Antibacterial Activity and Anti-Quorum Sensing Mediated Phenotype in Response to Essential Oil from Melaleuca bracteata Leaves
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Components in M. bracteata EO by GC-MS
2.2. Determination of the Antimicrobial Activity and MIC of M. bracteata EO
2.3. Quorum Sensing Inhibition (QSI) assays of M. bracteata EO
2.4. Growth Curve
2.5. Determination of Violacein
2.6. Biofilm Determination
2.7. Swarming Motility Assay
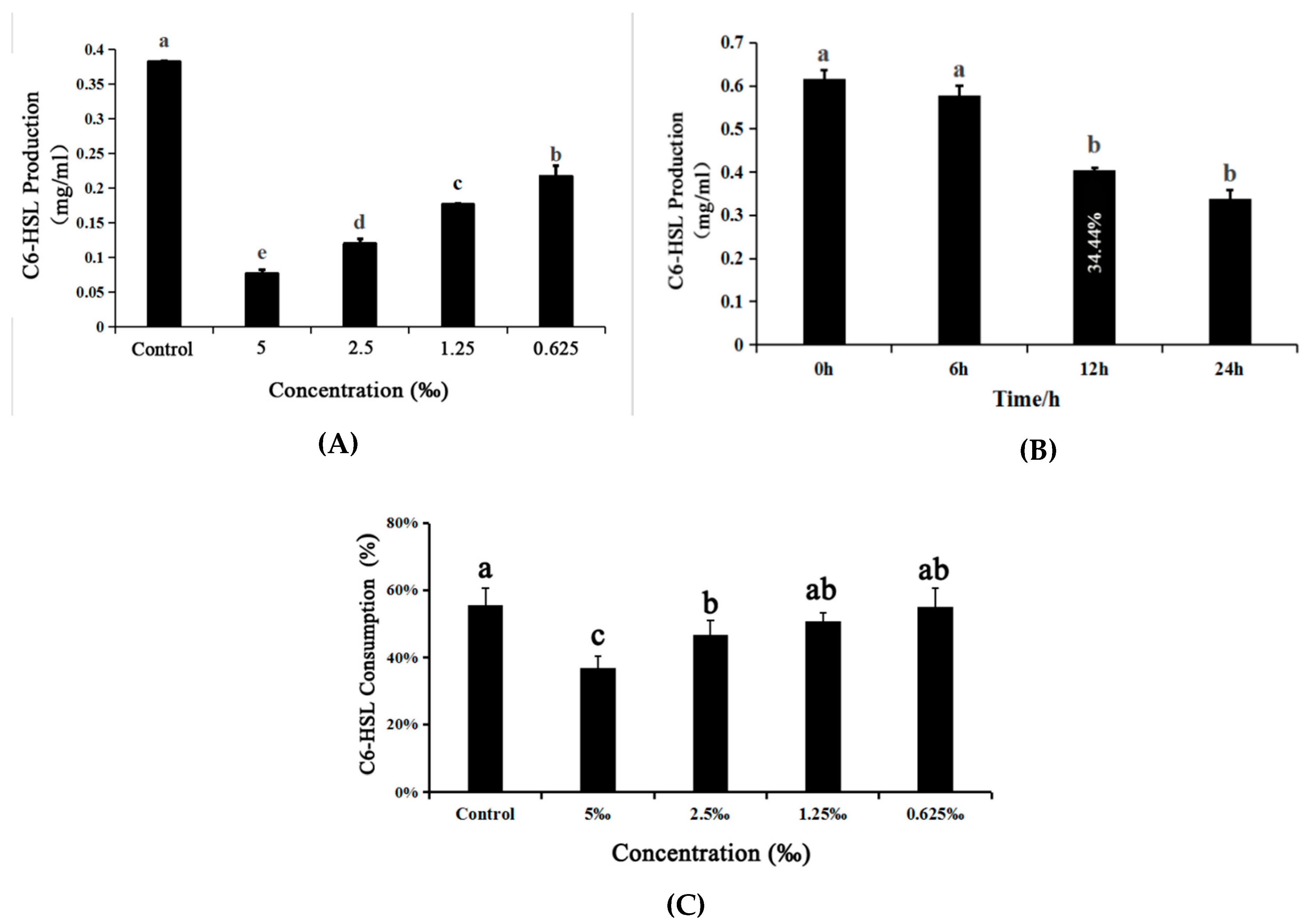
2.8. Detection of the Production of C6-HSL Signal Molecules
2.9. M. bracteata EO Reduced the Expression of the QS-Related Genes
3. Discussion
4. Materials and Methods
4.1. Essential Oil, Bacterial Strains, Medium, and Growth Conditions
4.2. Determination of Components of M. bracteata EO by GC-MS
4.3. Determination of Antimicrobial Activity and MIC of M. bracteata EO
4.4. Quorum Sensing Inhibition Assays
4.5. Growth Curve Analysis
4.6. Violacein Detection Assay
4.7. Effect of Essential Oil on Biofilm Development
4.8. Swarming Motility
4.9. Extraction and Detection of AHL
4.10. Effect of M. bracteata EO on Signaling Molecules (C6-AHL)
4.11. Gene Expression Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| QS | Quorum sensing |
| Anti-QS | Anti-quorum sensing |
| AHLs | N-acyl-homoserine lactones |
| C6-HSL | N-hexanoyl-l-homoserine lactone |
| MIC | Minimum inhibitory concentration |
| Sub-MIC | Sub-minimal inhibitory concentration |
| AI | Autoinducer |
| VF | virulence factor |
| EO | Essential oil |
| QSI | Quorum-sensing inhibitor |
References
- Iñiguez-Moreno, M. Resistance of pathogenic and spoilage microorganisms to disinfectants in the presence of organic matter and their residual effect on stainless steel and polypropylene. J. Glob. Antimicrob. Resist. 2018, 14, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, W.; Deng, J.; Liu, X.; Zhou, J.; Li, X. Antibacterial activity of Litsea cubeba essential oil and its mechanism against Botrytis cinerea. RSC Adv. 2019, 9, 28987–28995. [Google Scholar] [CrossRef]
- Chang, C.Y.; Krishnan, T.; Wang, H.; Chen, Y.; Yin, W.F.; Chong, Y.M.; Tan, L.Y.; Chong, T.M.; Chan, K.G. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep. 2014, 4, 7245. [Google Scholar] [CrossRef]
- Zhou, J.W.; Luo, H.Z.; Jiang, H.; Jian, T.K.; Chen, Z.Q.; Jia, A.Q. Hordenine, a novel quorum sensing inhibitor and anti-biofilm agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018, 66, 1620–1628. [Google Scholar] [CrossRef]
- Haque, S.; Ahmad, F.; Dar, S.A.; Jawed, A.; Mandal, R.K.; Wahid, M.; Lohani, M.; Khan, S.; Singh, V.; Akhter, N. Developments in strategies for Quorum Sensing virulence factor inhibition to combat drug resistant bacteria. Microb. Pathog. 2018, 121, 293–302. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Jamuna, B.A.; Vittal, R.R. Quorum Sensing Inhibitory and Anti-Biofilm Activity of Essential Oils and Their in vivo Efficacy in Food Systems. Food Biotechnol. 2014, 28, 269–292. [Google Scholar]
