Inositols’ Importance in the Improvement of the Endocrine–Metabolic Profile in PCOS
Abstract
:1. Introduction
2. Polycystic Ovary Syndrome (PCOS)
2.1. Definition and Classification
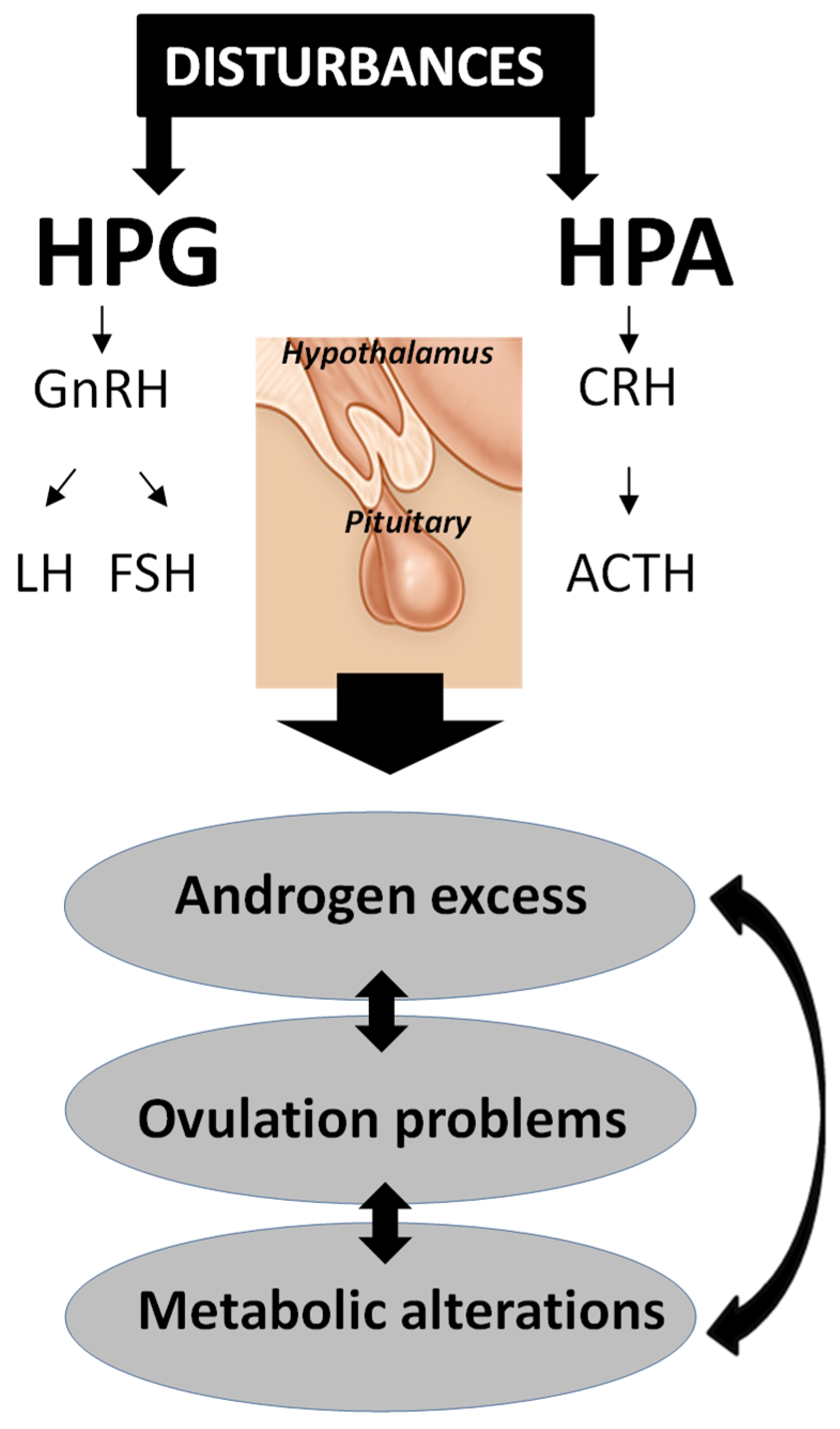
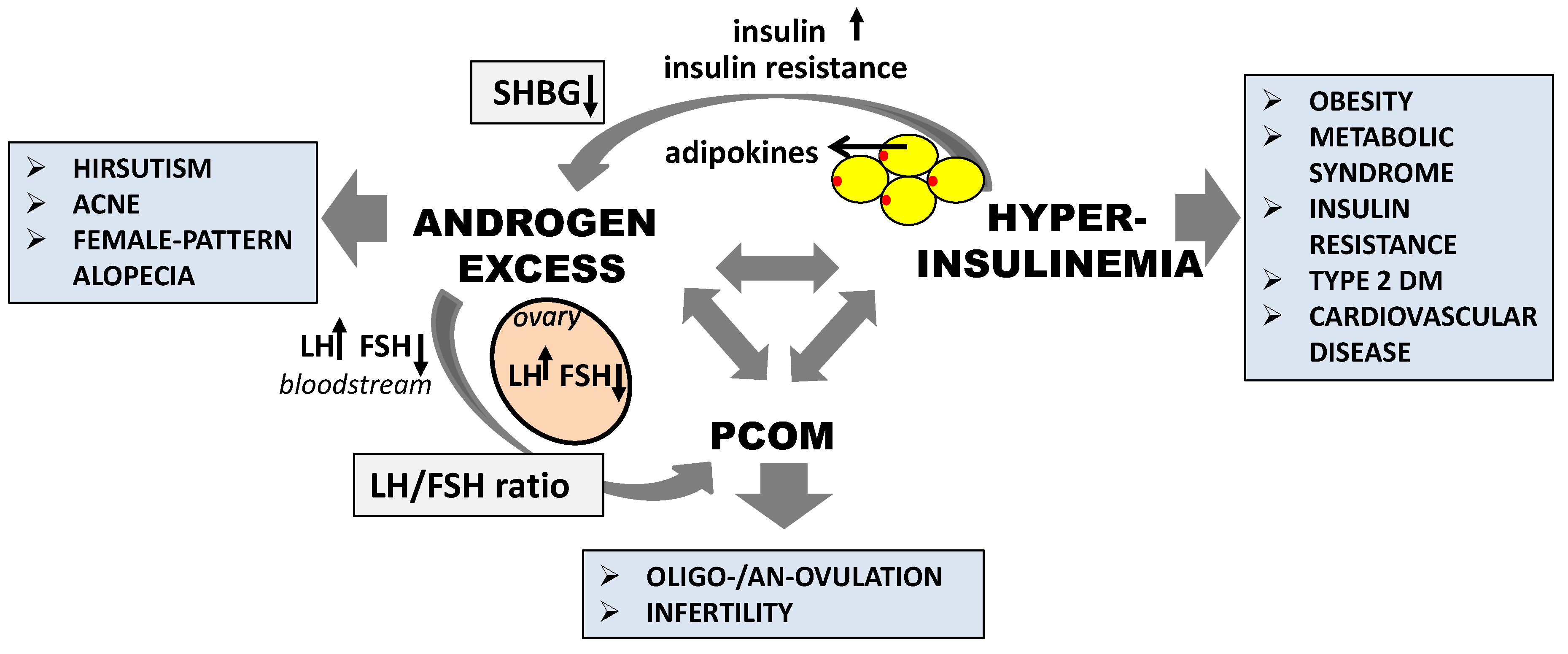
2.2. Pathogenesis of PCOS
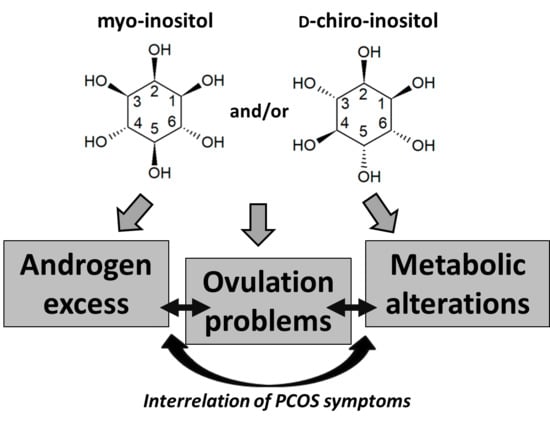
3. Inositol Characteristics
3.1. Isomers and Biosynthesis
3.2. Mechanism of Action
3.3. Clinical Relevance
4. The Role of Inositol in PCOS Treatment
4.1. The Effect of Inositol in Androgen Excess
4.2. The Effect of Inositol in Ovulation Disorders and Infertility
4.3. The Effect of Inositol in Polycystic Ovarian Morphology
4.4. The Effect of Inositol in Metabolic Abnormalities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic Ovary Syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef]
- Jin, P.; Xie, Y. Treatment Strategies for Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2018, 34, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Gateva, A.; Unfer, V.; Kamenov, Z. The Use of Inositol(s) Isomers in the Management of Polycystic Ovary Syndrome: A Comprehensive Review. Gynecol. Endocrinol. 2018, 34, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Facchinetti, F.; Orrù, B.; Giordani, B.; Nestler, J. Myo-Inositol Effects in Women with PCOS: A Meta-Analysis of Randomized Controlled Trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Orrù, B.; Grandi, G.; Unfer, V. Short-Term Effects of Metformin and Myo-Inositol in Women with Polycystic Ovarian Syndrome (PCOS): A Meta-Analysis of Randomized Clinical Trials. Facchinetti F1, Orrù B2, Grandi G1, Unfer V3. Gynecol. Endocrinol. 2019, 35, 198–206. [Google Scholar] [CrossRef]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- David, A.; Ehrmann, M.D. Polycystic Ovary Syndrome. N. Engl. J. Med. 2005, 352, 1223–1236. [Google Scholar]
- Michelmore, K.F.; Balen, A.H.; Dunger, D.B.; Vessey, M.P. Polycystic Ovaries and Associated Clinical and Biochemical Features in Young Women. Clin. Endocrinol. 1999, 51, 779–786. [Google Scholar] [CrossRef]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.W.; Norman, R.J.; Davies, M.J. The Prevalence of Polycystic Ovary Syndrome in a Community Sample Assessed under Contrasting Diagnostic Criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Position Statement: Criteria for Defining Polycystic Ovary Syndrome as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef] [PubMed]
- Zawadski, J.K.; Dunaif, A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach. In Polycystic Ovary Syndrome; Dunaif, A., Givens, J.R., Haseltine, F., Eds.; Blackwell Scientific: Boston, MA, USA, 1992; pp. 377–384. [Google Scholar]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The Polycystic Ovary Syndrome: A Position Statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [CrossRef] [PubMed]
- De Melo, A.S.; de Carvalho, C.R.; Ferriani, R.A.; Vieira, C.S.; Barbieri, M.A.; Dias, S.V.; Cardoso, V.C.; Bettiol, H. Pathogenesis of Polycystic Ovary Syndrome: Multifactorial Assessment from the Foetal Stage to Menopause. Reproduction 2015, 150, R11–R24. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of Polycystic Ovary Syndrome in a Dutch Twin-Family Study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Yarali, H.; Oguz, H.; Bayraktar, M. Glucose Intolerance, Insulin Resistance, and Hyperandrogenemia in First Degree Relatives of Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 2031–2036. [Google Scholar] [CrossRef]
