Perfluorooctanoate in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Micelle Formation and Structure
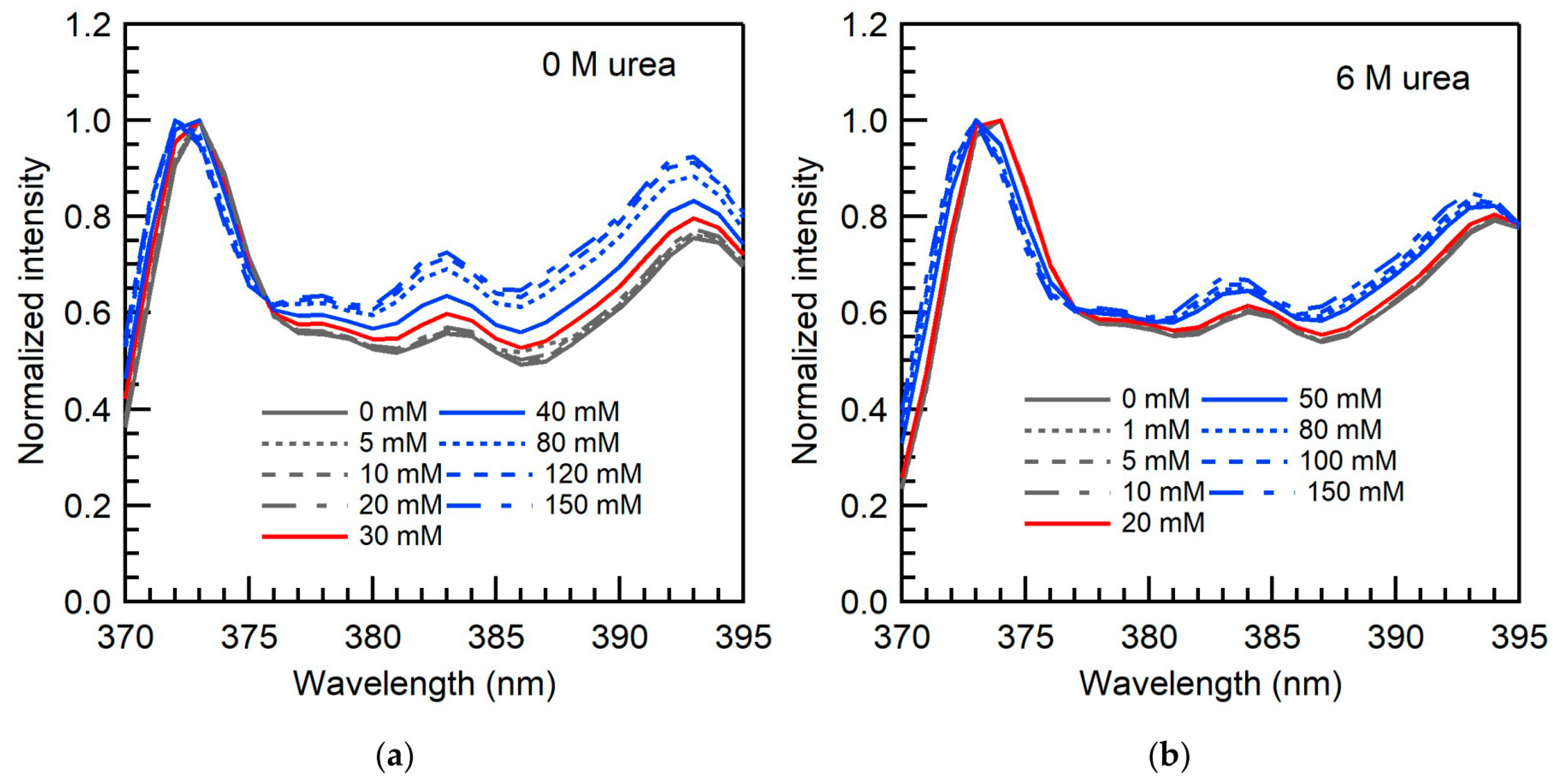
2.2. Micellar Microenvironment
2.3. Comparison of Fluorocarbon and Hydrocarbon Surfactants
3. Materials and Methods
3.1. Materials
3.2. Conductivity
3.3. Surface Tension
3.4. Micropolarity
3.5. Viscosity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wallqvist, A.; Covell, D.G.; Thirumalai, D. Hydrophobic interactions in aqueous urea solutions with implications for the mechanism of protein denaturation. J. Am. Chem. Soc. 1998, 120, 427–428. [Google Scholar] [CrossRef]
- Das, A.; Mukhopadhyay, C. Urea-mediated protein denaturation: A consensus view. J. Phys. Chem. B 2009, 113, 12816–12824. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Ghasemi, M.; Furlani, E.P.; Tsianou, M. Solvent processing of cellulose for effective bioresource utilization. Curr. Opin. Green Sustain. Chem. 2018, 14, 40–52. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Filipe, A.; Antunes, F.E.; Lindman, B.; Topgaard, D.; Davidovich, I.; Talmon, Y. New insights on the role of urea on the dissolution and thermally-induced gelation of cellulose in aqueous alkali. Gels 2018, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Walrafen, G.E. Raman spectral studies of the effects of urea and sucrose on water structure. J. Chem. Phys. 1966, 44, 3726–3727. [Google Scholar] [CrossRef]
- Sacco, A.; Holz, M. NMR studies on hydrophobic interactions in solution Part 2—Temperature and urea effect on the self-association of ethanol in water. J. Chem. Soc. Faraday Trans. 1997, 93, 1101–1104. [Google Scholar] [CrossRef]
- Soper, A.K.; Castner, E.W.; Luzar, A. Impact of urea on water structure: A clue to its properties as a denaturant? Biophys. Chem. 2003, 105, 649–666. [Google Scholar] [CrossRef]
- Chitra, R.; Smith, P.E. Molecular association in solution: A kirkwood−buff analysis of sodium chloride, ammonium sulfate, guanidinium chloride, urea, and 2,2,2-trifluoroethanol in water. J. Phys. Chem. B 2002, 106, 1491–1500. [Google Scholar] [CrossRef]
- Mayele, M.; Holz, M. NMR studies on hydrophobic interactions in solution Part 5. Effect of urea on the hydrophobic self-association of tert-butanol in water at different temperatures. Phys. Chem. Chem. Phys. 2000, 2, 2429–2434. [Google Scholar] [CrossRef]