- Kothari, V.; Sharma, S.; Padia, D. Recent research advances on Chromobacterium violaceum. Asian Pac. J. Trop. Med. 2017, 10, 810–818. [Google Scholar] [CrossRef]
- Durán, N.; Justo, G.Z.; Durán, M.; Brocchi, M.; Cordi, L.; Tasic, L.; Castro, G.R.; Nakazato, G. Advances in Chromobacterium violaceum and properties of violacein-Its main secondary metabolite: A review. Biotechnol. Adv. 2016, 34, 1030–1045. [Google Scholar] [CrossRef]
- Eris, R.; Ulusoy, S. Rose, clove, chamomile essential oils and pine turpentine inhibit quorum sensing in Chromobacterium violaceum and Pseudomonas aeruginosa. J. Essent. Oil Bear. Plants 2013, 16, 126–135. [Google Scholar] [CrossRef]
- Asghar, A.; Butt, M.S.; Shahid, M.; Huang, Q. Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum. J. Food Sci. Technol. 2017, 54, 2306–2315. [Google Scholar]
- Li, C.; Liu, H.; Zhao, L.; Zhang, W.; Qiu, S.; Yang, X.; Tan, H. Antibacterial neolignans from the leaves of Melaleuca bracteata. Fitoterapia 2017, 120, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhang, W.; Chen, G.; Luo, Y. Optimization of Extraction Conditions for Maximal Phenolic, Flavonoid and Antioxidant Activity from Melaleuca bracteata Leaves Using the Response Surface Methodology. PLoS ONE 2016, 11, e0162139. [Google Scholar] [CrossRef] [PubMed]
- Adesanwo, K.J.; Shode, F.O.; Aiyelaagbe, O.O.; Rabiu, O.O.; Oyede, R.T.; Oluwole, F.S. Antisecretory and antiulcerogenic activities of the stem bark extract of Melaleuca bracteata and isolation of principles. J. Med. Plants Res. 2009, 3, 822–824. [Google Scholar]
- Siddique, S.; Parveen, Z.; Mazhar, S. Chemical composition, antibacterial and antioxidant activities of essential oils from leaves of three Melaleuca species of Pakistani flora. Arab. J. Chem. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Wang, W.; Yang, C.; Liu, J.; Zhou, L.; Shen, Y.; Wang, Z.; Chen, J.; Wu, S. Composition Analysis of Essential Oil from Melaleuca bracteata Leaves Using Ultrasound-assisted Extraction and its Antioxidative and Antimicrobial Activities. BioResources 2018, 13, 8488–8504. [Google Scholar] [CrossRef]
- Durán, M.; Faljoni-Alario, A.; Durán, N. Chromobacterium violaceum and its important metabolites--review. Folia Microbiol. 2010, 55, 535–547. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Althubiani, A.S.; Abulreesh, H.H.; Alhazza, I.M.; Aqil, F. leaves Extracts of Mangifera indicaL. Inhibit Quorum Sensing—Regulated Production of Virulence Factors and Biofilm in Test Bacteria. Front. Microbiol. 2017, 8, 727. [Google Scholar] [CrossRef]
- Verstraeten, N.; Braeken, K.; Debkumari, B.; Fauvart, M.; Fransaer, J.; Vermant, J.; Michiels, J. Living on a surface: Swarming and biofilm formation. Trends Microbiol. 2008, 16, 496–506. [Google Scholar] [CrossRef]
