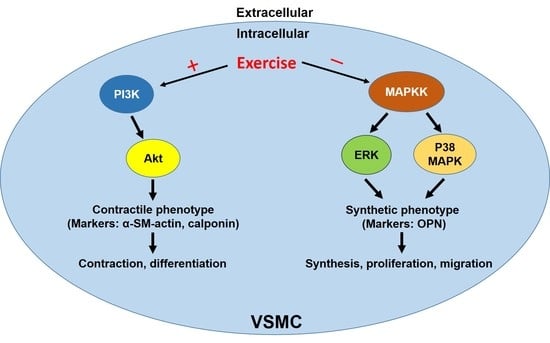
Role of the Balance of Akt and MAPK Pathways in the Exercise-Regulated Phenotype Switching in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Results
2.1. Effects of Aerobic Exercise on the Blood Pressure and Heart Rate of Rats
2.2. Aerobic Exercise Reduces the Wall Thickness of Thoracic Aortas in Spontaneously Hypertensive Rats
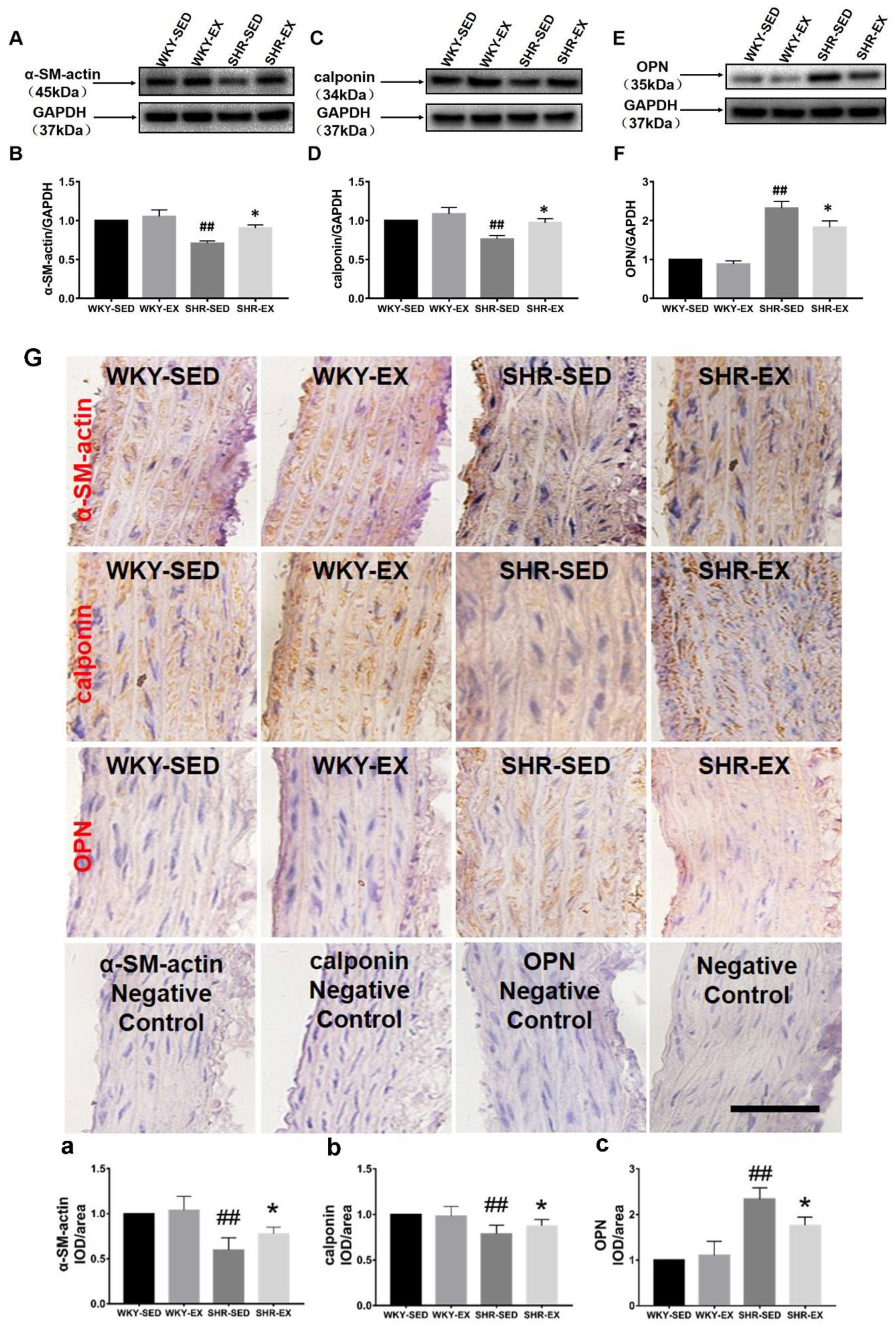
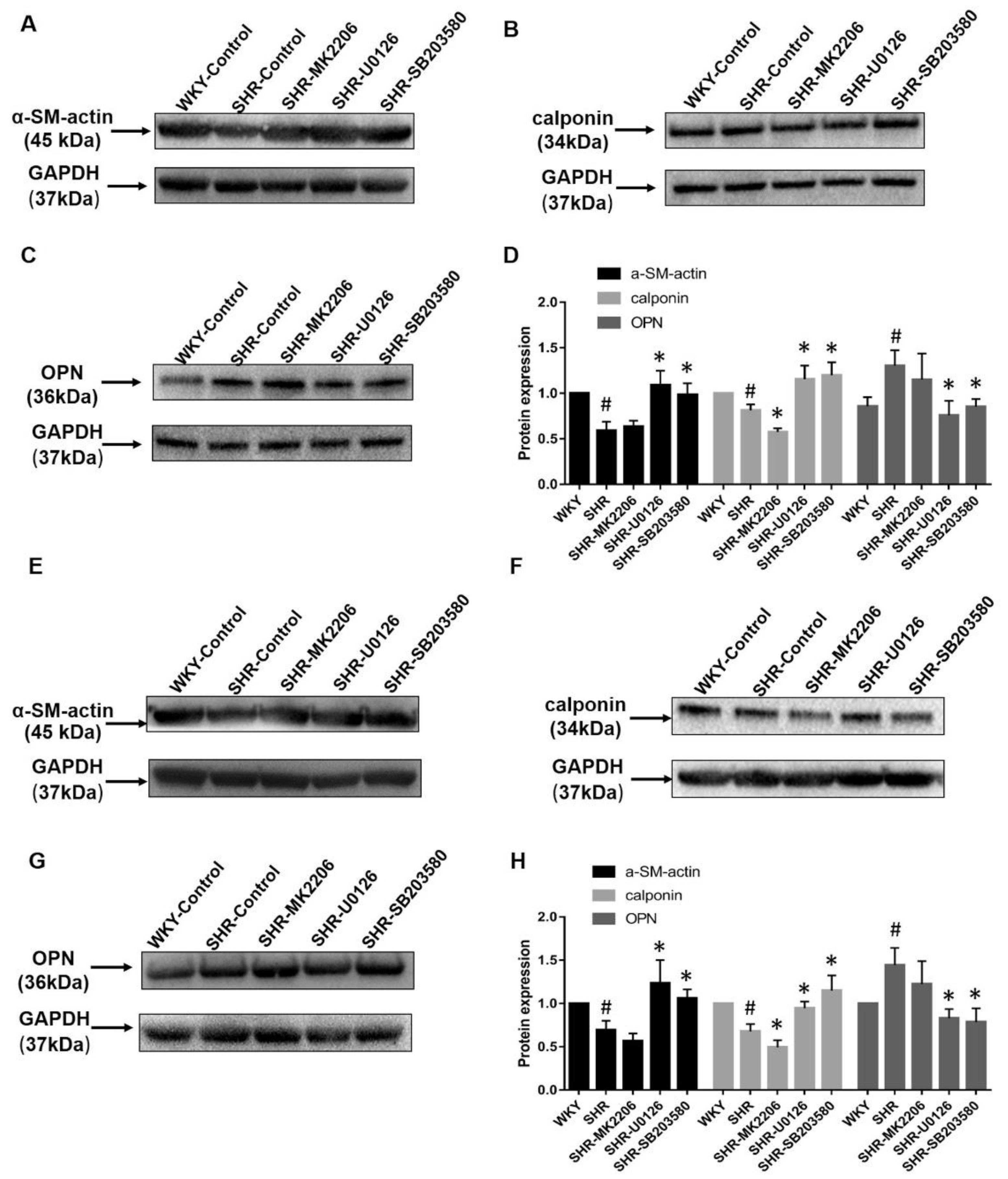
2.3. Aerobic Exercise Changes the VSMC Marker Protein Expression
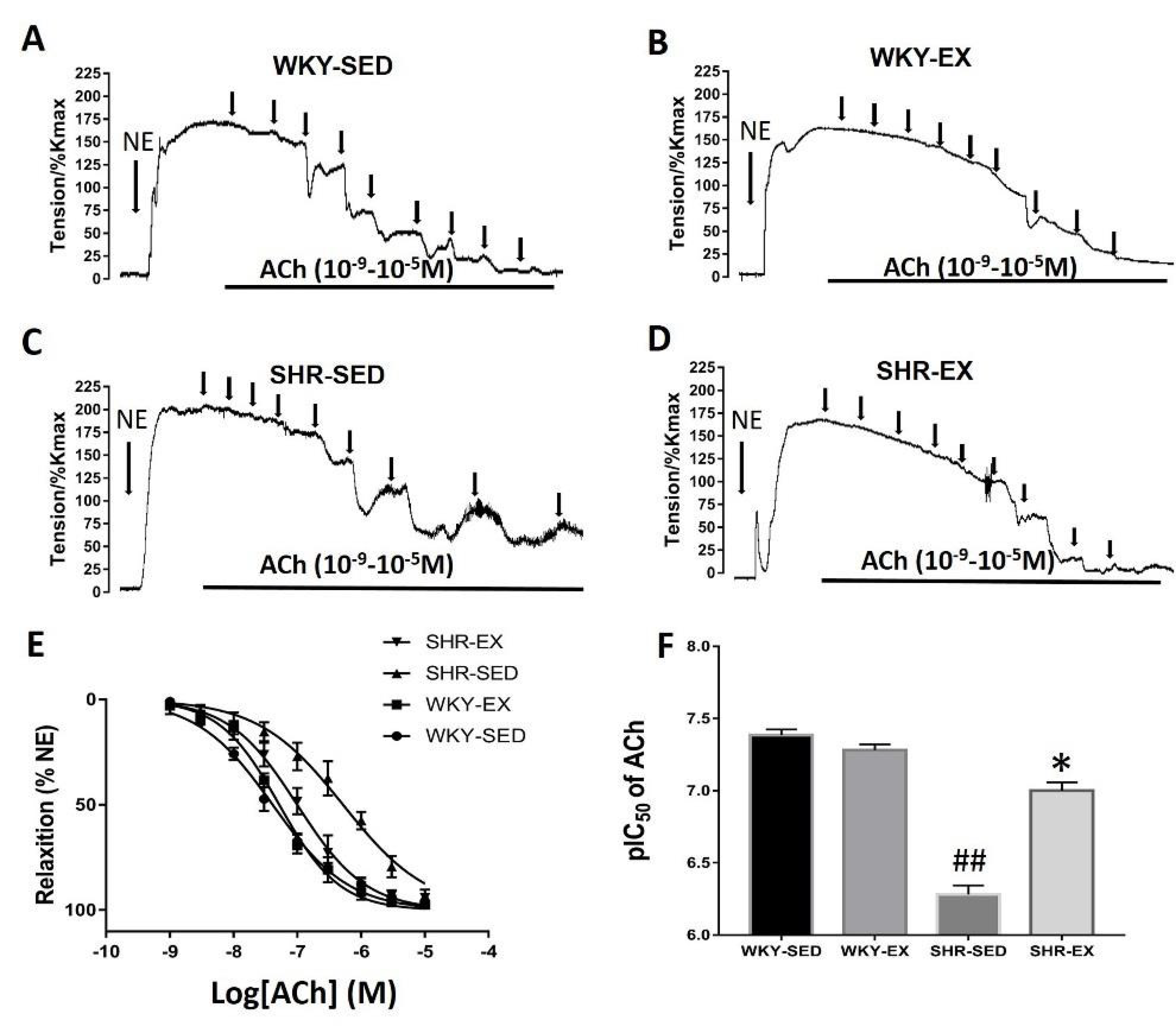
2.4. Aerobic Exercise Improves the Vasomotor Function of Mesenteric Arteries in Spontaneously Hypertensive Rats
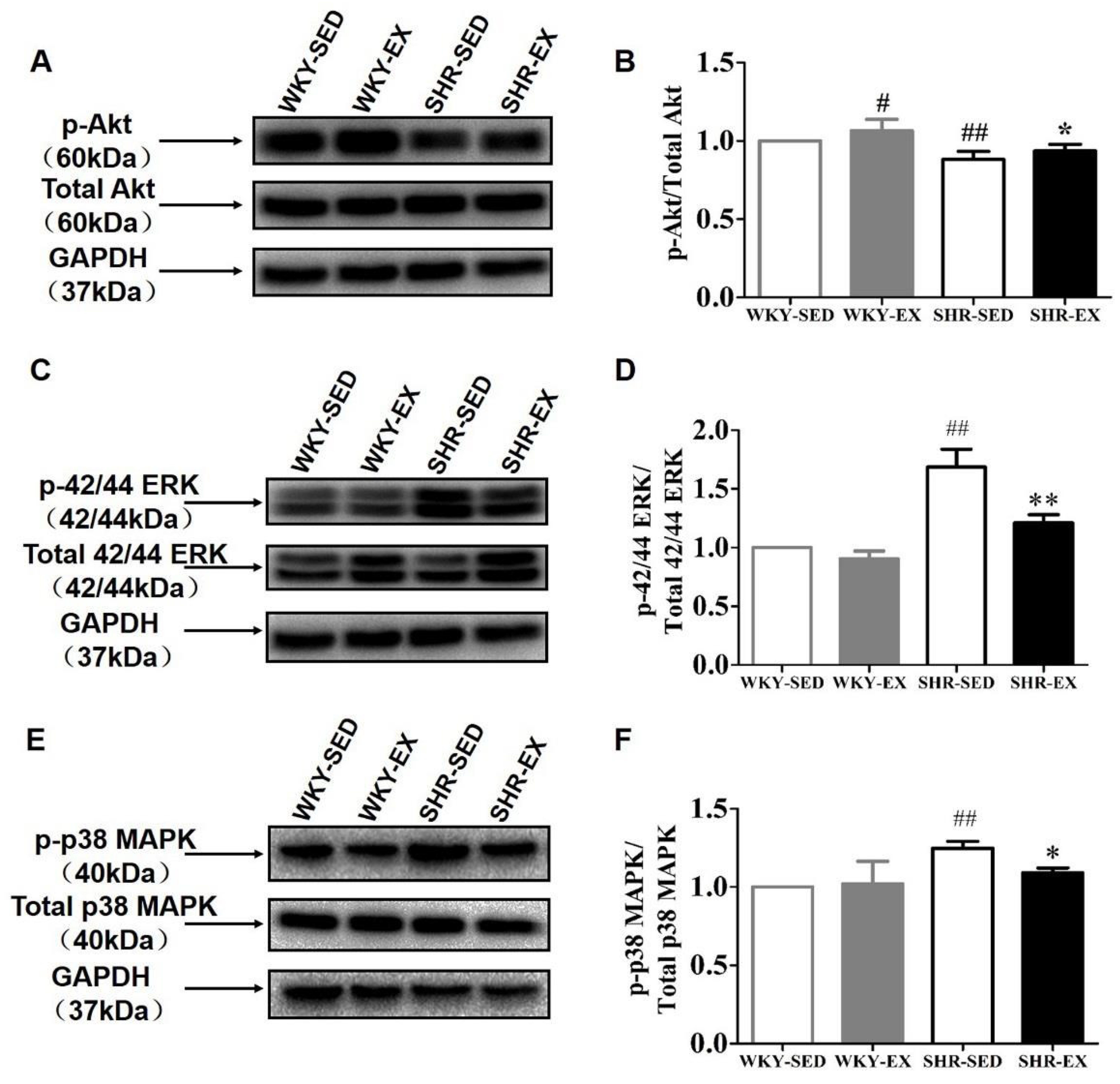
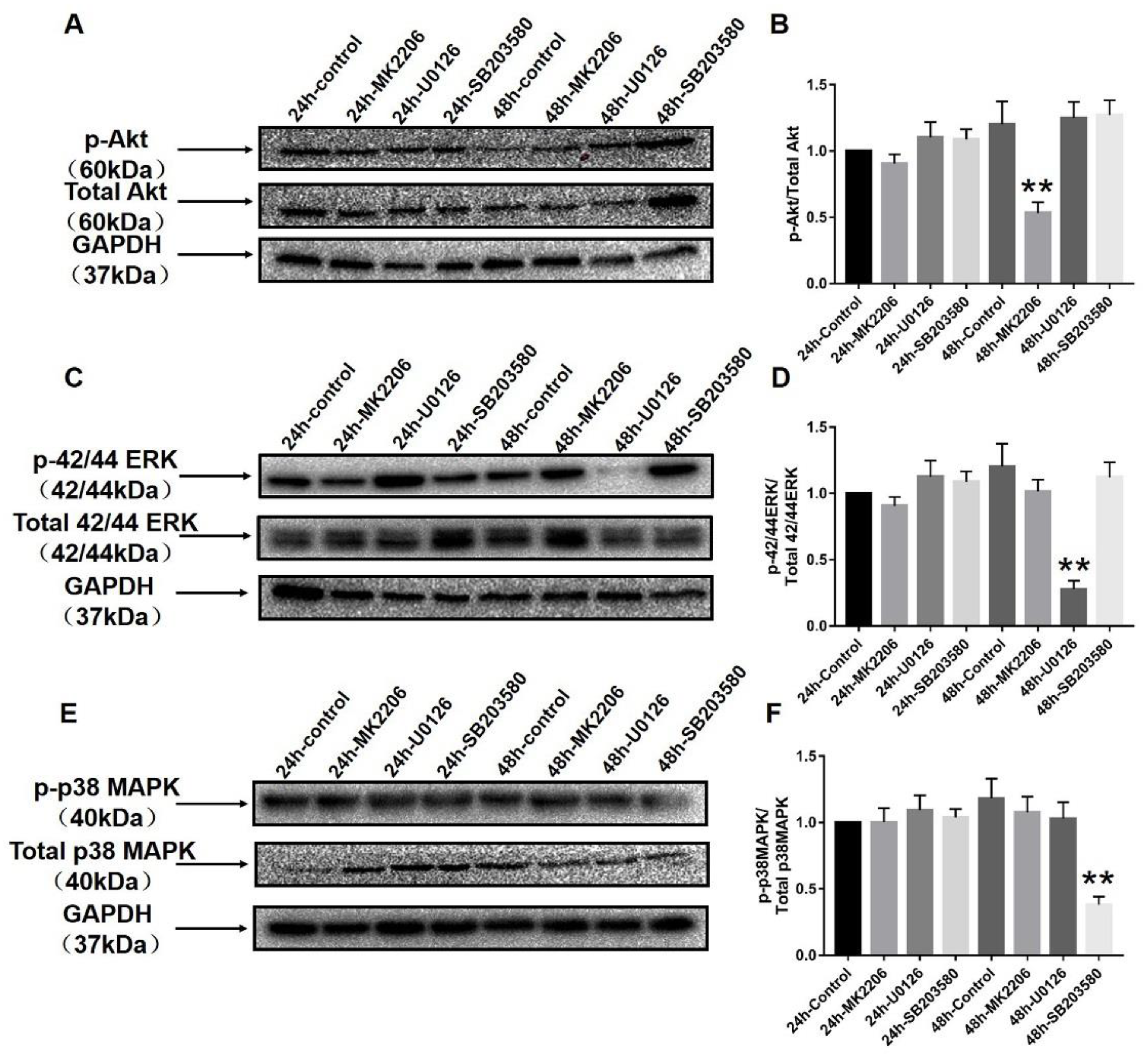
2.5. Aerobic Exercise Balances the Functional Role of Akt and MAPK Signaling Pathways in Phenotype Switching
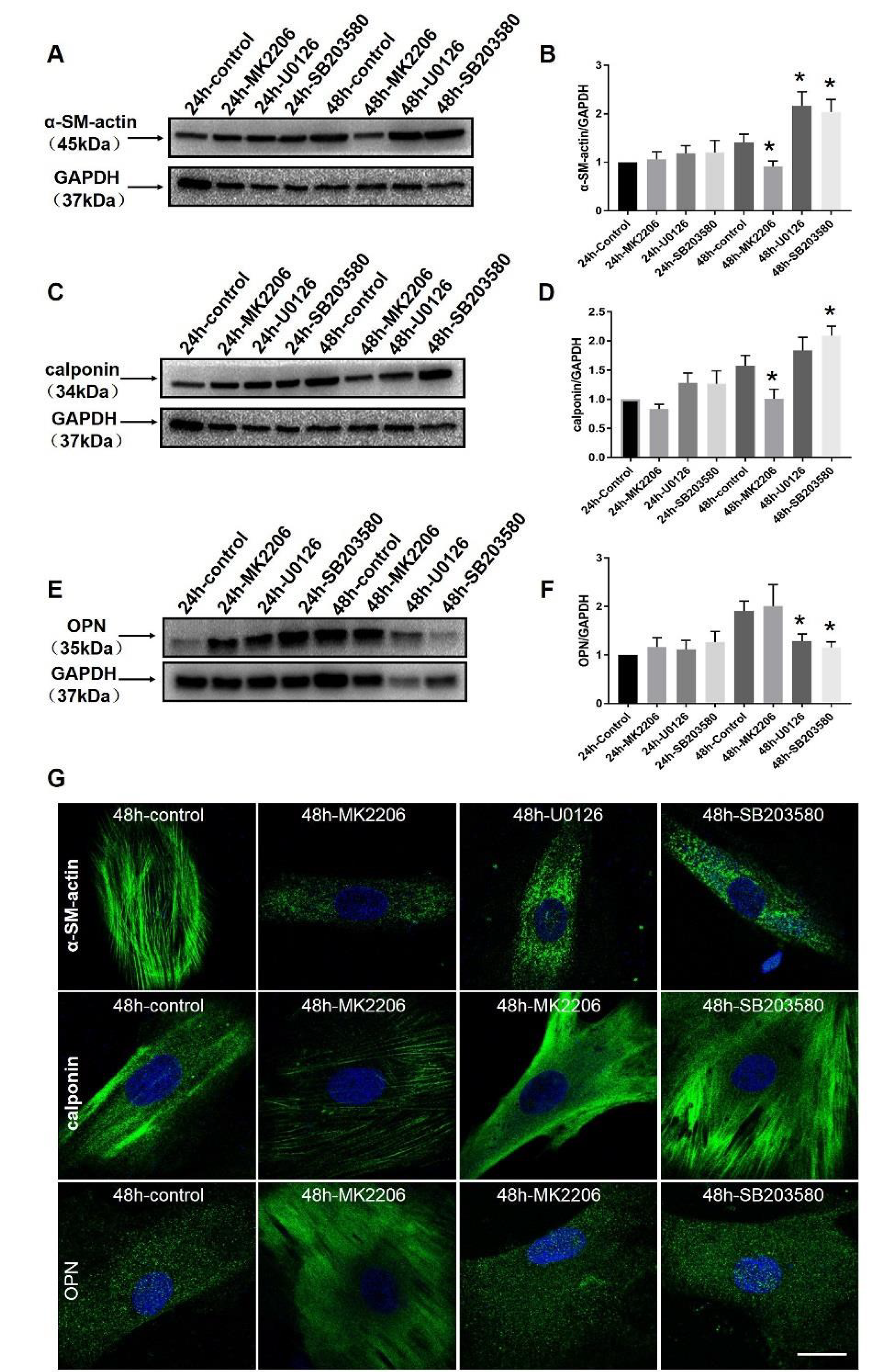
2.6. Specific Blockers Inhibit the Function of Akt and MAPK Signaling Pathways
2.7. Akt and MAPK Regulate Phenotype Switching of VSMCs in Wistar Rats and In Vitro
2.8. Phenotype Switching of the Pathological State of the Thoracic Aorta and Mesenteric Artery In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals and Exercise Protocol
4.2. Blood Pressure and Heart Rate Measurements
4.3. Material Preparation
4.4. Histological Assays
4.5. Immunohistochemistry
4.6. Wire Myography of Mesenteric Arteries
4.7. Western Blot Analysis
4.8. Cell Culture and Organ Culture
4.9. Immunofluorescence
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AMPK | AMP-activate protein kinase |
| BP | Blood pressure |
| DBP | Diastolic blood pressure |
| eNOS | Endothelial nitric oxide synthase |
| ERK | Extracellular regulated protein kinase |
| MAP | Mean arterial pressure |
| MAPK | Mitogen-activated protein kinase |
| NE | Norepinephrine |
| NO | Nitric oxide |
| PI3K | Phosphatidylinositol 3-kinase |
| PDGF–BB | Platelet derived growth factor-BB |
| PKB | Protein kinase B |
| ROS | Reactive oxygen species |
| SBP | Systolic pressure |
| SHR | Spontaneously hypertensive rat |
| VSMC | Vascular smooth muscle cell |
| WKY | Wistar-Kyoto rat |
References