- Shimizu, A.; Fumino, K.; Yukiyasu, K.; Taniguchi, Y. NMR studies on dynamic behavior of water molecule in aqueous denaturant solutions at 25 °C: Effects of guanidine hydrochloride, urea and alkylated ureas. J. Mol. Liq. 2000, 85, 269–278. [Google Scholar] [CrossRef]
- Hua, L.; Zhou, R.; Thirumalai, D.; Berne, B.J. Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding. Proc. Natl. Acad. Sci. USA 2008, 105, 16928–16933. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, H.; Pettitt, B.M. Preferential solvation in urea solutions at different concentrations: Properties from simulation studies. J. Phys. Chem. B 2007, 111, 5233–5242. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mukhopadhyay, C. Atomistic mechanism of protein denaturation by urea. J. Phys. Chem. B 2008, 112, 7903–7908. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Mohan, S.; Ghosh, S.K.; Choudhury, N. Molecular dynamics simulation of aqueous urea solution: Is urea a structure breaker? J. Phys. Chem. B 2014, 118, 11757–11768. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surface Chemistry of Surfactants and Polymers; John Wiley & Sons, Incorporated: Somerset, NJ, USA, 2014. [Google Scholar]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Sarkar, B.; Alexandridis, P. Adsorption of poly(ethylene oxide)-containing amphiphilic polymers on solid-liquid interfaces: Fundamentals and applications. Adv. Colloid Interface Sci. 2017, 244, 132–163. [Google Scholar] [CrossRef]
- He, Z.; Ma, Y.; Alexandridis, P. Comparison of ionic liquid and salt effects on the thermodynamics of amphiphile micellization in water. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 159–168. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F. Differential scanning calorimetry investigation of the effect of salts on aqueous solution properties of an amphiphilic block copolymer (Poloxamer). Langmuir 1997, 13, 6074–6082. [Google Scholar] [CrossRef]
- Kaizu, K.; Alexandridis, P. Micellization of polyoxyethylene–polyoxypropylene block copolymers in aqueous polyol solutions. J. Mol. Liq. 2015, 210, 20–28. [Google Scholar] [CrossRef]
