An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens
Abstract
1. Introduction
2. Serotonin Synthesis and Effects
3. Sex Hormone Influence on Serotonin
4. Irritable Bowel Syndrome (IBS)
- (1)
- Related to defecation;
- (2)
- Associated with a change in stool frequency;
- (3)
- Associated with a change in stool form (appearance).
5. Migraine
6. Primary Non-Migraine Headache
7. Fibromyalgia
8. Chronic Fatigue Syndrome
9. Serotonin Modulating Analgesics
10. Discussion
11. Conclusions
12. Limitations
Funding
Conflicts of Interest
References
- Maurer, A.J.; Lissounov, A.; Knezevic, I.; Candido, K.D.; Knezevic, N.N. Pain and sex hormones: A review of current understanding. Pain Manag. 2016, 6, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. In Center for Disease Control and Prevention: Morbidity and Mortality Weekly Report (MMWR); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018; pp. 1001–1006. [Google Scholar]
- Committe, I.P.R.C. National Pain Strategy: A Comprehensive Population Health-Level Strategy for Pain; US Department of Health and Human Services, Natoinal Institutes of Health: Washington, DC, USA, 2016. [Google Scholar]
- Ruan, X.; Kaye, A.D. A Call for Saving Interdisciplinary Pain Management. J. Orthop. Sports Phys. Ther. 2016, 46, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.S.; Smith, B.H.; Blyth, F.M. Pain and the global burden of disease. Pain 2016, 157, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.H.; Elliott, A.M.; Chambers, W.A.; Smith, W.C.; Hannaford, P.C.; Penny, K. The impact of chronic pain in the community. Fam. Pract. 2001, 18, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Gureje, O.; Von Korff, M.; Simon, G.E.; Gater, R. Persistent pain and well-being: A World Health Organization Study in Primary Care. JAMA 1998, 280, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Altamirano, J.L.; Olmos-Hernandez, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Feng, C.C.; Liu, Q.; Zhang, L.Y.; Dong, X.; Liu, Z.L.; Cao, Z.J.; Mo, J.Z.; Li, Y.; Fang, J.Y.; et al. Vagal afferents mediate antinociception of estrogen in a rat model of visceral pain: The involvement of intestinal mucosal mast cells and 5-hydroxytryptamine 3 signaling. J. Pain 2014, 15, 204–217. [Google Scholar] [CrossRef]
- Craft, R.M. Modulation of pain by estrogens. Pain 2007, 132 (Suppl. 1), S3–S12. [Google Scholar] [CrossRef]
- Marcus, D.A. Interrelationships of neurochemicals, estrogen, and recurring headache. Pain 1995, 62, 129–139. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Sutcliffe, J.G. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 2004, 25, 481–486. [Google Scholar] [CrossRef]
- Martin, V.T.; Behbehani, M. Ovarian hormones and migraine headache: Understanding mechanisms and pathogenesis—Part I. Headache 2006, 46, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Bardin, L.; Lavarenne, J.; Eschalier, A. Serotonin receptor subtypes involved in the spinal antinociceptive effect of 5-HT in rats. Pain 2000, 86, 11–18. [Google Scholar] [CrossRef]
- Obata, H.; Saito, S.; Sasaki, M.; Ishizaki, K.; Goto, F. Antiallodynic effect of intrathecally administered 5-HT(2) agonists in rats with nerve ligation. Pain 2001, 90, 173–179. [Google Scholar] [CrossRef]
- Sasaki, M.; Obata, H.; Kawahara, K.; Saito, S.; Goto, F. Peripheral 5-HT2A receptor antagonism attenuates primary thermal hyperalgesia and secondary mechanical allodynia after thermal injury in rats. Pain 2006, 122, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., III. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar]
- Gustafson, E.L.; Durkin, M.M.; Bard, J.A.; Zgombick, J.; Branchek, T.A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996, 117, 657–666. [Google Scholar] [CrossRef]
- Kukushkin, M.L.; Igon’kina, S.I. Role of 5-HT3 receptors in the mechanisms of central pain syndrome. Bull. Exp. Biol. Med. 2003, 135, 552–555. [Google Scholar] [CrossRef]
- Carpenter, D.O. Neural mechanisms of emesis. Can. J. Physiol. Pharmacol. 1990, 68, 230–236. [Google Scholar] [CrossRef]
- Lieba-Samal, D.; Wober, C. Sex hormones and primary headaches other than migraine. Curr. Pain Headache Rep. 2011, 15, 407–414. [Google Scholar] [CrossRef]
- Amandusson, A.; Blomqvist, A. Estrogenic influences in pain processing. Front. Neuroendocr. 2013, 34, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Bethea, C.L.; Lu, N.Z.; Gundlah, C.; Streicher, J.M. Diverse actions of ovarian steroids in the serotonin neural system. Front. Neuroendocr. 2002, 23, 41–100. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; Simonian, S.X.; Thanky, N.R.; Bicknell, R.J. Oestrogen modulation of noradrenaline neurotransmission. In Neuronal and Cognitive Effects of Oestrogens: Novartis Foundation Symposium; John Wiley & Sons: Hoboken, NJ, USA, 2000; Volume 230, pp. 74–85, Discussion 85–93. [Google Scholar]