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves–Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Rocic, P.; Gupte, S. Chapter 2–The role of vascular smooth muscle phenotype in coronary artery disease. Transla. Res. Coronary. Artery. Dis. 2016, 15–22. [Google Scholar] [CrossRef]
- Assunta, P.; Alfredo, G.; Cilli, C.; Antonia, P.; Andrea, G.; Sara, D.; Maria, A.; Giuliana, P.; Capani, F.; Agostino, C. Phenotype modulation in cultures of vascular smooth muscle cells from diabetic rats: Association with increased nitric oxide synthase expression and superoxide anion generation. J. Cell. Physiol. 2003, 196, 378–385. [Google Scholar]
- Huang, S.; Chen, P.; Shui, X.; He, Y.; Wang, H.; Zheng, J.; Zhang, L.; Li, J.; Xue, Y.; Chen, C.; et al. Baicalin attenuates transforming growth factor–β1–induced human pulmonary artery smooth muscle cell proliferation and phenotypic switch by inhibiting hypoxia inducible factor–1α and aryl hydrocarbon receptor expression. J. Pharm. Pharmacol. 2014, 66, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiao, Y.J.; Song, F.L.; Yang, Y.; Xiao, M.; Ling, W.H. Increased plasma S–adenosyl–homocysteine levels induce the proliferation and migration of VSMCs through an oxidative stress–ERK1/2 pathway in apoE–/– mice. Cardiovasc. Res. 2012, 95, 241–250. [Google Scholar] [CrossRef]
- Nishio, E.; Watanabe, Y. The involvement of reactive oxygen species and arachidonic acid in alpha 1–adrenoceptor–induced smooth muscle cell proliferation and migration. Br. J. Pharmacol. 2010, 121, 665–670. [Google Scholar] [CrossRef]
- Hald, E.S.; Alford, P.W. Smooth Muscle Phenotype switching in blast traumatic brain injury–induced cerebral vasospasm. Transl. Stroke. Res. 2014, 5, 385–393. [Google Scholar] [CrossRef]
- Deng, Y.; Lin, C.; Zhou, H.J.; Zhou, J.; Min, W. Smooth muscle cell differentiation: Mechanisms and models for vascular diseases. Front. Biol. 2017, 12, 392–405. [Google Scholar] [CrossRef]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Bohm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef]