- Sarkar, B.; Ravi, V.; Alexandridis, P. Micellization of amphiphilic block copolymers in binary and ternary solvent mixtures. J. Colloid Interface Sci. 2013, 390, 137–146. [Google Scholar] [CrossRef]
- Sarkar, B.; Lam, S.; Alexandridis, P. Micellization of alkyl-propoxy-ethoxylate surfactants in water-polar organic solvent mixtures. Langmuir 2010, 26, 10532–10540. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.R.; Jain, N.J.; Sharma, R.K.; Bahadur, P. Effect of additives on the micellization of PEO/PPO/PEO block copolymer F127 in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 57–69. [Google Scholar] [CrossRef]
- Ruiz, C.C. A photophysical study of the urea effect on micellar properties of sodium dodecylsulfate aqueous solutions. Colloid Polym. Sci. 1995, 273, 1033–1040. [Google Scholar] [CrossRef]
- Baglioni, P.; Rivara-Minten, E.; Dei, L.; Ferroni, E. ESR study of sodium dodecyl sulfate and dodecyltrimethylammonium bromide micellar solutions: Effect of urea. J. Phys. Chem. 1990, 94, 8218–8222. [Google Scholar] [CrossRef]
- Briganti, G.; Puvvada, S.; Blankschtein, D. Effect of urea on micellar properties of aqueous solutions of nonionic surfactants. J. Phys. Chem. 1991, 95, 8989–8995. [Google Scholar] [CrossRef]
- Kumar, S.; Parveen, N.; Kabir-ud, D. Effect of urea addition on micellization and the related phenomena. J. Phys. Chem. B 2004, 108, 9588–9592. [Google Scholar] [CrossRef]
- Abuin, E.B.; Lissi, E.A.; Aspée, A.; Gonzalez, F.D.; Varas, J.M. Fluorescence of 8-anilinonaphthalene-1-sulfonate and properties of sodium dodecyl sulfate micelles in water–urea mixtures. J. Colloid Interface Sci. 1997, 186, 332–338. [Google Scholar] [CrossRef]
- Almgren, M.; Swarup, S. Size of sodium dodecyl sulfate micelles in the presence of additives i. Alcohols and other polar compounds. J. Colloid Interface Sci. 1983, 91, 256–266. [Google Scholar] [CrossRef]
- De Moura, A.F.; Bernardino, K.; de Oliveira, O.V.; Freitas, L.C.G. Solvation of sodium octanoate micelles in concentrated urea solution studied by means of molecular dynamics simulations. J. Phys. Chem. B 2011, 115, 14582–14590. [Google Scholar] [CrossRef]