- Fuster, J.J.; Andrés, V. Telomere Biology and Cardiovascular Disease. Circ. Res. 2006, 99, 1167–1180. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Kandarakis, H.; Legro, R.S. The Role of Genes and Environment in the Etiology of PCOS. Endocrine 2006, 30, 19–26. [Google Scholar] [CrossRef]
- Fornes, R.; Maliqueo, M.; Hu, M.; Hadi, L.; Jimenez-Andrade, J.M.; Ebefors, K.; Nyström, J.; Labrie, F.; Jansson, T.; Benrick, A.; et al. The Effect of Androgen Excess on Maternal Metabolism, Placental Function and Fetal Growth in Obese Dams. Sci. Rep. 2017, 7, 8066. [Google Scholar] [CrossRef]
- Jahromi, M.S.; Tehrani, F.R.; Noroozzadeh, M.; Zarkesh, M.; Ghasemi, A.; Zadeh-Vakili, A. Elevated Expression of Steroidogenesis Pathway Genes; CYP17, GATA6 and StAR in Prenatally Androgenized Rats. Gene 2016, 593, 167–171. [Google Scholar] [CrossRef]
- Anderson, S.A.; Barry, J.A.; Hardiman, P.J. Risk of Coronary Heart Disease and Risk of Stroke in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2014, 176, 486–487. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; San Millán, J.L. The Molecular-Genetic Basis of Functional Hyperandrogenism and the Polycystic Ovary Syndrome. Endocr. Rev. 2005, 26, 251–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, J.; Guo, Z.; Chen, M.; Wang, C.; He, C.; Zuo, Z. A Pilot Study on Polycystic Ovarian Syndrome Caused by Neonatal Exposure to Tributyltin and Bisphenol A in Rats. Chemosphere 2019, 231, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Akin, L.; Kendirci, M.; Narin, F.; Kurtoglu, S.; Saraymen, R.; Kondolot, M.; Koçak, S.; Elmali, F. The Endocrine Disruptor Bisphenol A May Play a Role in the Aetiopathogenesis of Polycystic Ovary Syndrome in Adolescent Girls. Acta Paediatr. Int. J. Paediatr. 2015, 104, e171–e177. [Google Scholar] [CrossRef] [PubMed]
- Mitro, S.D.; Johnson, T.; Zota, A.R. Cumulative Chemical Exposures During Pregnancy and Early Development. Curr. Environ. Health Rep. 2015, 2, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Maity, A.; Missmer, S.A.; Williams, P.L.; Mahalingaiah, S.; Ehrlich, S.; Berry, K.F.; Altshul, L.; Perry, M.J.; Cramer, D.W.; et al. Serum Concentrations of Polychlorinated Biphenyls in Relation to in Vitro Fertilization Outcomes. Environ. Health Perspect. 2011, 119, 1010–1016. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.M.; Koenders, P.P.; Steegers-Theunissen, R.P.; ten Boekel, E.; ter Wee, M.M.; Louwers, Y.; Lips, P.; Laven, J.S.E.; Simsek, S. Vitamin D and Metabolic Disturbances in Polycystic Ovary Syndrome (PCOS): A Cross-Sectional Study. PLoS ONE 2018, 13, e0204748. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and Fertility: A Systematic Review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef]
- Nelson, V.L.; Qin, K.; Rosenfield, R.L.; Wood, J.R.; Penning, T.M.; Legro, R.S.; Strauss, J.F.; McAllister, J.M. The Biochemical Basis for Increased Testosterone Production in Theca Cells Propagated from Patients with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2014, 86, 5925–5933. [Google Scholar] [CrossRef]
- Unfer, V.; Porcaro, G. Updates on the Myo-Inositol plus d-Chiro-Inositol Combined Therapy in Polycystic Ovary Syndrome. Expert Rev. Clin. Pharmacol. 2014, 7, 623–631. [Google Scholar] [CrossRef]