- Ghosh, R.; Tiwary, B.K.; Kumar, A.; Chakraborty, R. Guava leaves Extract Inhibits Quorum-Sensing and Chromobacterium violaceum Induced Lysis of Human Hepatoma Cells: Whole Transcriptome Analysis Reveals Differential Gene Expression. PLoS ONE 2014, 9, e107703. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.F.; Carvalho, C.B.; Santos, F.; Gazzinelli, R.T.; Oliveira, S.C.; Azevedo, V.; Teixeira, S.M. Chromobacterium violaceum genome: Molecular mechanisms associated with pathogenicity. Genet. Mol. Res. 2004, 3, 148–161. [Google Scholar] [PubMed]
- Bacha, K.; Tariku, Y.; Gebreyesus, F.; Zerihun, S.; Mohammed, A.; Weiland-Bräuer, N.; Schmitz, R.A.; Mulat, M. Antimicrobial and anti-Quorum Sensing activities of selected medicinal plants of Ethiopia: Implication for development of potent antimicrobial agents. BMC Microbiol. 2016, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; López-Gálvez, F.; Gil, M.I.; Tomás-Barberán, F.A.; Allende, A. Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics. Food Chem. 2009, 115, 1337–1344. [Google Scholar] [CrossRef]
- Taganna, J.C.; Quanico, J.P.; Perono, R.M.G.; Amor, E.C.; Rivera, W.L. Tannin-rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilm maturation and LasA staphylolytic activity in Pseudomonas aeruginosa. J. Ethnopharmacol. 2011, 134, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; He, C.C.; Chu, Q.H. Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett. Appl. Microbiol. 2011, 52, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.S.V.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Adhavan, P.; Kaur, G.; Princy, A.; Murugan, R. Essential oil nanoemulsions of wild patchouli attenuate multi-drug resistant gram-positive, gram-negative and Candida albicans. Ind. Crops Prod. 2017, 100, 106–116. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012, 45, 85–92. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015, 6, 420. [Google Scholar] [CrossRef]
- Stauff, D.L.; Bassler, B.L. Quorum sensing in Chromobacterium violaceum: DNA recognition and gene regulation by the cviR receptor. J. Bacteriol. 2011, 193, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Morten, H.; Michael, G. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1300–1307. [Google Scholar]
- Swem, L.R.; Swem, D.L.; O’Loughlin, C.T.; Gatmaitan, R.; Zhao, B.; Ulrich, S.M.; Bassler, B.L. A Quorum-Sensing Antagonist Targets Both Membrane-Bound and Cytoplasmic Receptors and Controls Bacterial Pathogenicity. Mol. Cell 2009, 35, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Verma, S.K.; Chauhan, A.; Venkatesha, K.; Verma, R.S.; Singh, V.R.; Darokar, M.P.; Chanotiya, C.S.; Padalia, R.C. Chemical Composition and Antibacterial Activity of Melaleuca bracteata Essential Oil from India: A Natural Source of Methyl Eugenol. Nat. Prod. Commun. 2017, 12, 1934578X1701200633. [Google Scholar] [CrossRef]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2010, 20, 378–384. [Google Scholar] [CrossRef]