- Alexandridis, P.; Athanassiou, V.; Hatton, T.A. Pluronic-P105 PEO-PPO-PEO block copolymer in aqueous urea solutions: Micelle formation, structure, and microenvironment. Langmuir 1995, 11, 2442–2450. [Google Scholar] [CrossRef]
- Finer, E.G.; Franks, F.; Tait, M.J. Nuclear magnetic resonance studies of aqueous urea solutions. J. Am. Chem. Soc. 1972, 94, 4424–4429. [Google Scholar] [CrossRef]
- Schick, M.J. Effect of electrolyte and urea on micelle formation. J. Phys. Chem. 1964, 68, 3585–3592. [Google Scholar] [CrossRef]
- Emerson, M.F.; Holtzer, A. Hydrophobic bond in micellar systems. Effects of various additives on the stability of micelles of sodium dodecyl sulfate and of n-dodecyltrimethylammonium bromide. J. Phys. Chem. 1967, 71, 3320–3330. [Google Scholar] [CrossRef] [PubMed]
- Trzesniak, D.; van der Vegt, N.F.A.; van Gunsteren, W.F. Computer simulation studies on the solvation of aliphatic hydrocarbons in 6.9 M aqueous urea solution. Phys. Chem. Chem. Phys. 2004, 6, 697–702. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Nakamura, S.; Shimizu, K. Molecular dynamics study on hydrophobic effects in aqueous urea solutions. J. Am. Chem. Soc. 2001, 123, 677–682. [Google Scholar] [CrossRef]
- Graziano, G. On the solubility of aliphatic hydrocarbons in 7 M aqueous urea. J. Phys. Chem. B 2001, 105, 2632–2637. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated Surfactants and Repellents; CRC Press: Boca Raton, FL, USA, 2001; Volume 97. [Google Scholar]
- Krafft, M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv. Drug Deliv. Rev. 2001, 47, 209–228. [Google Scholar] [CrossRef]
- Wang, K.; Karlsson, G.; Almgren, M.; Asakawa, T. Aggregation behavior of cationic fluorosurfactants in water and salt solutions. A CryoTEM survey. J. Phys. Chem. B 1999, 103, 9237–9246. [Google Scholar] [CrossRef]
- Renner, R. The long and the short of perfluorinated replacements. Environ. Sci. Technol. 2006, 40, 12–13. [Google Scholar] [CrossRef] [Green Version]
- Kissa, E. Fluorinated Surfactants: Synthesis, Properties, Applications; Marcel Dekker: New York, NY, USA, 1994; Volume 50. [Google Scholar]
- Orizondo, R.A.; Nelson, D.L.; Fabiilli, M.L.; Cook, K.E. Effects of fluorosurfactant structure and concentration on drug availability and biocompatibility in water-in-perfluorocarbon emulsions for pulmonary drug delivery. Colloid Polym. Sci. 2017, 295, 2413–2422. [Google Scholar]