- Genazzani, A.D. Inositol as Putative Integrative Treatment for PCOS. Reprod. Biomed. Online 2016, 33, 770–780. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Sir-Petermann, T.; Codner, E.; Pérez, V.; Echiburú, B.; Maliqueo, M.; De Guevara, A.L.; Preisler, J.; Crisosto, N.; Sánchez, F.; Cassorla, F.; et al. Metabolic and Reproductive Features before and during Puberty in Daughters of Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 1923–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sir-Petermann, T.; Maliqueo, M.; Codner, E.; Echiburú, B.; Crisosto, N.; Pérez, V.; Pérez-Bravo, F.; Cassorla, F. Early Metabolic Derangements in Daughters of Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 4637–4642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maliqueo, M.; Sir-Petermann, T.; Pérez, V.; Echiburú, B.; De Guevara, A.L.; Gálvez, C.; Crisosto, N.; Azziz, R. Adrenal Function during Childhood and Puberty in Daughters of Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 3282–3288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibanez, L.; Dimartino-Nardi, J.; Potau, N.; Saenger, P. Premature Adrenarche-Normal Variant or Forerunner of Adult Disease? Endocr. Rev. 2000, 21, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Maliqueo, M.; Echiburú, B.; Crisosto, N.; Amigo, P.; Aranda, P.; Sánchez, F.; Sir-Petermann, T. Metabolic Parameters in Cord Blood of Newborns of Women with Polycystic Ovary Syndrome. Fertil. Steril. 2009, 92, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.C.; Gnatuk, C.L.; Kunselman, A.R.; Demers, L.M.; Lee, P.A.; Legro, R.S. Hyperandrogenism and Hyperinsulinism in Children of Women with Polycystic Ovary Syndrome: A Controlled Study. J. Clin. Endocrinol. Metab. 2008, 93, 1662–1669. [Google Scholar] [CrossRef] [Green Version]
- Al-Suod, H.; Ligor, M.; Rațiu, I.A.; Rafińska, K.; Górecki, R.; Buszewski, B. A Window on Cyclitols: Characterization and Analytics of Inositols. Phytochem. Lett. 2017, 20, 507–519. [Google Scholar] [CrossRef]
- Jóźwik, M.; Jóźwik, M.; Teng, C.; Jóźwik, M.; Battaglia, F.C. Human Breast Milk Sugars and Polyols over the First 10 Puerperium Days. Am. J. Hum. Biol. 2013, 25, 198–204. [Google Scholar] [CrossRef]
- Larner, J. D-Chiro-Inositol—Its Functional Role in Insulin resistance. Int. J. Exp. Diabetes Res. 2002, 3, 47–60. [Google Scholar] [CrossRef]
- Holub, B.J. The Nutritional Significance, Metabolism, and Function of Myo-Inositol and Phosphatidylinositol in Health and Disease. Adv. Nutr. Res. 1982, 4, 107–141. [Google Scholar] [PubMed]
- Asplin, I.; Galasko, G.; Larner, J. Chiro-Inositol Deficiency and Insulin Resistance: A Comparison of the Chiro-Inositol- and the Myo-Inositol-Containing Insulin Mediators Isolated from Urine, Hemodialysate, and Muscle of Control and Type II Diabetic Subjects. Proc. Natl. Acad. Sci. USA 2006, 90, 5924–5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unfer, V.; Proietti, S.; Gullo, G.; Porcaro, G.; Carlomagno, G.; Bizzarri, M. Polycystic Ovary Syndrome: Features, Diagnostic Criteria and Treatments. Endocrinol. Metab. Syndr. 2014, 3, 3. [Google Scholar]
- Croze, M.L.; Soulage, C.O. Potential Role and Therapeutic Interests of Myo-Inositol in Metabolic Diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Larner, J.; Huang, L.C.; Tang, G.; Suzuki, S.; Schwartz, C.F.; Romero, G.; Roulidis, Z.; Zeller, K.; Shen, T.Y.; Oswald, A.S.; et al. Insulin Mediators: Structure and Formation. Cold Spring Harb. Symp. Quant. Biol. 1988, 53, 965–971. [Google Scholar] [CrossRef]