- Malyala, A.; Kelly, M.J.; Ronnekleiv, O.K. Estrogen modulation of hypothalamic neurons: Activation of multiple signaling pathways and gene expression changes. Steroids 2005, 70, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Cyr, M.; Ghribi, O.; Thibault, C.; Morissette, M.; Landry, M.; Di Paolo, T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res. Rev. 2001, 37, 153–161. [Google Scholar] [CrossRef]
- Pecins-Thompson, M.; Brown, N.A.; Kohama, S.G.; Bethea, C.L. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J. Neurosci. 1996, 16, 7021–7029. [Google Scholar] [CrossRef]
- Smith, L.J.; Henderson, J.A.; Abell, C.W.; Bethea, C.L. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology 2004, 29, 2035–2045. [Google Scholar] [CrossRef]
- Pecins-Thompson, M.; Bethea, C.L. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience 1999, 89, 267–277. [Google Scholar] [CrossRef]
- Moses, E.L.; Drevets, W.C.; Smith, G.; Mathis, C.A.; Kalro, B.N.; Butters, M.A.; Leondires, M.P.; Greer, P.J.; Lopresti, B.; Loucks, T.L.; et al. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: A PET study. Biol. Psychiatry 2000, 48, 854–860. [Google Scholar] [CrossRef]
- Hirvonen, J.; Kajander, J.; Allonen, T.; Oikonen, V.; Någren, K.; Hietala, J. Measurement of serotonin 5-HT1A receptor binding using positron emission tomography and [carbonyl-(11)C]WAY-100635-considerations on the validity of cerebellum as a reference region. J. Cereb. Blood Flow Metab. 2007, 27, 185–195. [Google Scholar] [CrossRef]
- Martikainen, I.K.; Hirvonen, J.; Kajander, J.; Hagelberg, N.; Mansikka, H.; Någren, K.; Hietala, J.; Pertovaara, A. Correlation of human cold pressor pain responses with 5-HT(1A) receptor binding in the brain. Brain Res. 2007, 1172, 21–31. [Google Scholar] [CrossRef]
- Parsey, R.V.; Slifstein, M.; Hwang, D.R.; Abi-Dargham, A.; Simpson, N.; Mawlawi, O.; Guo, N.N.; Van Heertum, R.; Mann, J.J.; Laruelle, M. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: Comparison of arterial and reference tisssue input functions. J. Cereb. Blood Flow Metab. 2000, 20, 1111–1133. [Google Scholar] [CrossRef] [PubMed]
- El-Yassir, N.; Fleetwood-Walker, S.M.; Mitchell, R. Heterogeneous effects of serotonin in the dorsal horn of rat: The involvement of 5-HT1 receptor subtypes. Brain Res. 1988, 456, 147–158. [Google Scholar] [CrossRef]
- Zemlan, F.P.; Kow, L.M.; Pfaff, D.W. Spinal serotonin (5-HT) receptor subtypes and nociception. J. Pharmacol. Exp. Ther. 1983, 226, 477–485. [Google Scholar] [PubMed]
- Boonyaratanakornkit, V.; Edwards, D.P. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin. Reprod. Med. 2007, 25, 139–153. [Google Scholar] [CrossRef]
- Glatzle, J.; Sternini, C.; Robin, C.; Zittel, T.T.; Wong, H.; Reeve, J.R., Jr.; Raybould, H.E. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 2002, 123, 217–226. [Google Scholar] [CrossRef]
- Meseguer, A.; Puche, C.; Cabero, A. Sex steroid biosynthesis in white adipose tissue. Horm. Metab. Res. 2002, 34, 731–736. [Google Scholar] [CrossRef]
- Rybaczyk, L.A.; Bashaw, M.J.; Pathak, D.R.; Moody, S.M.; Gilders, R.M.; Holzschu, D.L. An overlooked connection: Serotonergic mediation of estrogen-related physiology and pathology. BMC Womens Health 2005, 5, 12. [Google Scholar] [CrossRef]
- Wissink, S.; van der Burg, B.; Katzenellenbogen, B.S.; van der Saag, P.T. Synergistic activation of the serotonin-1A receptor by nuclear factor-kappa B and estrogen. Mol. Endocrinol. 2001, 15, 543–552. [Google Scholar]
- Riad, M.; Watkins, K.C.; Doucet, E.; Hamon, M.; Descarries, L. Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors). J. Neurosci. 2001, 21, 8378–8386. [Google Scholar] [CrossRef]
- Zhang, Y.; D’Souza, D.; Raap, D.K.; Garcia, F.; Battaglia, G.; Muma, N.A.; Van de Kar, L.D. Characterization of the functional heterologous desensitization of hypothalamic 5-HT(1A) receptors after 5-HT(2A) receptor activation. J. Neurosci. 2001, 21, 7919–7927. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016. [Google Scholar] [CrossRef]
- Moayyedi, P.; Mearin, F.; Azpiroz, F.; Andresen, V.; Barbara, G.; Corsetti, M.; Emmanuel, A.; Hungin, A.P.S.; Layer, P.; Stanghellini, V.; et al. Irritable bowel syndrome diagnosis and management: A simplified algorithm for clinical practice. United Eur. Gastroenterol. J. 2017, 5, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Payne, S. Sex, gender, and irritable bowel syndrome: Making the connections. Gend. Med. 2004, 1, 18–28. [Google Scholar] [CrossRef]
