-Szmich, S.; Czernek, U.; Krakowska, M.; Frąckowiak, M.; Zięba, A.; Czyżykowski, R.; Kulejewska, D.; Potemski, P. Synchronous primary ovarian and endometrial cancers: A series of cases and a review of literature. Prz Menopauzalny 2014, 13, 6469. [Google Scholar] [CrossRef]
- Dogan, A.; Schultheis, B.; Rezniczek, G.A.; Hilal, Z.; Cetin, C.; Häusler, G.; Tempfer, C.B. Synchronous Endometrial and Ovarian Cancer in Young Women: Case Report and Review of the Literature. Anticancer Res. 2017, 37, 969–978. [Google Scholar] [Green Version]
- Rozov, S.V.; Porkka-Heiskanen, T.; Panula, P. On the Role of Histamine Receptors in the Regulation of Circadian Rhythms. PLoS ONE 2015, 10, e0144694. [Google Scholar] [CrossRef]
- Tabarean, I.V. Histamine receptor signaling in energy homeostasis. Neuropharmacology 2016, 106, 13–19. [Google Scholar] [CrossRef]
- Alvarez, E.O. The role of histamine on cognition. Behav. Brain Res. 2009, 199, 183–189. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Silberstein, S.D. Histamine and Migraine. Headache 2018, 58, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Bao, A.M.; Swaab, D.F. The human histaminergic system in neuropsychiatric disorders. Trends Neurosci. 2015, 38, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Y.; Ikeda, J.; Tian, T.; Sato, A.; Ohtsu, H.; Morii, E. Roles of histamine on the expression of aldehyde dehydrogenase 1 in endometrioid adenocarcinoma cell line. Cancer Med. 2014, 3, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Chanda, R.; Ganguly, A.K. Diamine-oxidase activity and tissue di-and poly-amine contents of human ovarian, cervical and endometrial carcinoma. Cancer Lett. 1995, 89, 23–28. [Google Scholar] [CrossRef]
- Wang, M.; Wei, X.; Shi, L.; Chen, B.; Zhao, G.; Yang, H. Integrative genomic analyses of the histamine H1 receptor and its role in cancer prediction. Int. J. Mol. Med. 2014, 33, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Batra, S.; Fadeel, I. Release of intracellular calcium and stimulation of cell growth by ATP and histamine in human ovarian cancer cells (SKOV-3). Cancer Lett. 1994, 77, 57–63. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.I.; Ferreira, R.; Gama, A.; Oliveira, P.A.; Ginja, M. Antihistamines as promising drugs in cancer therapy. Life Sci. 2017, 172, 27–41. [Google Scholar] [CrossRef]
- Verdoodt, F.; Pottegård, A.; Dehlendorff, C.; Jäättelä, M.; Hallas, J.; Friis, S.; Kjaer, S.K. Antihistamine use and risk of ovarian cancer: A population-based case-control study. Maturitas 2019, 120, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; Matulonis, U.A.; Castells, M. Chemotherapy hypersensitivity reactions in ovarian cancer. J. Natl. Compr. Cancer Netw. 2014, 12, 389–402. [Google Scholar] [CrossRef]
- Yahata, H.; Saito, M.; Sendo, T.; Itoh, Y.; Uchida, M.; Hirakawa, T.; Nakano, H.; Oishi, R. Prophylactic effect of pemirolast, an antiallergic agent, against hypersensitivity reactions to paclitaxel in patients with ovarian cancer. Int. J. Cancer 2006, 118, 2636–2638. [Google Scholar] [CrossRef]