- Brown, D.I.; Griendling, K.K. Regulation of Signal Transduction by Reactive Oxygen Species in the Cardiovascular System. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Guillermo, G.C.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Renna, N.F.; Heras, N.D.L.; Miatello, R.M. Pathophysiology of vascular remodeling in hypertension. Int. J. Hypertens. 2013, 2013, 808353. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.P.; Owens, G.K. Regulation of Smooth Muscle α–Actin Expression in vivo is dependent on CArG elements within the 5′and first intron promoter regions. Circ. Res. 1999, 84, 852–861. [Google Scholar] [CrossRef]
- Deruiter, M.C.; Poelmann, R.E.; Vanmunsteren, J.C.; Mironov, V.; Markwald, R.R.; Gittenbergerdegroot, A.C. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ. Res. 1997, 80, 444–451. [Google Scholar] [CrossRef]
- Duband, J.L.; Gimona, M.; Scatena, M.; Sartore, S.; Small, J.V. Calponin and SM22 as differentiation markers of smooth–muscle–spatiotemporal distribution during avian embryonic–development. Differentiation 1993, 55, 1–11. [Google Scholar] [CrossRef]
- Madsen, C.S.; Regan, C.P.; Hungerford, J.E.; White, S.L.; Manabe, I.; Owens, G.K. Smooth muscle–specific expression of the smooth muscle myosin heavy chain gene in transgenic mice requires 5’–flanking and first intronic DNA sequence. Circ. Res. 1998, 82, 908–917. [Google Scholar] [CrossRef]
- Kim, S.; Ip, H.S.; Lu, M.M.; Clendenin, C.; Parmacek, M.S. A serum response factor–dependent transcriptional regulatory program identifies distinct smooth muscle cell sublineages. Mol. Cell. Biol. 1997, 17, 2266–2278. [Google Scholar] [CrossRef]
- Speer, M.Y.; Mckee, M.D.; Guldberg, R.E.; Liaw, L.; Yang, H.Y.; Tung, E.; Karsenty, G.; Giachelli, C.M. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein–deficient mice: Evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J. Exp. Med. 2002, 196, 1047–1055. [Google Scholar] [CrossRef]
- Takahashi, M. Epiregulin as a major autocrine/paracrine Factor released from ERK– and p38MAPK–activated vascular smooth muscle cells. Circulation 2003, 108, 2524–2529. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Yang, P.; Cai, R.; Rhoda, E.; Lucy, Y.; Emily, J.A.; Colin, W.; Janet, J.; Anthony, P. Chemerin elicits potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat vasculature. J. Am. Heart Assoc. 2016, 5, e004421. [Google Scholar] [CrossRef] [PubMed]