- Dool, H.V.D.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Andotra, S.; Kalgotra, N.; Pandey, S.K. Syntheses, Characterization, Thermal, and Antimicrobial Studies of Lanthanum(III) Tolyl/Benzyldithiocarbonates. Bioinorg. Chem. Appl. 2014, 2014, 780631. [Google Scholar] [CrossRef] [Green Version]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.D. Anti-quorum sensing potential of Adenanthera pavonina. Pharmacogn. Res. 2015, 7, 105. [Google Scholar]
- Zhang, Y.; Kong, J.; Huang, F.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Hexanal as a QS inhibitor of extracellular enzyme activity of Erwinia carotovora and Pseudomonas fluorescens and its application in vegetables. Food Chem. 2018, 255, 1–7. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. Jove 2011, 47, e2437. [Google Scholar] [CrossRef]
- Blosser, R.S.; Gray, K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 2000, 40, 47–55. [Google Scholar] [CrossRef]
- Joshi, J.R.; Khazanov, N.; Senderowitz, H.; Burdman, S.; Lipsky, A.; Yedidia, I. Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci. Rep. 2016, 6, 38126. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| NO. | Compounds Name | Molecular Formula | Molecular Weight | Relative Content | Retention Time/min | Retention Index |
|---|---|---|---|---|---|---|
| 1 | Methyleugenol | C11H14O2 | 178.23 | 90.46% | 29.472 | 1399 |
| 2 | Methyl trans-cinnamate | C10H10O2 | 162.1852 | 4.25% | 28.864 | 1382 |
| 3 | Estragole | C10H12O | 148.2 | 0.32% | 21.064 | 1194 |
| 4 | alpha-Terpineol | C10H18O | 154.25 | 0.23% | 20.905 | 1190 |
| 5 | 3,7-Dimethyl-1,6-octadien-3-yl acetate 3,7 2-aminobenzoate | C17H23NO2 | 273.37 | 0.22% | 16.42 | 1098 |
| 6 | 2,7-Dimethyl-2,6-octadien-1-ol | C10H18O | 154.2493 | 0.18% | 23.219 | 1240 |
| 7 | Citronellol | C10H20O | 156.27 | 0.14% | 22.719 | 1229 |
| 8 | 2,2-Dimethoxybutane | C6H14O2 | 118.17 | 0.06% | 3.626 | - |
| 9 | 3-Hexen-1-ol | C6H12O | 100.16 | 0.05% | 5.735 | 838 |
| 10 | Z-Methyl geranate | C11H18O2 | 182.26 | 0.05% | 26.75 | 1321 |
| 11 | Myrcene | C10H16 | 136.23 | 0.05% | 23.411 | 1244 |
| 12 | Elemicin | C12H16O3 | 208.25 | 0.04% | 33.566 | 1544 |
| 13 | Citral | C10H16O | 152.23 | 0.04% | 24.591 | 1269 |
| 14 | Terpinen-4-ol | C10H18O | 154.25 | 0.03% | 20.159 | 1175 |
| 15 | Methyl propionate | C4H8O2 | 88.11 | 0.03% | 2.154 | - |
| 16 | Espatulenol | C15H24O | 220.3505 | 0.03% | 34.417 | 1575 |
| 17 | (3aS,3bR,4S,7R,7aR)-7-methyl-3 | C15H24 | 204.3511 | 0.03% | 31.836 | 1481 |
| 18 | Methyl 3,4,5-trimethoxybenzoate | C11H14O5 | 226.23 | 0.03% | 36.955 | 1718 |
| 19 | 4-epi-cubedol | C15H26O | 222 | 0.03% | 36.201 | 1664 |
| 20 | epi-a-Cadinol | C15H26O | 222.3663 | 0.03% | 36.368 | 1673 |
| 21 | (R)-Lavandulyl acetate | C12H20O2 | 196 | 0.02% | 21.881 | 1211 |
| 22 | 1,3,8-p-Menthatriene | C10H14 | 134.2182 | 0.02% | 12.847 | 1100 |
| 23 | 2-Carene(7CI,8CI) | C10H16 | 136.234 | 0.02% | 15.707 | 1083 |
| 24 | Methyl butyrate | C5H10O2 | 102.13 | 0.02% | 3.038 | - |