- Mach, C.M.; Lapp, E.A.; Weddle, K.J.; Hunter, R.J.; Burns, K.A.; Parker, C.; Brown, J.; Smith, J.A. Adjunct Histamine Blockers as Premedications to Prevent Carboplatin Hypersensitivity Reactions. Pharmacotherapy 2016, 36, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Sendo, T.; Sakai, N.; Itoh, Y.; Ikesue, H.; Kobayashi, H.; Hirakawa, T.; Nakano, H.; Oishi, R. Incidence and risk factors for paclitaxel hypersensitivity during ovarian cancer chemotherapy. Cancer Chemother. Pharmacol. 2005, 56, 91–96. [Google Scholar] [CrossRef] [PubMed]
- McCully, M.L.; Kouzeli, A.; Moser, B. Peripheral Tissue Chemokines: Homeostatic Control of Immune Surveillance T Cells. Trends Immunol. 2018, 39, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M. Chemokines and their receptors: Orchestrating a fine balance between health and disease. Crit Rev. Biotechnol. 2010, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Zhou, Y.; Iribarren, P.; Wang, J. Chemokines and chemokine receptors: Their manifold roles in homeostasis and disease. Cell Mol. Immunol. 2004, 1, 95–104. [Google Scholar]
- Kunkel, S.L.; Godessart, N. Chemokines in autoimmunity: From pathology to therapeutics. Autoimmun Rev. 2002, 1, 313–320. [Google Scholar] [CrossRef]
- Muralidhar, G.G.; Barbolina, M.V. Chemokine receptors in epithelial ovarian cancer. Int. J. Mol. Sci. 2014, 15, 361–376. [Google Scholar] [CrossRef]
- Mao, T.L.; Fan, K.F.; Liu, C.L. Targeting the CXCR4/CXCL12 axis in treating epithelial ovarian cancer. Gene Ther. 2017, 24, 621–629. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, C.C.; Yang, L.; Xu, Y.M.; Chen, Y.N. Role of CXCL12-CXCR4 axis in ovarian cancer metastasis and CXCL12-CXCR4 blockade with AMD3100 suppresses tumor cell migration and invasion in vitro. J. Cell Physiol. 2019, 234, 3897–3909. [Google Scholar] [CrossRef]
- Scotton, C.J.; Wilson, J.L.; Milliken, D.; Stamp, G.; Balkwill, F.R. Epithelial cancer cell migration: A role for chemokine receptors? Cancer Res. 2001, 61, 4961–4965. [Google Scholar]
- Jiang, Y.P.; Wu, X.H.; Xing, H.Y.; DU, X.Y. Role of CXCL12 in metastasis of human ovarian cancer. Chin. Med. J. (Engl.) 2007, 120, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Porcile, C.; Bajetto, A.; Barbero, S.; Pirani, P.; Schettini, G. CXCR4 activation induces epidermal growth factor receptor transactivation in an ovarian cancer cell line. Ann. N. Y. Acad. Sci. 2004, 1030, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Porcile, C.; Bajetto, A.; Barbieri, F.; Barbero, S.; Bonavia, R.; Biglieri, M.; Pirani, P.; Florio, T.; Schettini, G. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp. Cell. Res. 2005, 308, 241–253. [Google Scholar] [CrossRef]
- Venkatakrishnan, G.; Salgia, R.; Groopman, J.E. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J. Biol. Chem. 2000, 275, 6868–6875. [Google Scholar] [CrossRef] [PubMed]
- Popple, A.; Durrant, L.G.; Spendlove, I.; Rolland, P.; Scott, I.V.; Deen, S.; Ramage, J.M. The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Br. J. Cancer. 2012, 106, 1306–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, F.; Bajetto, A.; Florio, T. Role of chemokine network in the development and progression of ovarian cancer: A potential novel pharmacological target. J. Oncol. 2010, 2010, 426956. [Google Scholar] [CrossRef]
- Prat, J.; FIGO Committee on Gynecologic Oncology. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef]
- Werry, T.D.; Wilkinson, G.F.; Willars, G.B. Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem. J. 2003, 374 Pt 2, 281–296. [Google Scholar] [CrossRef]