- Unfer, V.; Carlomagno, G.; Papaleo, E.; Vailati, S.; Candiani, M.; Baillargeon, J.P. Hyperinsulinemia Alters Myoinositol to D-Chiroinositol Ratio in the Follicular Fluid of Patients with PCOS. Reprod. Sci. 2014, 21, 854–858. [Google Scholar] [CrossRef]
- Zulfarina, M.S.; Syarifah-Noratiqah, S.-B.; Nazrun, S.A.; Sharif, R.; Naina-Mohamed, I. Pharmacological Therapy in Panic Disorder: Current Guidelines and Novel Drugs Discovery for Treatment-Resistant Patient. Clin. Psychopharmacol. Neurosci. 2019, 17, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; McGrath, B.M.; Silverstone, P.H. A Review of the Possible Relevance of Inositol and the Phosphatidylinositol Second Messenger System (PI-Cycle) to Psychiatric Disorders—Focus on Magnetic Resonance Spectroscopy (MRS) Studies. Hum. Psychopharmacol. 2005, 20, 309–326. [Google Scholar] [CrossRef]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and Pharmacokinetics of Inositol(s) in Health and Disease. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef]
- Krishnan, A.; Muthusami, S. Hormonal Alterations in PCOS and Its Influence on Bone Metabolism. J. Endocrinol. 2017, 232, R99–R113. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Chung, S.K.; Miao, D.; Lau, K.S.; Chan, A.W.; Kung, A.W. Sodium/Myo-inositol Cotransporter 1 and Myo-inositol Are Essential for Osteogenesis and Bone Formation. J. Bone Miner. Res. 2011, 26, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Lahuta, L.; Ligor, M.; Placek, W.; Górecki, R.; Buszewski, B. The Healing-Promoting Properties of Selected Cyclitols—A Review. Nutrients 2018, 10, 1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laganà, A.S.; Rossetti, P.; Buscema, M.; La Vignera, S.; Condorelli, R.A.; Gullo, G.; Granese, R.; Triolo, O. Metabolism and Ovarian Function in PCOS Women: A Therapeutic Approach with Inositols. Int. J. Endocrinol. 2016, 2016, 6306410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzo, A.; Laganà, A.S.; Barbaro, L. Comparison between Effects of Myo-Inositol and d-Chiro-Inositol on Ovarian Function and Metabolic Factors in Women with PCOS. Gynecol. Endocrinol. 2014, 30, 205–208. [Google Scholar] [CrossRef]
- Nestler, J.E.; Unfer, V. Reflections on Inositol(s) for PCOS Therapy: Steps toward Success. Gynecol. Endocrinol. 2015, 31, 501–505. [Google Scholar] [CrossRef]
- Huang, L.C.; Fonteles, M.C.; Houston, D.B.; Zhang, C.; Larner, J. Chiroinositol Deficiency and Insulin Resistance. III. Acute Glycogenic and Hypoglycemic Effects of Two Inositol Phosphoglycan Insulin Mediators in Normal Streptozotocin-Diabetic Rats In Vivo. Endocrinology 1993, 132, 652–657. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Carlomagno, G.; Gerli, S.; Oliva, M.M.; Devroey, P.; Lanzone, A.; Soulange, C.; Facchinetti, F.; Di Renzo, G.C.; Bizzarri, M.; et al. Results from the International Consensus Conference on Myo-Inositol and D-Chiro-Inositol in Obstetrics and Gynecology-Assisted Reproduction Technology. Gynecol. Endocrinol. 2015, 31, 441–446. [Google Scholar] [CrossRef]
- Benelli, E.; Del Ghianda, S.; Di Cosmo, C.; Tonacchera, M. A Combined Therapy with Myo-Inositol and D-Chiro-Inositol Improves Endocrine Parameters and Insulin Resistance in PCOS Young Overweight Women. Int. J. Endocrinol. 2016, 2016, 3204083. [Google Scholar] [CrossRef] [Green Version]