- Drossman, D.A.; Thompson, W.G. The irritable bowel syndrome: Review and a graduated multicomponent treatment approach. Ann. Intern. Med. 1992, 116, 1009–1016. [Google Scholar] [CrossRef]
- Kane, S.V.; Sable, K.; Hanauer, S.B. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: A prevalence study. Am. J. Gastroenterol. 1998, 93, 1867–1872. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Barber, M.D.; Graff, L.A.; Shen, B. Symptomatology of irritable bowel syndrome and inflammatory bowel disease during the menstrual cycle. Gastroenterol. Rep. 2015, 3, 185–193. [Google Scholar] [CrossRef]
- Wald, A.; Van Thiel, D.H.; Hoechstetter, L.; Gavaler, J.S.; Egler, K.M.; Verm, R.; Scott, L.; Lester, R. Gastrointestinal transit: The effect of the menstrual cycle. Gastroenterology 1981, 80, 1497–1500. [Google Scholar] [CrossRef]
- Cheung, C.K.; Wu, J.C. Genetic polymorphism in pathogenesis of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 17693–17698. [Google Scholar] [CrossRef]
- Gu, Q.Y.; Zhang, J.; Feng, Y.C.; Dai, G.R.; Du, W.P. Association of genetic polymorphisms in HTR3A and HTR3E with diarrhea predominant irritable bowel syndrome. Int. J. Clin. Exp. Med. 2015, 8, 4581–4585. [Google Scholar]
- Kapeller, J.; Houghton, L.A.; Mönnikes, H.; Walstab, J.; Möller, D.; Bönisch, H.; Burwinkel, B.; Autschbach, F.; Funke, B.; Lasitschka, F.; et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum. Mol. Genet. 2008, 17, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Saria, A.; Javorsky, F.; Humpel, C.; Gamse, R. 5-HT3 receptor antagonists inhibit sensory neuropeptide release from the rat spinal cord. Neuroreport 1990, 1, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Zierau, O.; Zenclussen, A.C.; Jensen, F. Role of female sex hormones, estradiol and progesterone, in mast cell behavior. Front. Immunol. 2012, 3, 169. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Gargano, L.; Morselli-Labate, A.M.; Santini, D.; Cogliandro, R.F.; De Giorgio, R.; Stanghellini, V.; Corinaldesi, R.; Barbara, G. Mucosal immune activation in irritable bowel syndrome: Gender-dependence and association with digestive symptoms. Am. J. Gastroenterol. 2009, 104, 392–400. [Google Scholar] [CrossRef]
- Li, T.J.; Yu, B.P.; Dong, W.G.; Luo, H.S.; Xu, L.; Li, M.Q. Ovarian hormone modulates 5-hydroxytryptamine 3 receptors mRNA expression in rat colon with restraint stress-induced bowel dysfunction. World J. Gastroenterol. 2004, 10, 2723–2726. [Google Scholar] [CrossRef]
- Adeyemo, M.A.; Spiegel, B.M.; Chang, L. Meta-analysis: Do irritable bowel syndrome symptoms vary between men and women? Aliment. Pharmacol. Ther. 2010, 32, 738–755. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Abdollahi, M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: A meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin. Ther. 2008, 30, 884–901. [Google Scholar] [CrossRef]
- Cremonini, F.; Nicandro, J.P.; Atkinson, V.; Shringarpure, R.; Chuang, E.; Lembo, A. Randomised clinical trial: Alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment. Pharmacol. Ther. 2012, 36, 437–448. [Google Scholar] [CrossRef]
- Berman, S.; Munakata, J.; Naliboff, B.D.; Chang, L.; Mandelkern, M.; Silverman, D.; Kovalik, E.; Mayer, E.A. Gender differences in regional brain response to visceral pressure in IBS patients. Eur. J. Pain 2000, 4, 157–172. [Google Scholar] [CrossRef]
- Koch, K.M.; Corrigan, B.W.; Manzo, J.; James, C.D.; Scott, R.J.; Stead, A.G.; Kersey, K.E. Alosetron repeat dose pharmacokinetics, effects on enzyme activities, and influence of demographic factors. Aliment. Pharmacol. Ther. 2004, 20, 223–230. [Google Scholar] [CrossRef]
- Naliboff, B.D.; Berman, S.; Chang, L.; Derbyshire, S.W.; Suyenobu, B.; Vogt, B.A.; Mandelkern, M.; Mayer, E.A. Sex-related differences in IBS patients: Central processing of visceral stimuli. Gastroenterology 2003, 124, 1738–1747. [Google Scholar] [CrossRef]
- Kozlowski, C.M.; Green, A.; Grundy, D.; Boissonade, F.M.; Bountra, C. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut 2000, 46, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Cheskin, L.J.; Heller, B.R.; Robinson, J.C.; Crowell, M.D.; Benjamin, C.; Schuster, M.M. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology 1990, 98, 1485–1489. [Google Scholar] [CrossRef]