- Bennett, L.D.; Fox, J.M.; Signoret, N. Mechanisms regulating chemokine receptor activity. Immunology 2011, 134, 246–256. [Google Scholar] [CrossRef]





| GPCR | Receptor | Ca2+ Signaling # | Type of Sample | Expression/Functional Status of GPCRs in Ovarian Cancer Analyzed in Cell Lines or Patient Samples |
|---|---|---|---|---|
| GPCRs Activated by Neurotransmitters | ||||
| Muscarinic receptors | N/A | Yes | OVCAR-3 cells | Carbachol increases Ca45 uptake by 25% of the ovarian cancer cells [46] |
| N/A | Yes | OVCAR-3 cells | Atropine blocks the carbachol-induced ovarian cancer cell proliferating effect [46] | |
| N/AM3 | Yes | Normal human ovary Human ovarian tumors SKOV-3 cells OVCAR-3 cells | Muscarinic receptors are functionally expressed in ovarian cancer cells, M3 being predominant [44] | |
| Adrenergic receptors | β2 | No | Human ovarian tumors | 19% of the samples were immunopositive for β2-adrenergic receptors [47] |
| No | Skov3-ip1 cells HeyA8 cells | NE, isoproterenol, and terbutaline stimulate PGE2 production, contributing to cancer cell migration and invasion [48] Silencing prostaglandin-endoperoxide synthase 2 reduces the NE-induced cancer cell migration and invasion [48] | ||
| β2β3 | No | Myometrial strips from ovarian cancer patients or patients with ovarian cancer in combination with endometrial cancer | β-adrenoceptor agonists diminished spontaneous uterine contractility, but had contradictory effects in cumulative administration [49] β-adrenoceptor antagonists caused varied effects on spontaneous uterine contractility when co-administered with β-adrenoceptor agonists [49] | |
| Β | No | HeyA8 and SKOV3ip1 cells | Pre-exposure to NE prevents chemotherapy-induced apoptosis [51] Isoproterenol reduces apoptotic efficacy of chemotherapeutic agents, while propranolol reverts the effect [51] | |
| Β | No | HeyA8 and SKOV3ip1 cells | Propranolol reverts the NE-induced IL-6 production [52] | |
| α1B | Yes | Endometrioid ovarian tumors | α1B expression is a marker of reduced survival and increased tumor recurrence [53] | |
| Serotonin receptors | 5-HT1A 5-HT1B 5-HT1D 5-HT1E 5-HT2A 5-HT2B 5-HT4 | No No No No Yes Yes Yes/No | A2780-CP20, SKOV3, HEYA8, 2774, ES2, TOV112D, OV90, SW626, UWB1.298 and CaOV3 cells | 5-HT1A, 5-HT1B, and 5-HT1D have a low expression [67] 5-HT2A has a high expression; DOI increases clonogenic survival [67] 5-HT1E is expressed only in 2774 and CaOV3 cells [68] 5-HT1A, 5-HT1B, 5-HT2B and 5-HT4 expression strongly decreases in invasive cancer [68] |
| Dopamine receptors | D1 | No | SKOV3ip1 and HeyA8 tumor-bearing nude mice | Butaclamol has no efficacy against the inhibitory effect of dopamine on stress-mediated tumor growth [50] SKF 82958 increases the extent of pericyte coverage in the tumoral tissue [50] |
| D2 | No | SKOV3 and A2780 cells | Thioridazine suppresses cell proliferation, induces apoptosis, ROS production, DNA damage and autophagy [79] | |
| No | SKOV3 xenografts in nude mice | Thioridazine inhibits tumor growth [79] | ||
| No | CAOV3, COV362, COV504, EFO-27, A2780, OVCAR4, SKOV3, and TOV-21G cells | Upregulation of stonin 2 [80] | ||