- Minozzi, M.; Costantino, D.; Guaraldi, C.; Unfer, V. The Effect of a Combination Therapy with Myo-Inositol and a Combined Oral Contraceptive Pill versus a Combined Oral Contraceptive Pill Alone on Metabolic, Endocrine, and Clinical Parameters in Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2011, 27, 920–924. [Google Scholar] [CrossRef]
- Wu, S.; Divall, S.; Nwaopara, A.; Radovick, S.; Wondisford, F.; Ko, C.; Wolfe, A. Obesity-Induced Infertility and Hyperandrogenism Are Corrected by Deletion of the Insulin Receptor in the Ovarian Theca Cell. Diabetes 2014, 63, 1270–1282. [Google Scholar] [CrossRef] [Green Version]
- Genazzani, A.D.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Despini, G.; Marini, G.; Prati, A.; Simoncini, T. Modulatory Role of D-Chiro-Inositol (DCI) on LH and Insulin Secretion in Obese PCOS Patients. Gynecol. Endocrinol. 2014, 30, 438–443. [Google Scholar] [CrossRef]
- Artini, P.G.; Di Berardino, O.M.; Papini, F.; Genazzani, A.D.; Simi, G.; Ruggiero, M.; Cela, V. Endocrine and Clinical Effects of Myo-Inositol Administration in Polycystic Ovary Syndrome. A Randomized Study. Gynecol. Endocrinol. 2013, 29, 375–379. [Google Scholar] [CrossRef]
- Costantino, D.; Minozzi, G.; Minozzi, F.; Guaraldi, C. Metabolic and hormonal effects of myo-inositol in women with polycystic ovary syndrome: A double-blind trial. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 105–110. [Google Scholar]
- Nestler, J.E.; Jakubowicz, D.J.; Reamer, P.; Gunn, R.D.; Allan, G. Ovulatory and Metabolic Effects of D-Chiro-Inositol in the Polycystic Ovary Syndrome. N. Engl. J. Med. 1999, 340, 1314–1320. [Google Scholar] [CrossRef] [Green Version]
- Regidor, P.A.; Schindler, A.E.; Lesoine, B.; Druckman, R. Management of Women with PCOS Using Myo-Inositol and Folic Acid. New Clinical Data and Review of the Literature. Horm. Mol. Biol. Clin. Investig. 2018. [Google Scholar] [CrossRef]
- Carlomagno, G.; De Grazia, S.; Unfer, V.; Manna, F. Myo-Inositol in a New Pharmaceutical Form: A Step Forward to a Broader Clinical Use. Expert Opin. Drug Deliv. 2012, 9, 267–271. [Google Scholar] [CrossRef]
- Heimark, D.; Mcallister, J.; Larner, J. Decreased Myo-Inositol to Chiro-Inositol (M/C) Ratios and Increased M/C Epimerase Activity in PCOS Theca Cells Demonstrate Increased Insulin Sensitivity Compared to Controls. Endocr. J. 2014, 61, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Januszewski, M.; Issat, T.; Jakimiuk, A.A. Metabolic and Hormonal Effects of a Combined Myo-Inositol and D-Chiro-Inositol Therapy on Patients with Polycystic Ovary Syndrome (PCOS). Ginekol. Polska 2019, 90, 7–10. [Google Scholar] [CrossRef]
- Balen, A.H.; Rutherford, A.J. Managing Anovulatory Infertility and Polycystic Ovary Syndrome. Br. Med. J. 2007, 335, 663–666. [Google Scholar] [CrossRef] [Green Version]
- Chiu, T.T.Y.; Tam, P.P.L. A Correlation of the Outcome of Clinical in Vitro Fertilization with the Inositol Content and Embryotrophic Properties of Human Serum. J. Assist. Reprod. Genet. 1992, 9, 524–530. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Bizzarri, M. Physiological Role and Clinical Utility of Inositols in Polycystic Ovary Syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 37, 129–139. [Google Scholar] [CrossRef]
- Chiu, T.T.Y.; Rogers, M.S.; Law, E.L.K.; Briton-Jones, C.M.; Cheung, L.P.; Haines, C.J. Follicular Fluid and Serum Concentrations of Myo-Inositol in Patients Undergoing IVF: Relationship with Oocyte Quality. Hum. Reprod. 2002, 17, 1591–1596. [Google Scholar] [CrossRef] [Green Version]