- Sagisaka, M.; Oasa, J.; Hasegawa, S.; Toyokawa, R.; Yoshizawa, A. Novel fluorinated double-tail surfactant having high microemulsifying ability in water/supercritical CO2 system. J. Supercrit. Fluids 2010, 53, 131–136. [Google Scholar] [CrossRef]
- Xiao, F. Emerging poly-and perfluoroalkyl substances in the aquatic environment: A review of current literature. Water Res. 2017, 124, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A never-ending story of per-and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Adami, H.-O.; Boffetta, P.; Cole, P.; Starr, T.B.; Mandel, J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit. Rev. Toxicol. 2014, 44 (Suppl. 1), 1–81. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Amith, M.; Nishanth, T.; Tanju, K. The overlooked short-and ultrashort-chain poly-and perfluorinated substances: A review. Chemosphere 2019, 220, 866–882. [Google Scholar]
- United States Environmental Protection Agency. EPA’s Per-and Polyfluoroalkyl Substances (PFAS) Action Plan; EPA 823R18004; United States Environmental Protection Agency: Washington, DC, USA, 2019. [Google Scholar]
- Asakawa, T.; Hashikawa, M.; Amada, K.; Miyagishi, S. Effect of urea on micelle formation of fluorocarbon surfactants. Langmuir 1995, 11, 2376–2379. [Google Scholar] [CrossRef]
- Wang, C.; Yan, P.; Xing, H.; Jin, C.; Xiao, J.-X. Thermodynamics of aggregation of ammonium/tetraalkylammonium perfluorooctanoates: Effect of counterions. J. Chem. Eng. Data 2010, 55, 1994–1999. [Google Scholar] [CrossRef]
- Jin, C.; Yan, P.; Wang, C.; Xiao, J.-X. Effect of counterions on fluorinated surfactants 1. Surface activity and micellization. Acta Chim. Sin. 2005, 63, 279–282. [Google Scholar]
- Prosser, A.J.; Franses, E.I. Adsorption and surface tension of ionic surfactants at the air–water interface: Review and evaluation of equilibrium models. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 1–40. [Google Scholar] [CrossRef]
- Ito, A.; Sakai, H.; Kondo, Y.; Yoshino, N.; Abe, M. Micellar solution properties of fluorocarbon−hydrocarbon hybrid surfactants. Langmuir 1996, 12, 5768–5772. [Google Scholar] [CrossRef]