- Houghton, L.A.; Lea, R.; Jackson, N.; Whorwell, P.J. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut 2002, 50, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Grundy, D. Neuroanatomy of visceral nociception: Vagal and splanchnic afferent. Gut 2002, 51 (Suppl. 1), i2–i5. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Shechter, A.; Rasmussen, B.K. Migraine prevalence. A review of population-based studies. Neurology 1994, 44 (Suppl. 4), S17–S23. [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Johannes, C.B.; Linet, M.S.; Stewart, W.F.; Celentano, D.D.; Lipton, R.B.; Szklo, M. Relationship of headache to phase of the menstrual cycle among young women: A daily diary study. Neurology 1995, 45, 1076–1082. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Chia, H.; Vohrah, R.C.; Wilkinson, M. Migraine and menstruation: A pilot study. Cephalalgia 1990, 10, 305–310. [Google Scholar] [CrossRef]
- Stewart, W.F.; Lipton, R.B.; Chee, E.; Sawyer, J.; Silberstein, S.D. Menstrual cycle and headache in a population sample of migraineurs. Neurology 2000, 55, 1517–1523. [Google Scholar] [CrossRef]
- Eidelman, B.H.; Mendelow, A.D.; McCalden, T.A.; Bloom, D.S. Potentiation of the cerebrovascular response to intra-arterial 5-hydroxytryptamine. Am. J. Physiol. 1978, 234, H300–H304. [Google Scholar] [CrossRef] [PubMed]
- Kugaya, A.; Epperson, C.N.; Zoghbi, S.; van Dyck, C.H.; Hou, Y.; Fujita, M.; Staley, J.K.; Garg, P.K.; Seibyl, J.P.; Innis, R.B. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am. J. Psychiatry 2003, 160, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; McCarson, K.E.; Welch, K.M.; Berman, N.E. Mechanisms of pain modulation by sex hormones in migraine. Headache 2011, 51, 905–922. [Google Scholar] [CrossRef] [PubMed]
- Nahin, R.L.; Byers, M.R. Adjuvant-induced inflammation of rat paw is associated with altered calcitonin gene-related peptide immunoreactivity within cell bodies and peripheral endings of primary afferent neurons. J. Comp. Neurol. 1994, 349, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cheng, J.; Han, J.S.; Wan, Y. Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund’s adjuvant in rats. Neuroreport 2004, 15, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Dostrovsky, J.O.; Davis, K.D.; Kawakita, K. Central mechanisms of vascular headaches. Can. J. Physiol. Pharmacol. 1991, 69, 652–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asghari, R.; Lung, M.S.; Pilowsky, P.M.; Connor, M. Sex differences in the expression of serotonin-synthesizing enzymes in mouse trigeminal ganglia. Neuroscience 2011, 199, 429–437. [Google Scholar] [CrossRef]
- Todorovic, S.; Anderson, E.G. Serotonin preferentially hyperpolarizes capsaicin-sensitive C type sensory neurons by activating 5-HT1A receptors. Brain Res. 1992, 585, 212–218. [Google Scholar] [CrossRef]
- Cardenas, C.G.; Del Mar, L.P.; Cooper, B.Y.; Scroggs, R.S. 5HT4 receptors couple positively to tetrodotoxin-insensitive sodium channels in a subpopulation of capsaicin-sensitive rat sensory neurons. J. Neurosci. 1997, 17, 7181–7189. [Google Scholar] [CrossRef]
- Del Mar, L.P.; Cardenas, C.G.; Scroggs, R.S. Serotonin inhibits high-threshold Ca2+ channel currents in capsaicin-sensitive acutely isolated adult rat DRG neurons. J. Neurophysiol. 1994, 72, 2551–2554. [Google Scholar] [CrossRef]
- Loyd, D.R.; Weiss, G.; Henry, M.A.; Hargreaves, K.M. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain 2011, 152, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Ikemi, Y.; Murakami, M.; Imagawa, T.; Otsuguro, K.; Ito, S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J. Physiol. 2006, 576 Pt 3, 809–822. [Google Scholar] [CrossRef]
- Chugani, D.C.; Niimura, K.; Chaturvedi, S.; Muzik, O.; Fakhouri, M.; Lee, M.L.; Chugani, H.T. Increased brain serotonin synthesis in migraine. Neurology 1999, 53, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T.; Behbehani, M. Ovarian hormones and migraine headache: Understanding mechanisms and pathogenesis—Part 2. Headache 2006, 46, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Puri, S.; Svojanovsky, S.R.; Mathur, S.; Macgregor, R.R.; Klein, R.M.; Welch, K.M.; Berman, N.E. Effects of oestrogen on trigeminal ganglia in culture: Implications for hormonal effects on migraine. Cephalalgia 2006, 26, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Tognoloni, M.; Russo, S.; Vulcano, M.R.; Feleppa, M.; Malà, M.; Sartori, M.; Gallai, V. Variations in the platelet arginine/nitric oxide pathway during the ovarian cycle in females affected by menstrual migraine. Cephalalgia 1996, 16, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Brandes, J.L. The influence of estrogen on migraine: A systematic review. JAMA 2006, 295, 1824–1830. [Google Scholar] [CrossRef]