- Gerli, S.; Mignosa, M.; Di Renzo, G.C. Effects of Inositol on Ovarian Function and Metabolic Factors in Women with PCOS: A Randomized Double Blind Placebo-Controlled Trial. Eur. Rev. Med. Pharmacol. Sci. 2003, 7, 151–159. [Google Scholar]
- Dell’Edera, D.; Sarlo, F.; Allegretti, A.; Simone, F.; Lupo, M.G.; Epifania, A.A. The Influence of D-Chiro-Inositol and D-Myo-Inositol in Pregnant Women with Glucose Intolerance. Biomed. Rep. 2017, 7, 169–172. [Google Scholar] [CrossRef]
- Papaleo, E.; Unfer, V.; Baillargeon, J.P.; De Santis, L.; Fusi, F.; Brigante, C.; Marelli, G.; Cino, I.; Redaelli, A.; Ferrari, A. Myo-Inositol in Patients with Polycystic Ovary Syndrome: A Novel Method for Ovulation Induction. Gynecol. Endocrinol. 2007, 23, 700–703. [Google Scholar] [CrossRef]
- Nordio, M.; Proietti, E. The Combined Therapy with Myo-Inositol and D-Chiro-Inositol Reduces the Risk of Metabolic Disease in PCOS Overweight Patients Compared to Myo-Inositol Supplementation Alone. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 575–581. [Google Scholar]
- Galazis, N.; Galazi, M.; Atiomo, W. D-Chiro-Inositol and Its Significance in Polycystic Ovary Syndrome: A Systematic Review. Gynecol. Endocrinol. 2011, 27, 256–262. [Google Scholar] [CrossRef]
- Isabella, R.; Raffone, E. Does Ovary Need D-Chiro-Inositol? J. Ovarian Res. 2012, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Arya, B.K.; Haq, A.U.; Chaudhury, K. Oocyte Quality Reflected by Follicular Fluid Analysis in Poly Cystic Ovary Syndrome (PCOS): A Hypothesis Based on Intermediates of Energy Metabolism. Med. Hypotheses 2012, 78, 475–478. [Google Scholar] [CrossRef]
- Colazingari, S.; Treglia, M.; Najjar, R.; Bevilacqua, A. The Combined Therapy Myo-Inositol plus D-Chiro-Inositol, Rather than D-Chiro-Inositol, Is Able to Improve IVF Outcomes: Results from a Randomized Controlled Trial. Arch. Gynecol. Obstet. 2013, 288, 1405–1411. [Google Scholar] [CrossRef]
- Balen, A.; Homburg, R.; Franks, S. Defining Polycystic Ovary Syndrome. BMJ 2009, 338, a2968. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Lujan, M.E.; Carmina, E.; Cedars, M.I.; Laven, J.; Norman, R.J.; Escobar-morreale, H.F. Definition and Significance of Polycystic Ovarian Morphology: A Task Force Report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2014, 20, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Bhide, P.; Homburg, R. Anti-Müllerian Hormone and Polycystic Ovary Syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 37, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between Androgens, FSH, Anti-Mullerian Hormone and Estradiol during Folliculogenesis in the Human Normal and Polycystic Ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef] [Green Version]
- Dinicola, S.; Chiu, T.T.Y.; Unfer, V.; Carlomagno, G.; Bizzarri, M. The Rationale of the Myo-Inositol and D-Chiro-Inositol Combined Treatment for Polycystic Ovary Syndrome. J. Clin. Pharmacol. 2014, 54, 1079–1092. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Dragotto, J.; Giuliani, A.; Bizzarri, M. Myo-Inositol and D-Chiro-Inositol (40:1) Reverse Histological and Functional Features of Polycystic Ovary Syndrome in a Mouse Model. J. Cell. Physiol. 2019, 234, 9387–9398. [Google Scholar] [CrossRef]