- Nagarajan, R. Molecular packing parameter and surfactant self-assembly: The neglected role of the surfactant tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Srinivasan, V.; Blankschtein, D. Prediction of conformational characteristics and micellar solution properties of fluorocarbon surfactants. Langmuir 2005, 21, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, S.J.; Ottewill, R.H.; Hayter, J.B.; Ingram, B.T. Small angle neutron scattering studies on micellar systems part 1. Ammonium octanoate, ammonium decanoate and ammonium perfluoroocatanoate. Colloid Polym. Sci. 1987, 265, 619–627. [Google Scholar] [CrossRef]
- Xing, H.; Yan, P.; Xiao, J.-X. Unusual location of the pyrene probe solubilized in the micellar solutions of tetraalkylammonium perfluorooctanoates. Soft Matter 2013, 9, 1164–1171. [Google Scholar] [CrossRef]
- López-Fontán, J.L.; Sarmiento, F.; Schulz, P.C. The aggregation of sodium perfluorooctanoate in water. Colloid Polym. Sci. 2005, 283, 862–871. [Google Scholar] [CrossRef]
- Nagarajan, R. Are large micelles rigid or flexible? A reinterpretation of viscosity data for micellar solutions. J. Colloid Interface Sci. 1982, 90, 477–486. [Google Scholar] [CrossRef]
- Zoeller, N.; Blankschtein, D. Experimental determination of micelle shape and size in aqueous solutions of dodecyl ethoxy sulfates. Langmuir 1998, 14, 7155–7165. [Google Scholar] [CrossRef]
- Boden, N.; Clements, J.; Jolley, K.W.; Parker, D.; Smith, M.H. Nematic-lamellar tricritical behavior and structure of the lamellar phase in the ammonium pentadecafluorooctanoate (APFO)/water system. J. Chem. Phys. 1990, 93, 9096–9105. [Google Scholar] [CrossRef]
- Jakli, G.; van Hook, W.A. Isotope effects in aqueous systems. 12. Thermodynamics of urea-h4/water and urea-d4/water-d2 solutions. J. Phys. Chem. 1981, 85, 3480–3493. [Google Scholar] [CrossRef]
- Zangi, R.; Zhou, R.; Berne, B.J. Urea’s action on hydrophobic interactions. J. Am. Chem. Soc. 2009, 131, 1535–1541. [Google Scholar] [CrossRef]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Cui, P.; Liu, C.-B.; Yuan, S.-L. Molecular dynamics simulation of pyrene solubilized in a sodium dodecyl sulfate micelle. Langmuir 2012, 28, 4931–4938. [Google Scholar] [CrossRef] [PubMed]