- Cassidy, E.M.; Tomkins, E.; Dinan, T.; Hardiman, O.; O’Keane, V. Central 5-HT receptor hypersensitivity in migraine without aura. Cephalalgia 2003, 23, 29–34. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Bonifazi, M. Sex hormones, central nervous system and pain. Horm. Behav. 2006, 50, 1–7. [Google Scholar] [CrossRef]
- Silberstein, S.D. Hormone-related headache. Med. Clin. N. Am. 2001, 85, 1017–1035. [Google Scholar] [CrossRef]
- Rasmussen, B.K.; Jensen, R.; Schroll, M.; Olesen, J. Epidemiology of headache in a general population—A prevalence study. J. Clin. Epidemiol. 1991, 44, 1147–1157. [Google Scholar] [CrossRef]
- Barea, L.M.; Tannhauser, M.; Rotta, N.T. An epidemiologic study of headache among children and adolescents of southern Brazil. Cephalalgia 1996, 16, 545–549, discussion 523. [Google Scholar] [CrossRef] [PubMed]
- Laurell, K.; Larsson, B.; Eeg-Olofsson, O. Prevalence of headache in Swedish schoolchildren, with a focus on tension-type headache. Cephalalgia 2004, 24, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Langemark, M.; Olesen, J. Pericranial tenderness in tension headache. A blind, controlled study. Cephalalgia 1987, 7, 249–255. [Google Scholar] [CrossRef]
- Jensen, R.; Rasmussen, B.K.; Pedersen, B.; Olesen, J. Muscle tenderness and pressure pain thresholds in headache. A population study. Pain 1993, 52, 193–199. [Google Scholar] [CrossRef]
- Spierings, E.L.; Ranke, A.H.; Honkoop, P.C. Precipitating and aggravating factors of migraine versus tension-type headache. Headache 2001, 41, 554–558. [Google Scholar] [CrossRef]
- Zivadinov, R.; Willheim, K.; Sepic-Grahovac, D.; Jurjevic, A.; Bucuk, M.; Brnabic-Razmilic, O.; Relja, G.; Zorzon, M. Migraine and tension-type headache in Croatia: A population-based survey of precipitating factors. Cephalalgia 2003, 23, 336–343. [Google Scholar] [CrossRef]
- Torelli, P.; Beghi, E.; Manzoni, G.C. Cluster headache prevalence in the Italian general population. Neurology 2005, 64, 469–474. [Google Scholar] [CrossRef]
- Ekbom, K.; Waldenlind, E. Cluster headache in women: Evidence of hypofertility(?) Headaches in relation to menstruation and pregnancy. Cephalalgia 1981, 1, 167–174. [Google Scholar] [CrossRef]
- Peatfield, R.C.; Petty, R.G.; Rose, F.C. Cluster headache in women. Cephalalgia 1982, 2, 171–172. [Google Scholar] [CrossRef]
- Fanciullacci, M.; Alessandri, M.; Figini, M.; Geppetti, P.; Michelacci, S. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain 1995, 60, 119–123. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain 1994, 117 Pt 3, 427–434. [Google Scholar] [CrossRef]
- Malick, A.; Strassman, R.M.; Burstein, R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J. Neurophysiol. 2000, 84, 2078–2112. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.L. Fibromyalgia syndrome: An emerging but controversial condition. JAMA 1987, 257, 2782–2787. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Martinez-Jauand, M.; Sitges, C.; Femenia, J.; Cifre, I.; González, S.; Chialvo, D.; Montoya, P. Age-of-onset of menopause is associated with enhanced painful and non-painful sensitivity in fibromyalgia. Clin. Rheumatol. 2013, 32, 975–981. [Google Scholar] [CrossRef]
- Vincent, A.; Lahr, B.D.; Wolfe, F.; Clauw, D.J.; Whipple, M.O.; Oh, T.H.; Barton, D.L.; St Sauver, J. Prevalence of fibromyalgia: A population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res. 2013, 65, 786–792. [Google Scholar] [CrossRef]
- Wolfe, F.; Brähler, E.; Hinz, A.; Häuser, W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: Results from a survey of the general population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Pomares, F.B.; Funck, T.; Feier, N.A.; Roy, S.; Daigle-Martel, A.; Ceko, M.; Narayanan, S.; Araujo, D.; Thiel, A.; Stikov, N.; et al. Histological Underpinnings of Grey Matter Changes in Fibromyalgia Investigated Using Multimodal Brain Imaging. J. Neurosci. 2017, 37, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J. Further strategies for treating fibromyalgia: The role of serotonin and norepinephrine reuptake inhibitors. Am. J. Med. 2009, 122, S44–S55. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Rodriguez, M.E. Mechanisms of disease: Pain in fibromyalgia syndrome. Nat. Clin. Pract. Rheumatol. 2006, 2, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Houvenagel, E.; Forzy, G.; Leloire, O.; Gallois, P.; Hary, S.; Hautecoeur, P.; Convain, L.; Henniaux, M.; Vincent, G.; Dhondt, J.L. Cerebrospinal fluid monoamines in primary fibromyalgia. Rev. Rhum. Mal. Osteoartic. 1990, 57, 21–23. [Google Scholar] [PubMed]