| 25 | Decane | C10H22 | 142.28 | 0.02% | 11.826 | 999 |
| 26 | Dispiro[2.0.2.5]undecane, 8-methylene | C12H18 | 162.27132 | 0.02% | 23.878 | 1254 |
| 27 | Copaene(6CI) | C15H24 | 204.3511 | 0.02% | 32.245 | 1495 |
| 28 | 2-(4-Methylphenyl)propan-2-ol | C10H14O | 150.22 | 0.02% | 20.484 | 1182 |
| 29 | trans-α-Bergamotene | C15H24 | 204.35106 | 0.02% | 23.382 | 1243 |
| Total | 96.49% |
| Bacterial Strains | Concentration/Antimicrobial Diameters (mm) | MIC | |||||
|---|---|---|---|---|---|---|---|
| 80‰ | 40‰ | 20‰ | 10‰ | Methanol | Kanamycin (250 µg/mL) | ||
| Dickeya dadantii Onc5 | 11.89 ± 0.246 ah | 10.70 ± 0.291 abg | 10.03 ± 0.303 bcfgh | 9.00 ± 0.518 cfg | 6.00 ± 0.00 | 20.21 ± 0.11 | 10‰ |
| Staphylococcus aureus ATCC25933 | 15.28 ± 1.083 ae | 13.05 ± 0.323 be | 10.98 ± 0.520 cef | 9.21 ± 0.078 def | 6.00 ± 0.00 | 26.01 ± 0.131 | 2.5‰ |
| Escherichia coli ATCC25922 | 11.38 ± 0.286 ai | 10.15 ± 0.451 bgh | 9.325 ± 0.343 bh | 8.33 ± 0.354 cgh | 6.00 ± 0.00 | 18.78 ± 1.032 | 10‰ |
| Pseudomonas aeruginosa PAO1 | 10.47 ± 0.186 aj | 9.82 ± 0.279 bh | 9.45 ± 0.236 ch | 8.15 ± 0.193 dh | 6.00 ± 0.00 | 17.23 ± 0.187 | 20‰ |
| Serratia marcescens MG1 | 14.11 ± 0.789 af | 11.81 ± 0.363 bf | 10.57 ± 0.191 cefg | 9.87 ± 0.484 de | 6.00 ± 0.00 | 25.08 ± 1.31 | 2.5‰ |
| Pseudomonas spp. | 13.42 ± 0.715 ag | 12.49 ± 0.308 bef | 11.50 ± 0.236 ce | 9.69 ± 0.315 def | 6.00 ± 0.00 | 23.17 ± 0.33 | 5‰ |
| Chromobacterium violaceum ATCC31532 | 11.55 ± 0.34 ai | 10.86 ± 0.49 ag | 9.80 ± 0.27 bgh | 8.03 ± 0.26 ch | 6.00 ± 0.00 | 21.61 ± 1.029 | 10‰ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Huang, X.; Yang, H.; Niu, X.; Li, D.; Yang, C.; Li, L.; Zou, L.; Qiu, Z.; Wu, S.; et al. Antibacterial Activity and Anti-Quorum Sensing Mediated Phenotype in Response to Essential Oil from Melaleuca bracteata Leaves. Int. J. Mol. Sci. 2019, 20, 5696. https://doi.org/10.3390/ijms20225696
Wang W, Huang X, Yang H, Niu X, Li D, Yang C, Li L, Zou L, Qiu Z, Wu S, et al. Antibacterial Activity and Anti-Quorum Sensing Mediated Phenotype in Response to Essential Oil from Melaleuca bracteata Leaves. International Journal of Molecular Sciences. 2019; 20(22):5696. https://doi.org/10.3390/ijms20225696
Chicago/Turabian StyleWang, Wenting, Xiaoqin Huang, Huixiang Yang, Xianqian Niu, Dongxiang Li, Chao Yang, Liang Li, Liting Zou, Ziwen Qiu, Shaohua Wu, and et al. 2019. "Antibacterial Activity and Anti-Quorum Sensing Mediated Phenotype in Response to Essential Oil from Melaleuca bracteata Leaves" International Journal of Molecular Sciences 20, no. 22: 5696. https://doi.org/10.3390/ijms20225696
APA StyleWang, W., Huang, X., Yang, H., Niu, X., Li, D., Yang, C., Li, L., Zou, L., Qiu, Z., Wu, S., & Li, Y. (2019). Antibacterial Activity and Anti-Quorum Sensing Mediated Phenotype in Response to Essential Oil from Melaleuca bracteata Leaves. International Journal of Molecular Sciences, 20(22), 5696. https://doi.org/10.3390/ijms20225696