- Wynne, B.M.; Chiao, C.W.; Webb, R.C. Vascular smooth muscle cell signaling mechanisms for contraction to Angiotensin II and endothelin–1. J. Am. Soc. Hypertens. 2009, 3, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; Leeuwen, F.N.; Touyz, R.M. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef]
- Dong, L.H.; Wen, J.K.; Liu, G.; McNutt, M.A.; Miao, S.B.; Gao, R.; Zheng, B.; Zhang, H.; Han, M. Blockade of the Ras–extracellular signal–regulated kinase 1/2 pathway is involved in smooth muscle 22[alpha]–mediated suppression of vascular smooth muscle cell proliferation and neointima hyperplasia. Arterioscl. Throm. Vas. 2010, 30, 683–691. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Kong, D.; Li, S.; Li, C.; Wang, H.; Sun, Y. Atorvastatin calcium inhibits phenotypic modulation of PDGF–BB–induced VSMCs via down–regulation the Akt signaling pathway. PLoS ONE 2015, 10, e0122577. [Google Scholar] [CrossRef]
- Ouyang, Q.F.; Han, Y.; Lin, Z.H.; Xie, H.; Xu, C.S.; Xie, L.D. Fluvastatin upregulates the alpha (1C) subunit of CaV1.2 channel expression in vascular smooth muscle cells via RhoA and ERK/p38 MAPK pathways. Dis. Markers 2014, 2014, 237067. [Google Scholar] [CrossRef]
- Ma, B.; Wells, A. The Mitogen–activated protein (MAP) kinases p38 and extracellular signal–regulated kinase (ERK) are involved in hepatocyte–mediated phenotypic switching in prostate cancer cells. J. Biol. Chem. 2014, 289, 11153–11161. [Google Scholar] [CrossRef]
- Xue, X.H.; Zhou, X.M.; Wei, W.; Chen, T.; Su, Q.P.; Tao, J.; Chen, L.D. Alisol A 24–Acetate, a Triterpenoid derived from Alisma orientale, inhibits Ox–LDL–induced phenotypic transformation and migration of rat vascular smooth muscle cells through suppressing ERK1/2 Signaling. J. Vasc. Res. 2016, 53, 291–300. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.X.; Wu, Y.; Liao, J.W.; Zeng, F.X.; Shi, L.J. Akt/eNOS and MAPK signaling pathways mediated the phenotypic switching of thoracic aorta vascular smooth muscle cells in aging/hypertensive rats. Physiol. Res. 2018, 67, 543–553. [Google Scholar] [CrossRef]