- Russell, I.J.; Vaeroy, H.; Javors, M.; Nyberg, F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992, 35, 550–556. [Google Scholar] [CrossRef]
- Buskila, D.; Press, J. Neuroendocrine mechanisms in fibromyalgia-chronic fatigue. Best Pract. Res. Clin. Rheumatol. 2001, 15, 747–758. [Google Scholar] [CrossRef]
- Kranzler, J.D.; Gendreau, J.F.; Rao, S.G. The psychopharmacology of fibromyalgia: A drug development perspective. Psychopharmacol. Bull. 2002, 36, 165–213. [Google Scholar]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef]
- Cassisi, G.; Sarzi-Puttini, P.; Casale, R.; Cazzola, M.; Boccassini, L.; Atzeni, F.; Stisi, S. Pain in fibromyalgia and related conditions. Reumatismo 2014, 66, 72–86. [Google Scholar] [CrossRef][Green Version]
- Hernandez-Leon, A.; De la Luz-Cuellar, Y.E.; Granados-Soto, V.; González-Trujano, M.E.; Fernández-Guasti, A. Sex differences and estradiol involvement in hyperalgesia and allodynia in an experimental model of fibromyalgia. Horm. Behav. 2018, 97, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J.; Engleberg, N.C.; Demitrack, M.A. Neurohormonal perturbations in fibromyalgia. Baillieres Clin. Rheumatol. 1996, 10, 365–378. [Google Scholar] [CrossRef]
- Arnold, L.M.; Clauw, D.J.; Dunegan, L.J.; Turk, D.C.; FibroCollaborative. A framework for fibromyalgia management for primary care providers. Mayo Clin. Proc. 2012, 87, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.L.; Kroenke, K.; Mease, P.; Williams, D.A.; Chen, Y.; D’Souza, D.; Wohlreich, M.; McCarberg, B. Burden of illness and treatment patterns for patients with fibromyalgia. Pain Med. 2012, 13, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Clauw, D.J.; Gendreau, R.M.; Rao, S.G.; Kranzler, J.; Chen, W.; Palmer, R.H. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J. Rheumatol. 2009, 36, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Almenar-Perez, E.; Sánchez-Fito, T.; Ovejero, T.; Nathanson, L.; Oltra, E. Impact of Polypharmacy on Candidate Biomarker miRNomes for the Diagnosis of Fibromyalgia and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Striking Back on Treatments. Pharmaceutics 2019, 11, 126. [Google Scholar] [CrossRef]
- Arnold, L.M.; Lu, Y.; Crofford, L.J.; Wohlreich, M.; Detke, M.J.; Iyengar, S.; Goldstein, D.J. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004, 50, 2974–2984. [Google Scholar] [CrossRef]
- Branco, J.C.; Zachrisson, O.; Perrot, S.; Mainguy, Y.; Multinational Coordinator Study Group. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in treatment of fibromyalgia. J. Rheumatol. 2010, 37, 851–859. [Google Scholar] [CrossRef]
- Arnold, L.M.; Gendreau, R.M.; Palmer, R.H.; Gendreau, J.F.; Wang, Y. Efficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: Results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010, 62, 2745–2756. [Google Scholar] [CrossRef]
- Lunn, M.P.; Hughes, R.A.; Wiffen, P.J. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst. Rev. 2014, 1, CD007115. [Google Scholar] [CrossRef]
- Russell, I.J.; Mease, P.J.; Smith, T.R.; Kajdasz, D.K.; Wohlreich, M.M.; Detke, M.J.; Walker, D.J.; Chappell, A.S.; Arnold, L.M. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 2008, 136, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Osada, K.; Ichibayashi, H.; Mizuno, H.; Ochiai, T.; Ishida, M.; Alev, L.; Nishioka, K. An open-label, long-term, phase III extension trial of duloxetine in Japanese patients with fibromyalgia. Mod. Rheumatol. 2017, 27, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 2008, 85, 355–375. [Google Scholar] [CrossRef]
- Sprott, H.; Franke, S.; Kluge, H.; Hein, G. Pain treatment of fibromyalgia by acupuncture. Rheumatol. Int. 1998, 18, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, K.J.; Blease, C. Myalgic encephalomyelitis/chronic fatigue syndrome and the biopsychosocial model: A review of patient harm and distress in the medical encounter. Disabil. Rehabil. 2018. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D. Chronic fatigue syndrome and quality of life. Patient Relat. Outcome Meas. 2018, 9, 253–262. [Google Scholar] [CrossRef]
- Straus, S.E.; Hickie, I.; Komaroff, A.; Fukuda, K.; Sharpe, M.C.; Dobbins, J.G. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar]
- Carruthers, B.M.; Van De Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgia Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2002, 11, 7–115. [Google Scholar] [CrossRef]
- Noda, M.; Ifuku, M.; Hossain, M.S.; Katafuchi, T. Glial Activation and Expression of the Serotonin Transporter in Chronic Fatigue Syndrome. Front. Psychiatry 2018, 9, 589. [Google Scholar] [CrossRef]
