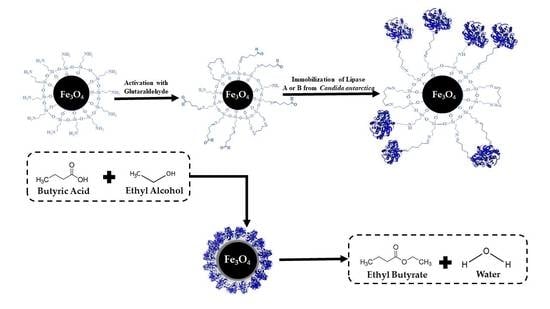
Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation
Abstract
1. Introduction
2. Results and Discussion
2.1. Immobilization Performance
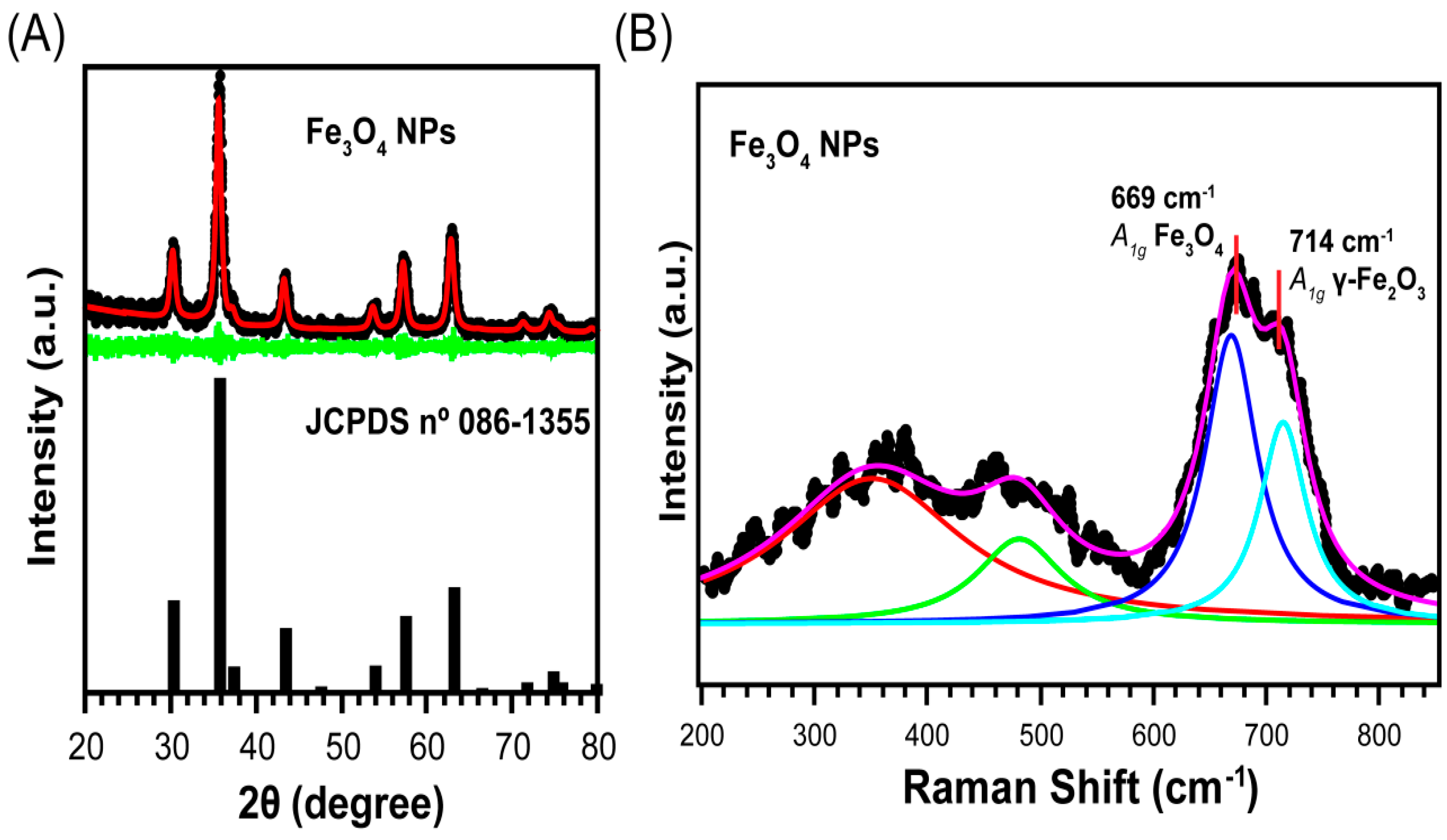
2.2. Characterization of Fe3O4 NPs