- Madonna, R.; Caterina, R.D.; Geng, Y.J. Aerobic exercise–related attenuation of arterial pulmonary hypertension: A right arrow targets the disease? Vasc. Pharmacol. 2016, 87, 6–9. [Google Scholar] [CrossRef]
- Weinstein, A.A.; Chin, L.M.K.; Keyser, R.E.; Kennedy, M.; Nathan, S.D.; Woolstenhulme, G.C.; Leighton, C. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Resp. Med. 2013, 107, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.P.; Moraes, R.S.; Vieira, P.J.; Ash, G.I.; Waclavowsky, G.; Pescatello, L.S.; Umpierre, D. Effects of aerobic exercise intensity on ambulatory blood pressure and vascular responses in resistant hypertension, a crossover trial. J. Hypertens. 2016, 34, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, S.A.; Koufaki, P.; Mercer, T.H.; Maclaughlin, H.L.; Rush, R.; Lindup, H.; Connor, E.; Jones, C.; Hendry, B.M.; Macdougall, I.C.; et al. Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: A pilot randomized controlled trial. Am. J. Kidney Dis. 2015, 65, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Roque, F.R.; Briones, A.M.; García–Redondo, A.B.; Galan, M.; Martinez–Revelles, S.; Avendario, M.S.; Cachofeiro, V.; Feman, D.T.; Vassallo, D.V.; Oliveira, E.M.; et al. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br. J. Pharmacol. 2014, 168, 686–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Zhang, H.; Chen, Y.; Liu, Y.; Zhao, T.; Zhang, L. Chronic exercise normalizes changes of Cav 1.2 and KCa 1.1 channels in mesenteric arteries from spontaneously hypertensive rats. Br. J. Pharmacol. 2015, 172, 1846–1858. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.P.; Wai, J.P.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Vina, J.; Sanchis–Gomar, F.; Martinez–Bello, V.; Gomez–Cabrera, M.C. Exercise acts as a drug: The pharmacological benefits of exercise. Br. J. Pharmacol. 2012, 167, 1–12. [Google Scholar] [CrossRef]
- Owens, G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 1995, 75, 487–517. [Google Scholar] [CrossRef]