| No | Epithelial ovarian cancer patient samples | Upregulation of stonin-2 is associated with progression and unfavorable cancer prognosis, being correlated with intestinal and intraperitoneal metastasis [80] | ||
| No | SKOV3ip1 or HeyA8 tumor-bearing nude mice | Eticlopride suppresses the inhibitory effect of dopamine on tumor growth and angiogenesis in stress conditions [81] Parlodel has no effect on the pericyte coverage in the tumoral tissue [81] | ||
| GPCRs Activated by Inflammation-Associated Molecules | ||||
| Bradykinin receptors | B2 | Yes/No | PEO4 cells | Low expression [93] |
| Yes | TOV-21 cells TOV-112 cells | Prominent expression in TOV-21 cells [94] BKM-570 effect is comparable to cisplastin [94] BK, but not des-Arg9-BK, triggers intracellular Ca2+ release in TOV-21 cells [94] | ||
| Histamine receptors | H1 | Yes | OVCAR-3 cells | H1-mediated Ca2+ mobilization stimulates cell growth [47] Pyrilamine blocks the histamine-induced cell proliferating effect [47] |
| Yes | SKOV-3 cells | Pyrilamine, but not cimetidine, completely abolish the intracellular Ca2+ rise induced by histamine [106] | ||
| N/A | Yes | SKOV-3 cells | Histamine induces a monophasic rise of intracellular Ca2+ both in the presence/absence of external Ca2+ [107] Histamine stimulates cell proliferation at high concentrations (micromolar) [107] | |
| Chemokine receptors | CXCR1 CXCR2 | Yes/No | SKOV-3 cells | Activate MAP kinase via EGF receptor; stimulate cell migration and proliferation [125] |
| CXCR4 | Yes | CAOV-3 cells | Stimulate secretion of integrin beta-1 and VEGF-C [122] | |
| Yes | IGROV cells CAOV-3 cells Human ovarian tumors | Strong CXCR4 receptors expression in cell lines and human ovarian tumors [121] Blocking CXCR4 receptors with AMD3100 inhibits ovarian cancer progression [119,120] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predescu, D.-V.; Crețoiu, S.M.; Crețoiu, D.; Pavelescu, L.A.; Suciu, N.; Radu, B.M.; Voinea, S.-C. G Protein-Coupled Receptors (GPCRs)-Mediated Calcium Signaling in Ovarian Cancer: Focus on GPCRs activated by Neurotransmitters and Inflammation-Associated Molecules. Int. J. Mol. Sci. 2019, 20, 5568. https://doi.org/10.3390/ijms20225568
Predescu D-V, Crețoiu SM, Crețoiu D, Pavelescu LA, Suciu N, Radu BM, Voinea S-C. G Protein-Coupled Receptors (GPCRs)-Mediated Calcium Signaling in Ovarian Cancer: Focus on GPCRs activated by Neurotransmitters and Inflammation-Associated Molecules. International Journal of Molecular Sciences. 2019; 20(22):5568. https://doi.org/10.3390/ijms20225568
Chicago/Turabian StylePredescu, Dragoș-Valentin, Sanda Maria Crețoiu, Dragoș Crețoiu, Luciana Alexandra Pavelescu, Nicolae Suciu, Beatrice Mihaela Radu, and Silviu-Cristian Voinea. 2019. "G Protein-Coupled Receptors (GPCRs)-Mediated Calcium Signaling in Ovarian Cancer: Focus on GPCRs activated by Neurotransmitters and Inflammation-Associated Molecules" International Journal of Molecular Sciences 20, no. 22: 5568. https://doi.org/10.3390/ijms20225568
APA StylePredescu, D.-V., Crețoiu, S. M., Crețoiu, D., Pavelescu, L. A., Suciu, N., Radu, B. M., & Voinea, S.-C. (2019). G Protein-Coupled Receptors (GPCRs)-Mediated Calcium Signaling in Ovarian Cancer: Focus on GPCRs activated by Neurotransmitters and Inflammation-Associated Molecules. International Journal of Molecular Sciences, 20(22), 5568. https://doi.org/10.3390/ijms20225568