- Jiao, J.; Sagnelli, M.; Shi, B.; Fang, Y.; Shen, Z.; Tang, T.; Dong, B.; Li, D.; Wang, X. Genetic and Epigenetic Characteristics in Ovarian Tissues from Polycystic Ovary Syndrome Patients with Irregular Menstruation Resemble Those of Ovarian Cancer. BMC Endocr. Disord. 2019, 19, 30. [Google Scholar] [CrossRef]
- DeUgarte, C.M.; Bartolucci, A.A.; Azziz, R. Prevalence of Insulin Resistance in the Polycystic Ovary Syndrome Using the Homeostasis Model Assessment. Fertil. Steril. 2005, 83, 1454–1460. [Google Scholar] [CrossRef]
- Orio, F.; Muscogiuri, G.; Ascione, A.; Marciano, F.; Volpe, A.; La Sala, G.; Savastano, S.; Colao, A.; Palomba, S. Effects of Physical Exercise on the Female Reproductive System. Minerva Endocrinol. 2013, 38, 305–319. [Google Scholar]
- Szydlarska, D.; Grzesiuk, W.; Bar-andziak, E. Kontrowersje Wokół Patogenezy Zespołu Policystycznych Jajników. Endokrynol. Otyłość Zaburzenia Przemiany Mater. 2010, 6, 141–146. [Google Scholar]
- Panidis, D.; Tziomalos, K.; Papadakis, E.; Vosnakis, C.; Chatzis, P.; Katsikis, I. Lifestyle Intervention and Anti-Obesity Therapies in the Polycystic Ovary Syndrome: Impact on Metabolism and Fertility. Endocrine 2012, 44, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Nybacka, Å.; Carlström, K.; Ståhle, A.; Nyrén, S.; Hellström, P.M.; Hirschberg, A.L. Randomized Comparison of the Influence of Dietary Management and/or Physical Exercise on Ovarian Function and Metabolic Parameters in Overweight Women with Polycystic Ovary Syndrome. Fertil. Steril. 2011, 96, 1508–1513. [Google Scholar] [CrossRef]
- Domecq, J.P.; Prutsky, G.; Mullan, R.J.; Hazem, A.; Sundaresh, V.; Elamin, M.B.; Phung, O.J.; Wang, A.; Hoeger, K.; Pasquali, R.; et al. Lifestyle Modification Programs in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2013, 98, 4655–4663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillargeon, J.P.; Diamanti-Kandarakis, E.; Ostlund, R.E.; Apridonidze, T.; Iuorno, M.J.; Nestler, J.E. Altered D-Chiro-Inositol Urinary Clearance in Women with Polycystic Ovary Syndrome. Diabetes Care 2006, 29, 300–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, R.A.; Rizzo, M.; Clifton, S.; Carmina, E. Lipid Levels in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Fertil. Steril. 2011, 95, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Falbo, A.; Chiossi, G.; Muscogiuri, G.; Fornaciari, E.; Orio, F.; Tolino, A.; Colao, A.; Sala La, B.G.; Zullo, F. Lipid Profile in Nonobese Pregnant Women with Polycystic Ovary Syndrome: A Prospective Controlled Clinical Study. Steroids 2014, 88, 36–43. [Google Scholar] [CrossRef]
- Gerli, S.; Papaleo, E.; Ferrari, A.; Di Renzo, G.C. Randomized, Double Blind Placebo-Controlled Trial: Effects of Myo-Inositol on Ovarian Function and Metabolic Factors in Women with PCOS. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 347–354. [Google Scholar]
- Minozzi, M.; Nordio, M.; Pajalich, R. The Combined Therapy Myo-Inositol plus D-Chiro-Inositol, in a Physiological Ratio, Reduces the Cardiovascular Risk by Improving the Lipid Profile in PCOS Patients. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 537–540. [Google Scholar]
- Crawford, T.J.; Crowther, C.A.; Alsweiler, J.; Brown, J. Antenatal Dietary Supplementation with Myo-Inositol in Women during Pregnancy for Preventing Gestational Diabetes. Cochrane Database Syst. Rev. 2015, 12, CD011507. [Google Scholar] [CrossRef]
- Brusati, V.; Jóźwik, M.; Jóźwik, M.; Teng, C.; Paolini, C.; Marconi, A.M.; Battaglia, F.C. Fetal and Maternal Non-Glucose Carbohydrates and Polyols Concentrations in Normal Human Pregnancies at Term. Pediatr. Res. 2005, 58, 700–704. [Google Scholar] [CrossRef] [Green Version]