- Fajalia, A.I.; Tsianou, M. Charging and uncharging a neutral polymer in solution: A small-angle neutron scattering investigation. J. Phys. Chem. B 2014, 118, 10725–10739. [Google Scholar] [CrossRef] [PubMed]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Moroi, Y. Micelles: Theoretical and Applied Aspects; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Shinoda, K. Colloidal Surfactants: Some Physicochemical Properties; Academic Press: Cambridge, MA, USA, 1963. [Google Scholar]
- Blanco, E.; González-Pérez, A.; Ruso, J.M.; Pedrido, R.; Prieto, G.; Sarmiento, F. A comparative study of the physicochemical properties of perfluorinated and hydrogenated amphiphiles. J. Colloid Interface Sci. 2005, 288, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Shanks, P.C.; Franses, E.I. Estimation of micellization parameters of aqueous sodium dodecyl sulfate from conductivity data. J. Phys. Chem. 1992, 96, 1794–1805. [Google Scholar] [CrossRef]
- Nivaggioli, T.; Alexandridis, P.; Hatton, T.A.; Yekta, A.; Winnik, M.A. Fluorescence probe studies of pluronic copolymer solutions as a function of temperature. Langmuir 1995, 11, 730–737. [Google Scholar] [CrossRef]
- An, Y.-J.; Jeong, S.-W. Interactions of perfluorinated surfactant with polycyclic aromatic hydrocarbons: Critical micelle concentration and solubility enhancement measurements. J. Colloid Interface Sci. 2001, 242, 419–424. [Google Scholar] [CrossRef]
- Damas, C.; Naejus, R.; Coudert, R.; Frochot, C.; Brembilla, A.; Viriot, M.L. Fluorocarbon and hydrocarbon short-chain nonionic amphiphiles: A comparative study of their behavior in aqueous medium. J. Phys. Chem. B 1998, 102, 10917–10924. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Pyrene fluorescence as a probe of fluorocarbon micelles and their mixed micelles with hydrocarbon surfactants. Langmuir 1988, 4, 942–945. [Google Scholar] [CrossRef]
- Antoniou, E.; Alexandridis, P. Polymer conformation in mixed aqueous-polar organic solvents. Eur. Polym. J. 2010, 46, 324–335. [Google Scholar] [CrossRef]
- Plucinsky, S.M.; Root, K.T.; Glover, K.J. Efficient solubilization and purification of highly insoluble membrane proteins expressed as inclusion bodies using perfluorooctanoic acid. Protein Expr. Purif. 2018, 143, 34–37. [Google Scholar] [CrossRef] [PubMed]