- Fujii, K.; Umemoto, S.; Fujii, A.; Yonezawa, T.; Sakumura, T.; Matsuzaki, M. Angiotensin II type 1 receptor antagonist downregulates nonmuscle myosin heavy chains in spontaneously hypertensive rat aorta. Hypertension 1999, 33, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Quindry, J.C.; Kavazis, A.N. Exercise–induced cardioprotection against myocardial ischemia–reperfusion injury. Free Radic. Biol. Med. 2008, 44, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.O.; Lee, K.H.; Kozyreva, O. The effect of complex exercise rehabilitation program on body composition, blood pressure, blood sugar, and vessel elasticity in elderly women with obesity. J. Exerc. Rehabil. 2013, 9, 514–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.D.; Mcmorris, C.A.; Longman, R.S.; Leigh, R.; Hill, M.D.; Friedenreich, C.M.; Poulin, M.J. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol. Aging 2010, 31, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Véronique, A.C.; Fagard, R.H. Effects of endurance training on blood pressure, blood pressure–regulating mechanisms, and cardiovascular risk factors. Hypertension 2005, 46, 667–675. [Google Scholar]
- Dimeo, F.; Pagonas, N.; Seibert, F.; Arndt, R.; Zidek, W.; Westhoff, T.H. Aerobic Exercise reduces blood pressure in resistant hypertension novelty and significance. Hypertension 2012, 60, 653–658. [Google Scholar] [CrossRef]
- Konukoglu, D.; Uzun, H. Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 2017, 965, 511–540. [Google Scholar]
- Li, Q.; Shen, L.; Wang, Z.; Jiang, H.P.; Liu, L.X. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacot. 2016, 84, 106–114. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, Z.Y.; Meng, Q.T.; Zhu, J.; Lei, S.Q.; Xu, J.; Dou, J. Shen–Fu injection preconditioning inhibits myocardial ischemia–reperfusion injury in diabetic rats: Activation of eNOS via the PI3K/Akt pathway. Biomed. Res. Int. 2011, 2011, 384627. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Gatta, D.M.; Patruno, A.; Lutiis, M.A.; Quiles, J.L.; Grilli, A.; Felaco, M.; Speranza, L. Biological effect of Licochalcone C on the regulation of PI3K/Akt/eNOS and NF–κB/iNOS/NO signaling pathways in H9c2 cells in response to LPS stimulation. Int. J. Mol. Sci. 2017, 18, 690. [Google Scholar] [CrossRef]
- Alexander, M.R.; Owens, G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Ann. Rev. Physiol. 2012, 74, 13–40. [Google Scholar] [CrossRef]