- Ishii, A.; Tanaka, M.; Watanabe, Y. The neural mechanisms of re-experiencing physical fatigue sensation: A magnetoencephalography study. Exp. Brain Res. 2016, 234, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Yamano, E.; Sugimoto, M.; Hirayama, A.; Kume, S.; Yamato, M.; Jin, G.; Tajima, S.; Goda, N.; Iwai, K.; Fukuda, S.; et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci Rep. 2016, 6, 34990. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, Y.; Zhang, Y.; Li, S.; Sun, Y.; Chen, Z.; Teng, L.; Wang, D. Antifatigue Potential Activity of Sarcodon imbricatus in Acute Excise-Treated and Chronic Fatigue Syndrome in Mice via Regulation of Nrf2-Mediated Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 9140896. [Google Scholar] [CrossRef] [PubMed]
- Almenar-Pérez, E.; Ovejero, T.; Sánchez-Fito, T.; Espejo, J.A.; Nathanson, L.; Oltra, E. Epigenetic Components of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Uncover Potential Transposable Element Activation. Clin. Ther. 2019, 41, 675–698. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Tamura, Y.; Eguchi, A.; Kume, S.; Miyashige, Y.; Nakano, M.; Watanabe, Y.; Kataoka, Y. Brain interleukin-1beta and the intrinsic receptor antagonist control peripheral Toll-like receptor 3-mediated suppression of spontaneous activity in rats. PLoS ONE 2014, 9, e90950. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, M.; Hossain, S.M.; Noda, M.; Katafuchi, T. Induction of interleukin-1beta by activated microglia is a prerequisite for immunologically induced fatigue. Eur. J. Neurosci. 2014, 40, 3253–3263. [Google Scholar] [CrossRef]
- Bal, N.; Figueras, G.; Vilaró, M.T.; Suñol, C.; Artigas, F. Antidepressant drugs inhibit a glial 5-hydroxytryptamine transporter in rat brain. Eur. J. Neurosci. 1997, 9, 1728–1738. [Google Scholar] [CrossRef]
- Hirst, W.D.; Price, G.W.; Rattray, M.; Wilkin, G.P. Serotonin transporters in adult rat brain astrocytes revealed by [3H]5-HT uptake into glial plasmalemmal vesicles. Neurochem. Int. 1998, 33, 11–22. [Google Scholar] [CrossRef]
- Katafuchi, T.; Kondo, T.; Take, S.; Yoshimura, M. Enhanced expression of brain interferon-alpha and serotonin transporter in immunologically induced fatigue in rats. Eur. J. Neurosci. 2005, 22, 2817–2826. [Google Scholar] [CrossRef]
- Cevik, R.; Gur, A.; Acar, S.; Nas, K.; Sarac, A.J. Hypothalamic-pituitary-gonadal axis hormones and cortisol in both menstrual phases of women with chronic fatigue syndrome and effect of depressive mood on these hormones. BMC Musculoskelet. Disord. 2004, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Veldman, J.; van Houdenhove, B.; Verguts, J. Chronic fatigue syndrome: A hormonal origin? A rare case of dysmenorrhea membranacea. Arch. Gynecol. Obstet. 2009, 279, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.-N.; Wang, Y.; Zhang, Y.; Yang, C.-X. Duloxetine for pain in fibromyalgia in adults: A systematic review and a meta-analysis. Int. J. Neurosci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla-Vaca, A.; Stone, A.; Caballero-Lozada, A.F.; Paredes, S.; Grant, M.C. Perioperative duloxetine for acute postoperative analgesia: A meta-analysis of randomized trials. Reg. Anesth. Pain Med. 2019, 44, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Zychowska, M.; Makuch, W.; Rojewska, E.; Przewlocka, B. Neuronal and immunological basis of action of antidepressants in chronic pain—Clinical and experimental studies. Pharmacol. Rep. 2013, 65, 1611–1621. [Google Scholar] [CrossRef]
- Miyauchi, T.; Tokura, T.; Kimura, H.; Ito, M.; Umemura, E.; Sato, B.A.; Nagashima, W.; Tonoike, T.; Yamamoto, Y.; Saito, K.; et al. Effect of antidepressant treatment on plasma levels of neuroinflammation-associated molecules in patients with somatic symptom disorder with predominant pain around the orofacial region. Hum. Psychopharmacol. 2019, 34, e2698. [Google Scholar] [CrossRef] [PubMed]
- Burch, R. Antidepressants for Preventive Treatment of Migraine. Curr. Treat. Option. Neurol. 2019, 21, 18. [Google Scholar] [CrossRef]
- Macian, N.; Pereira, B.; Shinjo, C.; DuBray, C.; Pickering, G. Fibromyalgia, milnacipran and experimental pain modulation: Study protocol for a double blind randomized controlled trial. Trials 2015, 16, 134. [Google Scholar] [CrossRef]
- Yasuda, H.; Hotta, N.; Kasuga, M.; Kashiwagi, A.; Kawamori, R.; Yamada, T.; Baba, Y.; Alev, L.; Nakajo, K. Efficacy and safety of 40 mg or 60 mg duloxetine in Japanese adults with diabetic neuropathic pain: Results from a randomized, 52-week, open-label study. J. Diabetes Investig. 2016, 7, 100–108. [Google Scholar] [CrossRef]