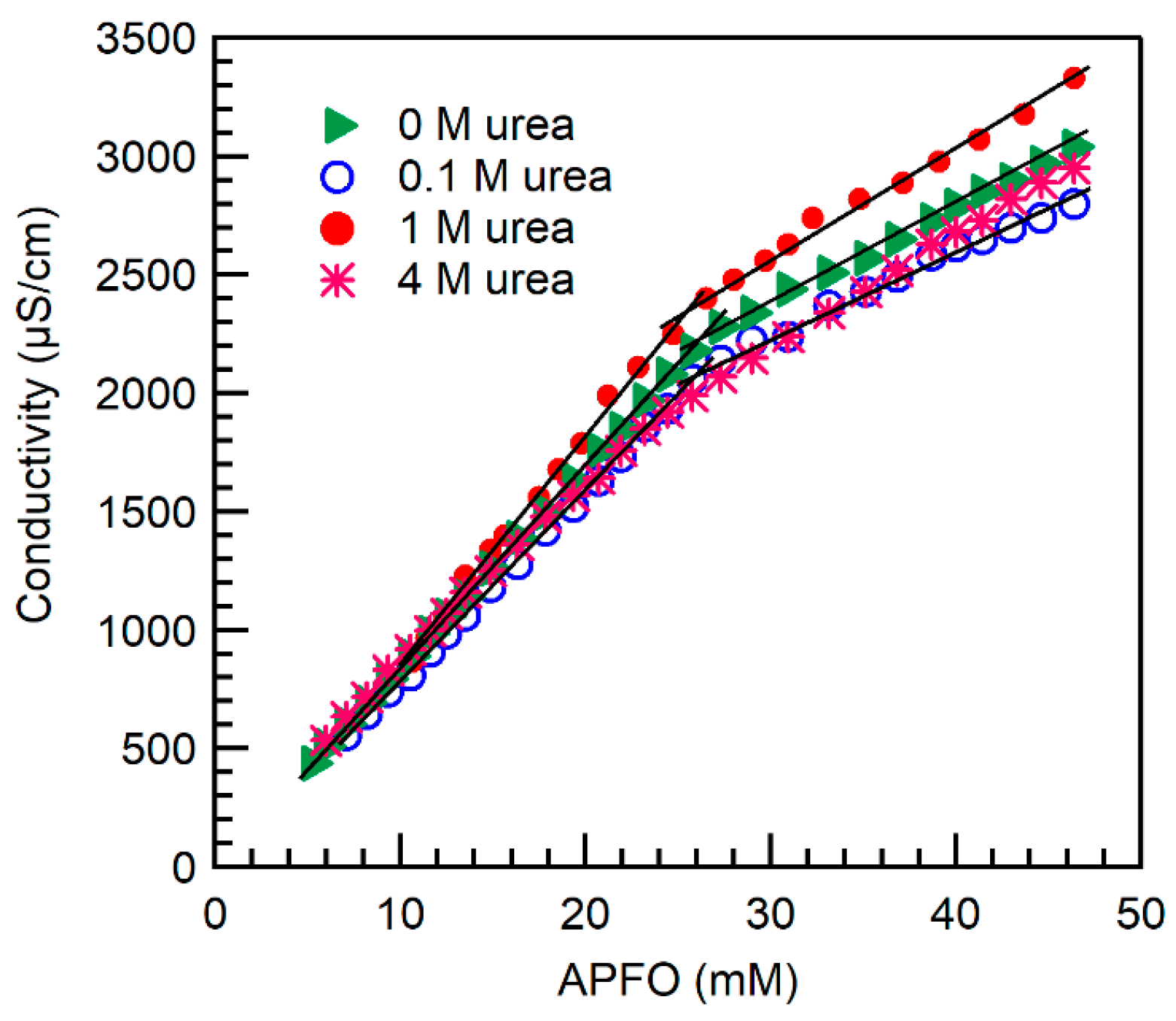
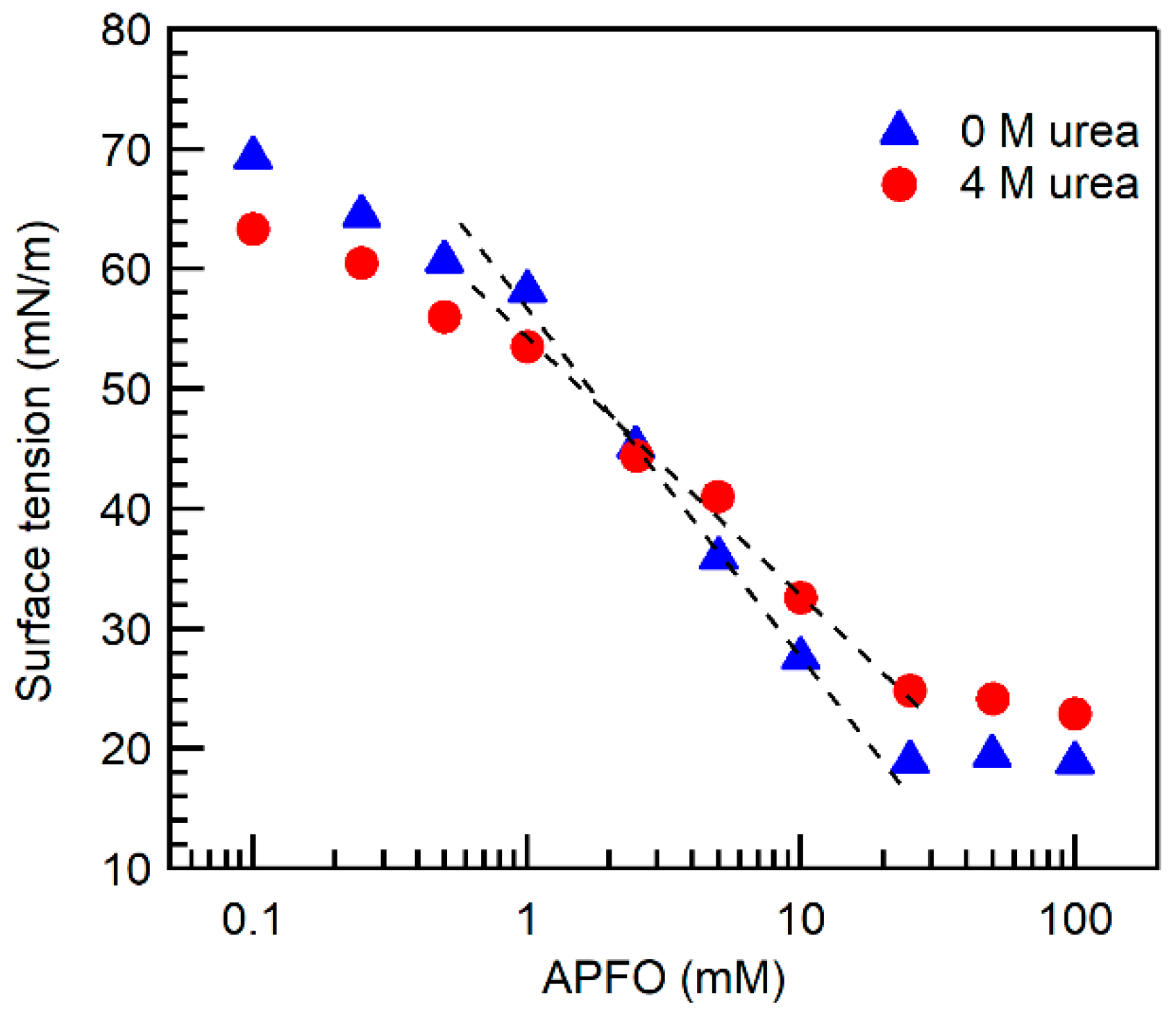
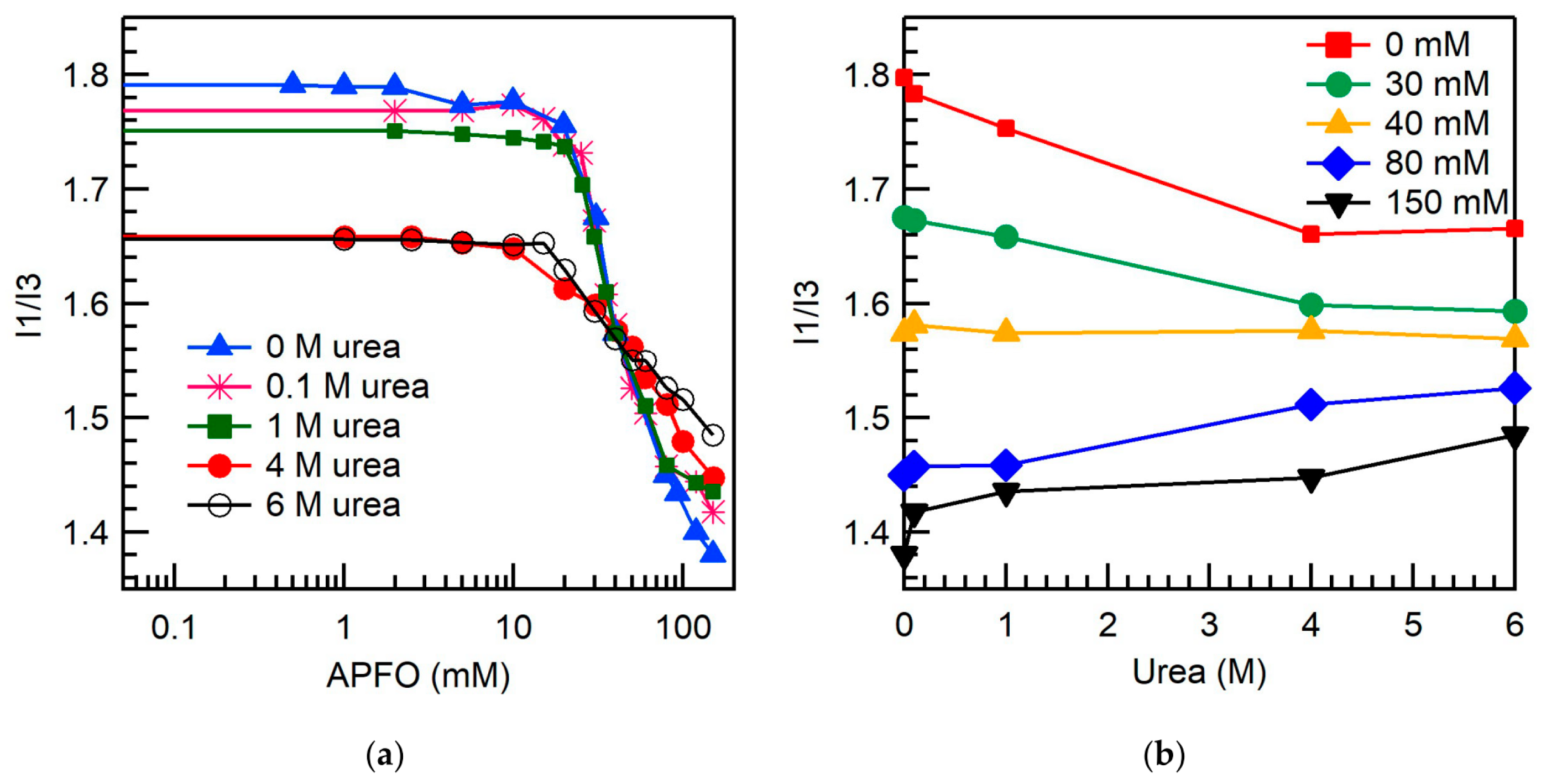
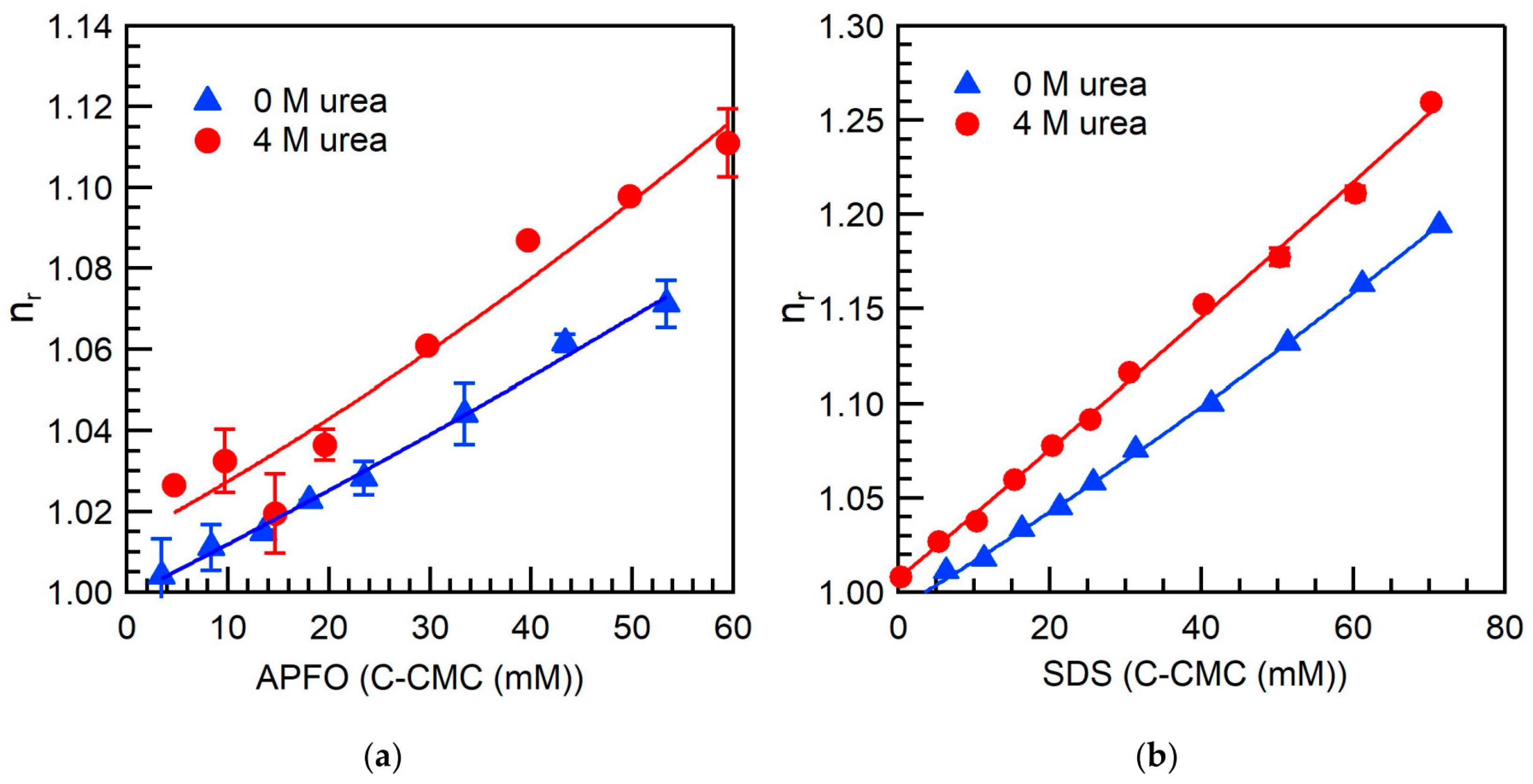
| Surfactant | Urea Concentration (M) | Temperature (°C) | CMC (mM) | α |
|---|---|---|---|---|
| APFO | 0 | 24 | 26.5 (±0.1) a,24.5 (±0.7) b, 25 [51] | 0.47, 0.40 [51] |
| APFO | 0.1 | 22 | 27.4 (±0.3) a, 22.5 (±2) b | 0.45 |
| APFO | 1 | 22 | 25.5 (±0.5) a, 22.5 (±0.2) b | 0.45 |
| APFO | 4 | 24 | 20.6 (±0.3) a, 20 (±0.2) b | 0.56 |
| APFO | 6 | 22 | 15.5 (±0.5)b | - |
| LiPFN | 0 | 25 | 11.3 [50] | 0.57 [50] |
| LiPFN | 3 | 25 | 9.5 [50] | 0.70 [50] |
| LiPFN | 6 | 25 | 9.3 [50] | 0.71 [50] |
| LiPFN | 8 | 25 | 10.0 [50] | 0.81 [50] |
| LiPFOS | 0 | 25 | 7.1 [50] | 0.63 [50] |
| LiPFOS | 3 | 25 | 5.8 [50] | 0.77 [50] |
| LiPFOS | 6 | 25 | 5.2 [50] | 0.90 [50] |
| SDS | 0 | 24 | 8.7 (±0.2) a, 9(±1) b, 8.2 [24] | 0.41, 0.30 [28] |
| SDS | 0.1 | 22 | 7.5 c [27] | 0.48 c [27] |
| SDS | 1 | 24 | 8.6 (±0.1) a, 8.5 (±1) b, 8.75 c [27] | 0.45, 0.43 c [27] |
| SDS | 4 | 24 | 10 (±0.3) a, 10.5 (±1) b, 9.6 [28], 10.5 [24] | 0.64, 0.40 [28] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kancharla, S.; Canales, E.; Alexandridis, P. Perfluorooctanoate in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment. Int. J. Mol. Sci. 2019, 20, 5761. https://doi.org/10.3390/ijms20225761
Kancharla S, Canales E, Alexandridis P. Perfluorooctanoate in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment. International Journal of Molecular Sciences. 2019; 20(22):5761. https://doi.org/10.3390/ijms20225761
Chicago/Turabian StyleKancharla, Samhitha, Emmanuel Canales, and Paschalis Alexandridis. 2019. "Perfluorooctanoate in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment" International Journal of Molecular Sciences 20, no. 22: 5761. https://doi.org/10.3390/ijms20225761
APA StyleKancharla, S., Canales, E., & Alexandridis, P. (2019). Perfluorooctanoate in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment. International Journal of Molecular Sciences, 20(22), 5761. https://doi.org/10.3390/ijms20225761