- Gerthoffer, W.T. Mechanisms of Vascular Smooth Muscle Cell Migration. Circ. Res. 2007, 100, 607–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davisdusenbery, B.N.; Wu, C.; Hata, A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscl. Throm. Vas. 2011, 31, 2370–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemoto, S.; Kawahara, S.; Hashimoto, R.; Umeji, K.; Matsuda, S.; Tanaka, M.; Kubo, M.; Matsuzaki, M. Different effects of amlodipine and enalapril on the mitogen–activated protein kinase/extracellular signal–regulated kinase kinase–extracellular signal–regulated kinase pathway for induction of vascular smooth muscle cell differentiation in vivo. Hypertens. Res. 2006, 29, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.W.; Holland, J.A.; Ziegler, L.M.; Chang, M.M.; Beebe, G.; Schmitt, M.E. Identification of a functional leukocyte–type NADPH Oxidase in human endothelial cells: A potential atherogenic source of reactive oxygen species. Endothelium 1999, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Takahashi, M.; Kimura, K.; Nishida, W.; Saga, H.; Sobue, K. Changes in the balance of phosphoinositide 3–kinase/protein kinase B (Akt) and the mitogen–activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J. Cell. Biol. 1999, 145, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Juhua, D.; Xin, S.; Wanlu, L.; Yun, Z.; Xuesong, L.; Haobo, X.; Zhitao, L.; Zhen, T.; Shuyuan, G.; Jianting, Y.; et al. 5–Aminolevulinic acid–mediated sonodynamic therapy promotes phenotypic switching from dedifferentiated to differentiated phenotype via reactive oxygen species and p38 mitogen–activated protein kinase in vascular smooth muscle cells. Ultrasound Med. Biol. 2015, 41, 1681–1689. [Google Scholar]
- Zhang, X.; Chen, J.; Wang, S. Serum Amyloid A induces a vascular smooth muscle cell phenotype switch through the p38 MAPK signaling pathway. Biomed. Res. Int. 2017, 8, 4941379. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.P.; Zhang, L.N.; Tian, G. Perivascular adipose tissue–derived leptin promotes vascular smooth muscle cell phenotypic switching via p38 mitogen–activated protein kinase in metabolic syndrome rats. Exp. Biol. Med. 2014, 239, 954–965. [Google Scholar] [CrossRef]