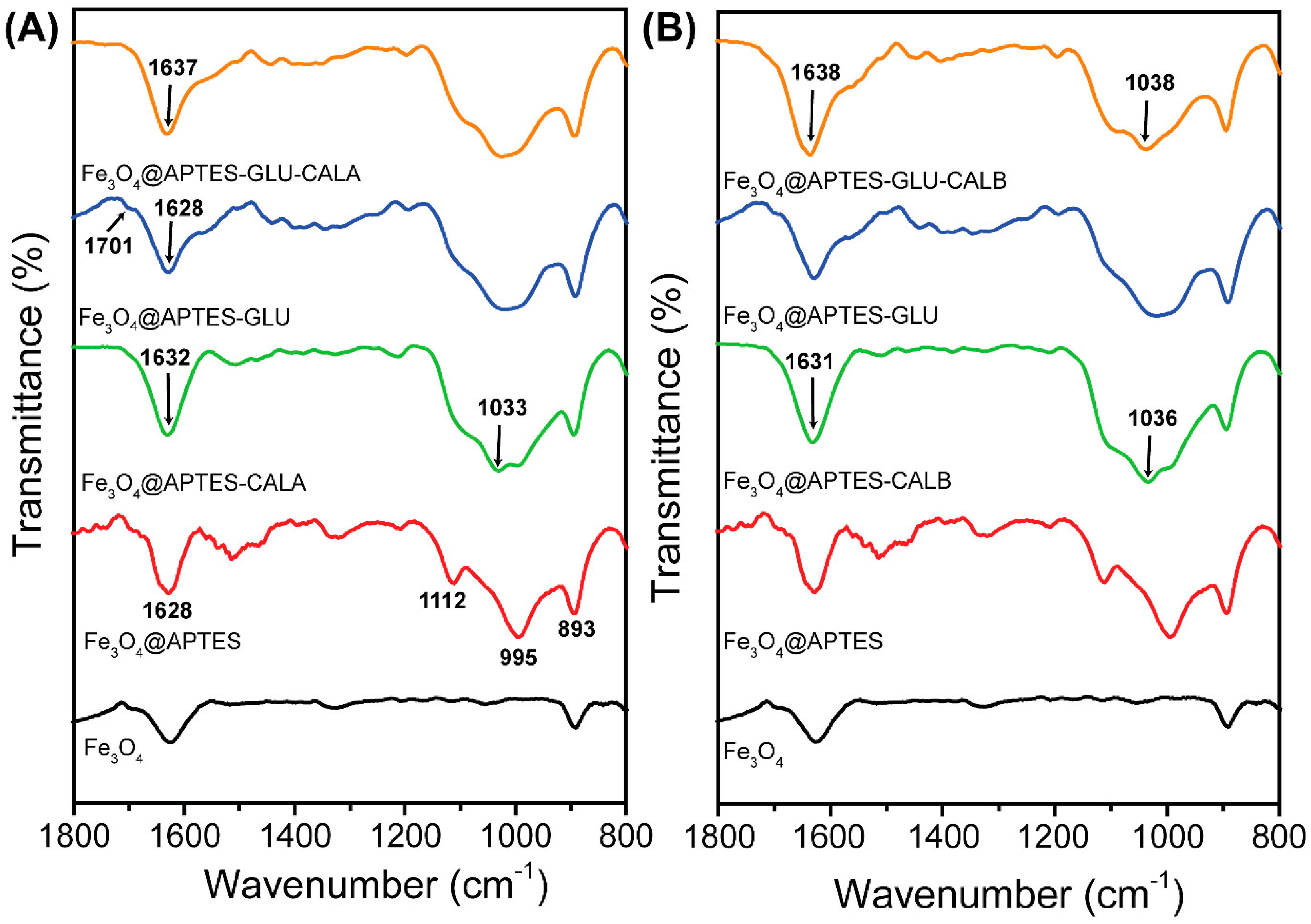
2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
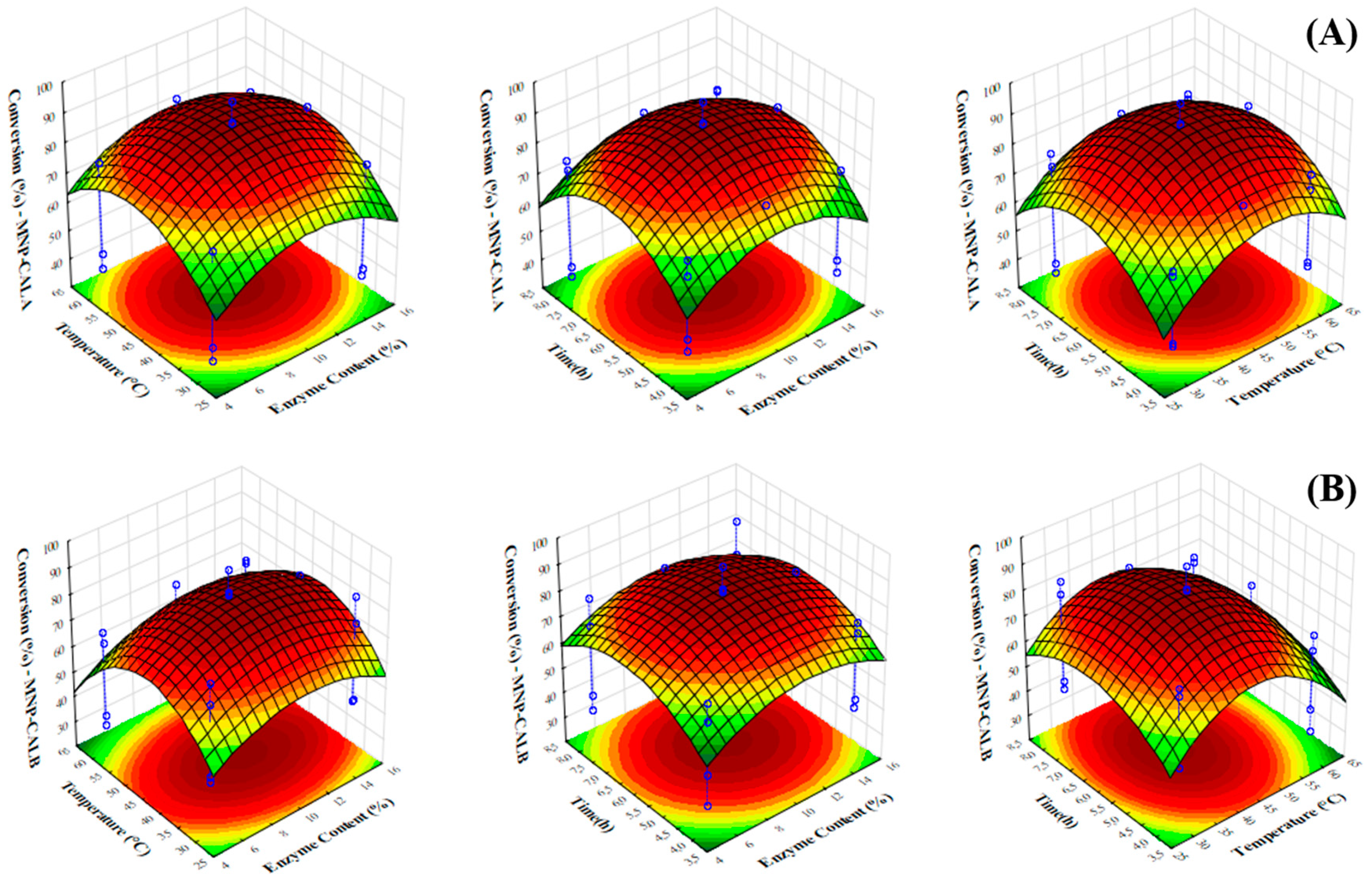