| Diagnostic Criteria | ||
|---|---|---|
| Rotterdam (at least two of three features) | AES (both features) | NIH (both features) |
| Oligo-/anovulation | Hyperandrogenism/hyperandrogenemia | Oligo-/anovulation |
| Hyperandrogenemia/hyperandrogenism | Oligo-/anovulation and/or Polycystic ovaries | Hyperandrogenism/hyperandrogenemia |
| Polycystic ovaries | ||
| PCOS Symptom | Inositol | Effect | Authors |
|---|---|---|---|
| Androgen excess | MI | -total and free T level reduction | [64] |
| -free T decrease; E2, SHBG increase | [59] | ||
| -T level reduction | [63] | ||
| DCI | -T level reduction and P4 level increase | [66] | |
| -free T decrease | [65] | ||
| both | -free T reduction; SHBG increase | [69] | |
| Ovulation and fertility disorders; ovaries dysfunction | MI | -better development of mouse embryos | [71] |
| -restored spontaneous ovulation; elevated rate of fertilization/pregnancy | [66] | ||
| -better embryo quality, no side-effects | [73] | ||
| -increased FSH sensitivity, better fertilization rates and embryo quality; reduced LH:FSH ratio | [63] | ||
| -restored spontaneous ovarian activity and fertility | [76] | ||
| -higher ovulation frequency, shorter time to first ovulation | [98] | ||
| DCI | -elevated E2; rapid follicular maturation | [74] | |
| -ovulation improvement | [78] | ||
| both | -poor oocyte quality; worse ovarian response | [79] | |
| -increased ovulation cases | [75] | ||
| -ovarian function improvement | [77] | ||
| -improved oocyte/embryo quality and pregnancy rates | [81] | ||
| -restoration of ovarian proper histological features, TGR ratio and fertility in mice | [87] | ||
| Metabolic abnormalities | MI | -improved glucose-to-insulin ratio and HOMA index | [63] |
| -increased circulating HDL level, weight loss, and leptin reduction | [98] | ||
| DCI | -improved insulin resistance, blood pressure, and plasma TG concentration | [65] | |
| -better insulin sensitivity, BMI reduction | [62] | ||
| both | -body weight and insulin level reduction | [69] | |
| -reduced fasting insulin and HOMA index | [59] | ||
| -improved LDL, HDL, and TG levels, HOMA index reduction | [99] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowska, A.; Osowski, A.; Jóźwik, M.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Inositols’ Importance in the Improvement of the Endocrine–Metabolic Profile in PCOS. Int. J. Mol. Sci. 2019, 20, 5787. https://doi.org/10.3390/ijms20225787
Wojciechowska A, Osowski A, Jóźwik M, Górecki R, Rynkiewicz A, Wojtkiewicz J. Inositols’ Importance in the Improvement of the Endocrine–Metabolic Profile in PCOS. International Journal of Molecular Sciences. 2019; 20(22):5787. https://doi.org/10.3390/ijms20225787
Chicago/Turabian StyleWojciechowska, Anna, Adam Osowski, Marcin Jóźwik, Ryszard Górecki, Andrzej Rynkiewicz, and Joanna Wojtkiewicz. 2019. "Inositols’ Importance in the Improvement of the Endocrine–Metabolic Profile in PCOS" International Journal of Molecular Sciences 20, no. 22: 5787. https://doi.org/10.3390/ijms20225787
APA StyleWojciechowska, A., Osowski, A., Jóźwik, M., Górecki, R., Rynkiewicz, A., & Wojtkiewicz, J. (2019). Inositols’ Importance in the Improvement of the Endocrine–Metabolic Profile in PCOS. International Journal of Molecular Sciences, 20(22), 5787. https://doi.org/10.3390/ijms20225787