- Millar, P.J.; Mcgowan, C.L.; Cornelissen, V.A.; Araujo, C.G.; Swaine, I.L. Evidence for the role of isometric exercise training in reducing blood pressure: Potential mechanisms and future directions. Sports Med. 2014, 44, 345–356. [Google Scholar] [CrossRef]

| Items | Stage | WKY-SED (n = 12) | WKY-EX (n = 12) | SHR-SED (n = 12) | SHR-EX (n = 12) |
|---|---|---|---|---|---|
| SBP | Initial | 134.9 ± 3.6 | 134.9 ± 3.4 | 191.6 ± 7.3 ## | 192.1 ± 3.9 |
| Final | 137.6 ± 3.8 | 131.2 ± 5.3 # | 195.8 ± 9.02 ## | 182.9 ± 3.6 **$$ | |
| DBP | Initial | 93.7 ± 4.6 | 94.0 ± 2.5 | 145.6 ± 4.2 ## | 145.4 ± 2.4 |
| Final | 98.2 ± 2.8 | 95.1 ± 3.8 | 148.4 ± 3.7 ## | 139.6 ± 2.7 *$ | |
| MAP | Initial | 107.4 ± 2.8 | 107.6 ± 3.9 | 160.9 ± 3.2 ## | 161.0 ± 2.8 |
| Final | 111.3 ± 4.03 | 107.1 ± 4.3 | 164.9 ± 5.6 ## | 154.2 ± 5.0 *$ | |
| HR | Initial | 355.4 ± 9.6 | 359.4 ± 7.3 | 412.1 ± 7.0 ## | 418.5 ± 10.1 |
| Final | 364.1 ± 8.0 | 358.5 ± 6.4 | 421.2 ± 9.0 ## | 400.2 ± 11.4 *$ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, Y.; Wu, Y.; Yu, J.; Zhang, Y.; Zeng, F.; Shi, L. Role of the Balance of Akt and MAPK Pathways in the Exercise-Regulated Phenotype Switching in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2019, 20, 5690. https://doi.org/10.3390/ijms20225690
Zhang L, Zhang Y, Wu Y, Yu J, Zhang Y, Zeng F, Shi L. Role of the Balance of Akt and MAPK Pathways in the Exercise-Regulated Phenotype Switching in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences. 2019; 20(22):5690. https://doi.org/10.3390/ijms20225690
Chicago/Turabian StyleZhang, Lin, Yanyan Zhang, Ying Wu, Jingjing Yu, Yimin Zhang, Fanxing Zeng, and Lijun Shi. 2019. "Role of the Balance of Akt and MAPK Pathways in the Exercise-Regulated Phenotype Switching in Spontaneously Hypertensive Rats" International Journal of Molecular Sciences 20, no. 22: 5690. https://doi.org/10.3390/ijms20225690
APA StyleZhang, L., Zhang, Y., Wu, Y., Yu, J., Zhang, Y., Zeng, F., & Shi, L. (2019). Role of the Balance of Akt and MAPK Pathways in the Exercise-Regulated Phenotype Switching in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences, 20(22), 5690. https://doi.org/10.3390/ijms20225690