- Engel, E.R.; Kudrow, D.; Rapoport, A.M. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol. Sci. 2014, 35, 429–435. [Google Scholar] [CrossRef]
- Vahedi, H.; Merat, S.; Rashidioon, A.; Ghoddoosi, A.; Malekzadeh, R. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: A double-blind randomized-controlled study. Aliment. Pharmacol. Ther. 2005, 22, 381–385. [Google Scholar] [CrossRef]
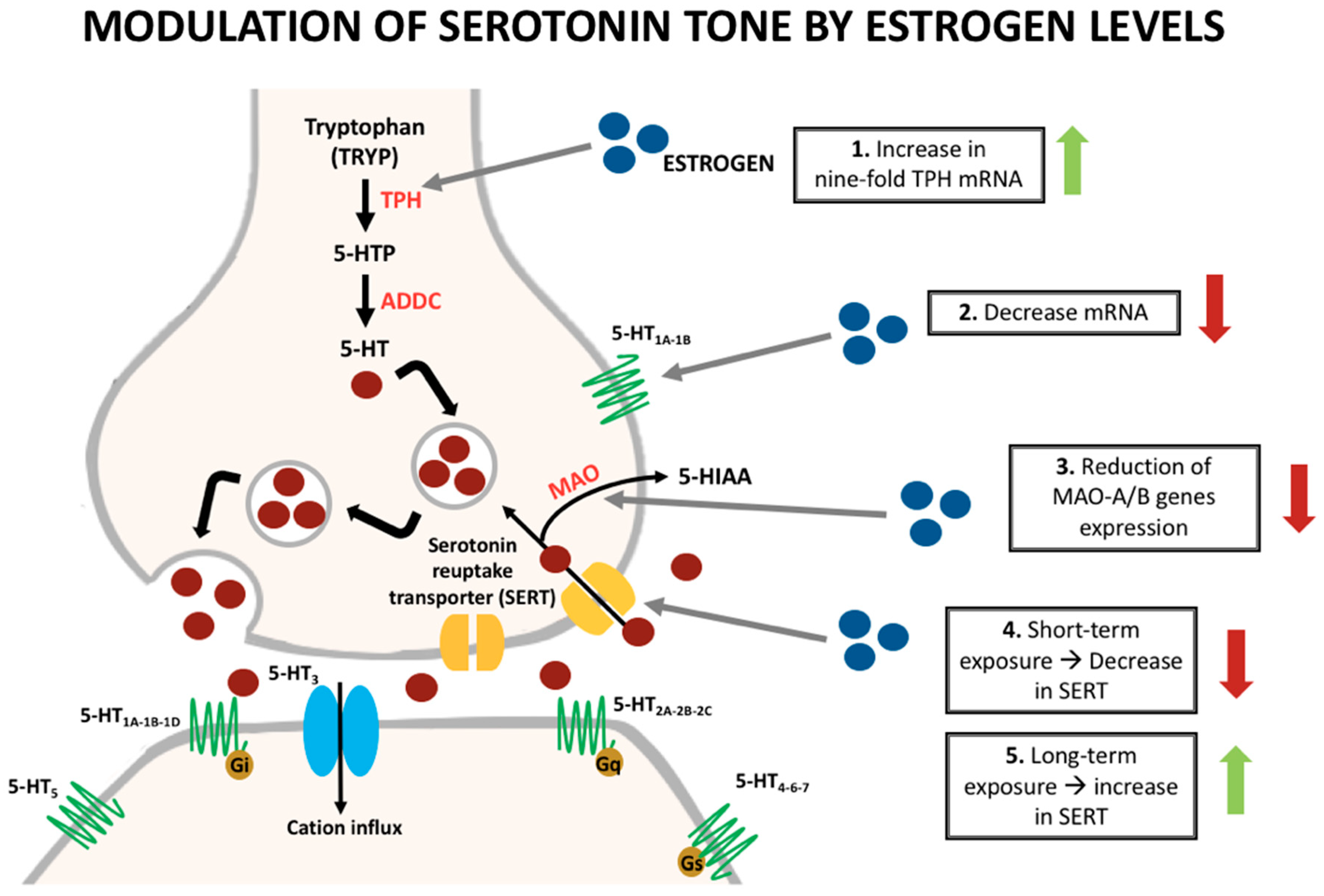
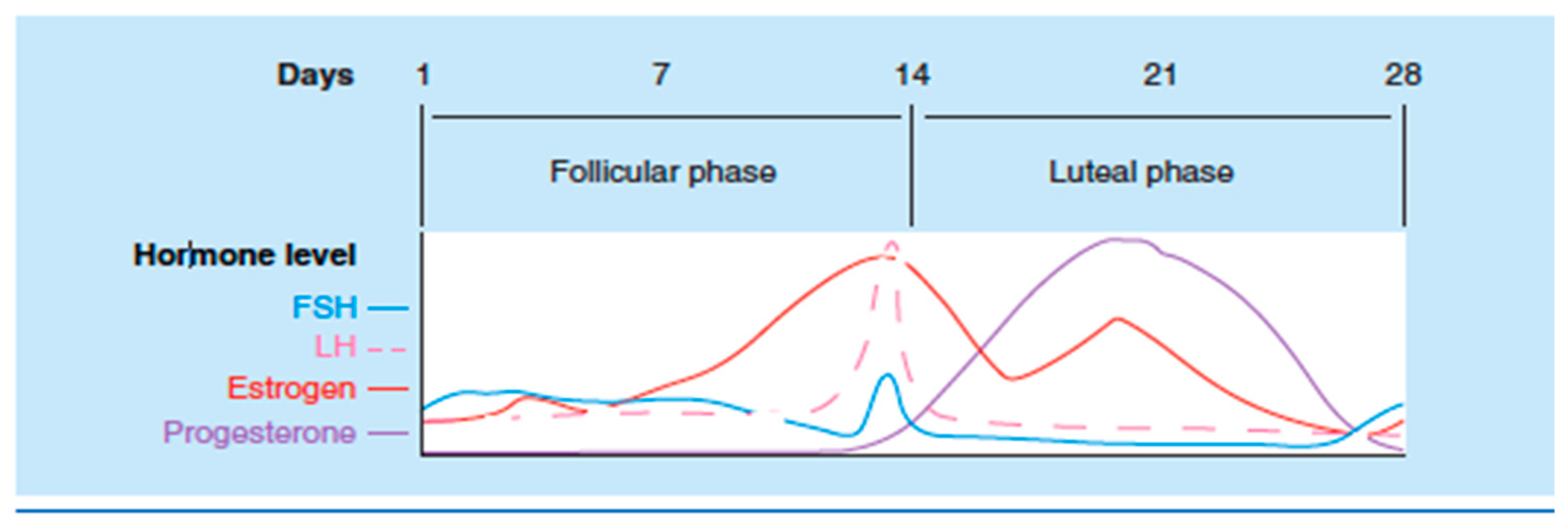
| Condition | What We know |
|---|---|
| Irritable Bowel Syndrome | - Increase of symptoms during luteal phase and menses - Increase levels of serotonin synthesis and its receptors - 5 HT3 receptor polymorphisms cause increased IBS/diarrhea risk - 5-HT3 receptor activation induces release of substance P, CGRP, and neurokinin - The interference of serotonin in pain modulation depends on the receptors, route of administration, and type of pain: ✓ Antinociceptive action of estrogen on the serotoninergic system due to afferent-driven vagal inhibition of pain ✓ The increase in serotonin levels in the periphery caused by the activation of estrogen receptors in IMMCs and spinal receptors is pro-nociceptive - During the estrogen withdrawal phase, there is an increase in pain perception in patients with IBS - Serotonin antagonist (Alosetron) has a demonstrated efficacy in IBS patients, with predominance in women |
| Migraine | - More common in women during childbearing ages - Estrogen causes activation of 5-HT1B without the compensation of the 5-HT2A receptor; this causes vasodilation and thus headache - Estrogen causes release of nociceptive substances including calcitonin gene-related peptide CGRP and TrpV1 - Estrogen can modify the c-Fos protein, which regulates the transcription of serotonin and norepinephrine - Estrogen increases the synthesis of serotonin by modulation of the rate-limiting enzyme TPH - Bimodal theory: an abrupt decrease in estrogen levels or a chronically high plasma estrogen concentration modulates the trigeminal pain pathway - Serotonin agonists such as Sumatriptan are effective for acute migraine |
| Primary Non-Migraine Headache | - Increase of headache female incidence after puberty - Estrogen upregulates serotonin - Estrogen withdrawal is associated with an increase in the intensity of pain - Activation of nociceptors induced by serotonin - Activation of trigeminal autonomic reflex is responsible for pain in cluster headaches |
| Fibromyalgia | - The prevalence is six-times more frequent among women - Serotonin and norepinephrine modulate the transmission of pain in the descending nociceptive modulatory pathways in the brain and spinal cord - Low serotonin synthesis level in patients with fibromyalgia - Estrogen deficiency as a promoter of the disease - Shorter exposure time to estrogens influences the pain hypersensitivity - Duloxetine and milnacipran, selective SNRIs, are FDA approved drugs for fibromyalgia |
| Chronic Fatigue Syndrome | - Role of glutaminergic and serotonergic pathways in the pathophysiology - Activation of IL-1β receptors in the disease, increases the expression of serotonin transporter (5-HTT) in glial cells - Reuptake increase of serotonin by astrocytes, increase the deactivation of serotonin by monoamine oxidase A in fibromyalgia patients - Imipramine (non-selective 5-HT reuptake inhibitor) has been used in this condition - Resolution of fatigue and decrease of inflammatory mediators in experiments in patients with CFS, after the discontinuation of hormone therapy |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes, S.; Cantillo, S.; Candido, K.D.; Knezevic, N.N. An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. Int. J. Mol. Sci. 2019, 20, 5729. https://doi.org/10.3390/ijms20225729
Paredes S, Cantillo S, Candido KD, Knezevic NN. An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. International Journal of Molecular Sciences. 2019; 20(22):5729. https://doi.org/10.3390/ijms20225729
Chicago/Turabian StyleParedes, Stephania, Santiago Cantillo, Kenneth D. Candido, and Nebojsa Nick Knezevic. 2019. "An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens" International Journal of Molecular Sciences 20, no. 22: 5729. https://doi.org/10.3390/ijms20225729
APA StyleParedes, S., Cantillo, S., Candido, K. D., & Knezevic, N. N. (2019). An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. International Journal of Molecular Sciences, 20(22), 5729. https://doi.org/10.3390/ijms20225729