2.4. Model Fitting and ANOVA
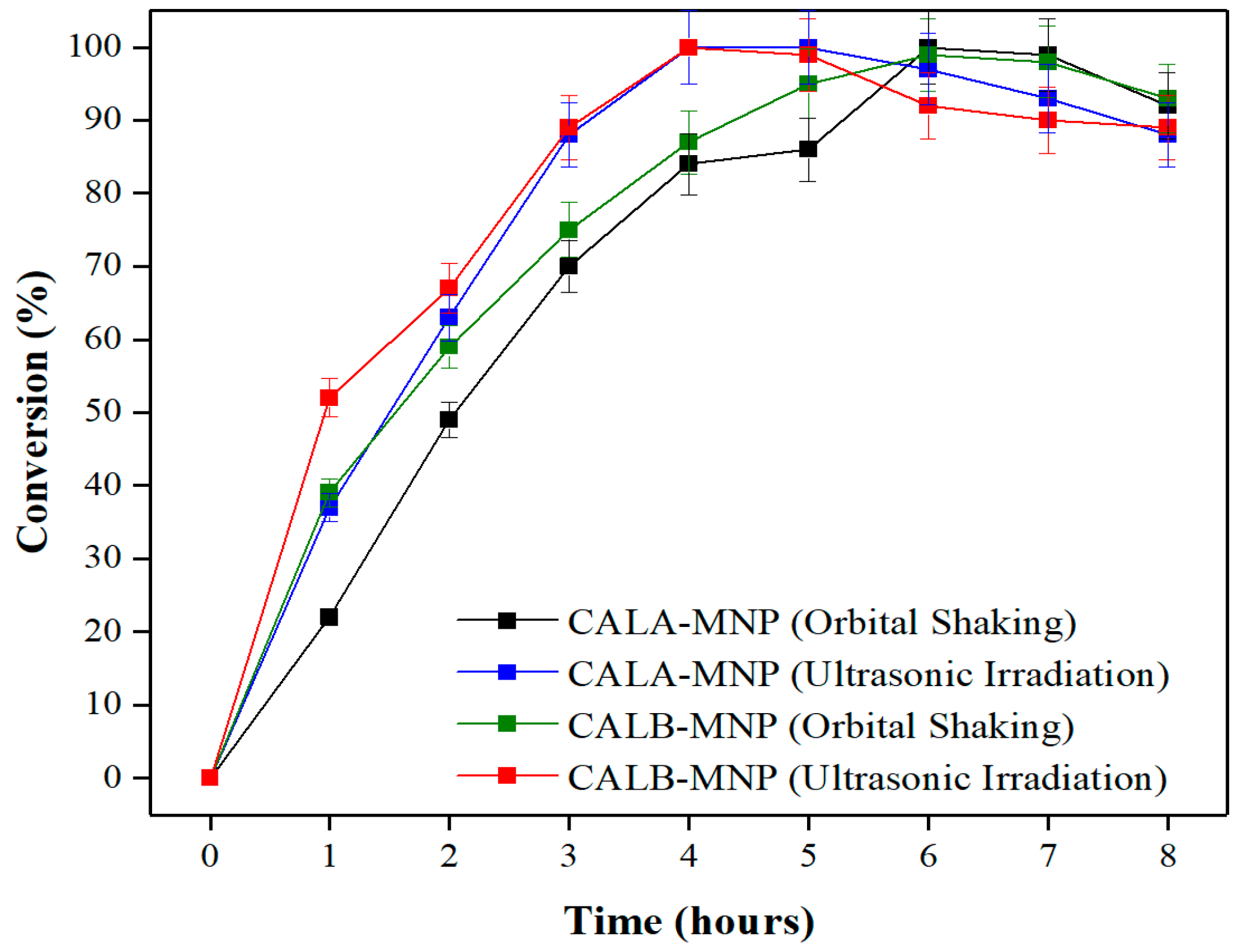
2.5. Time Course of Esterification
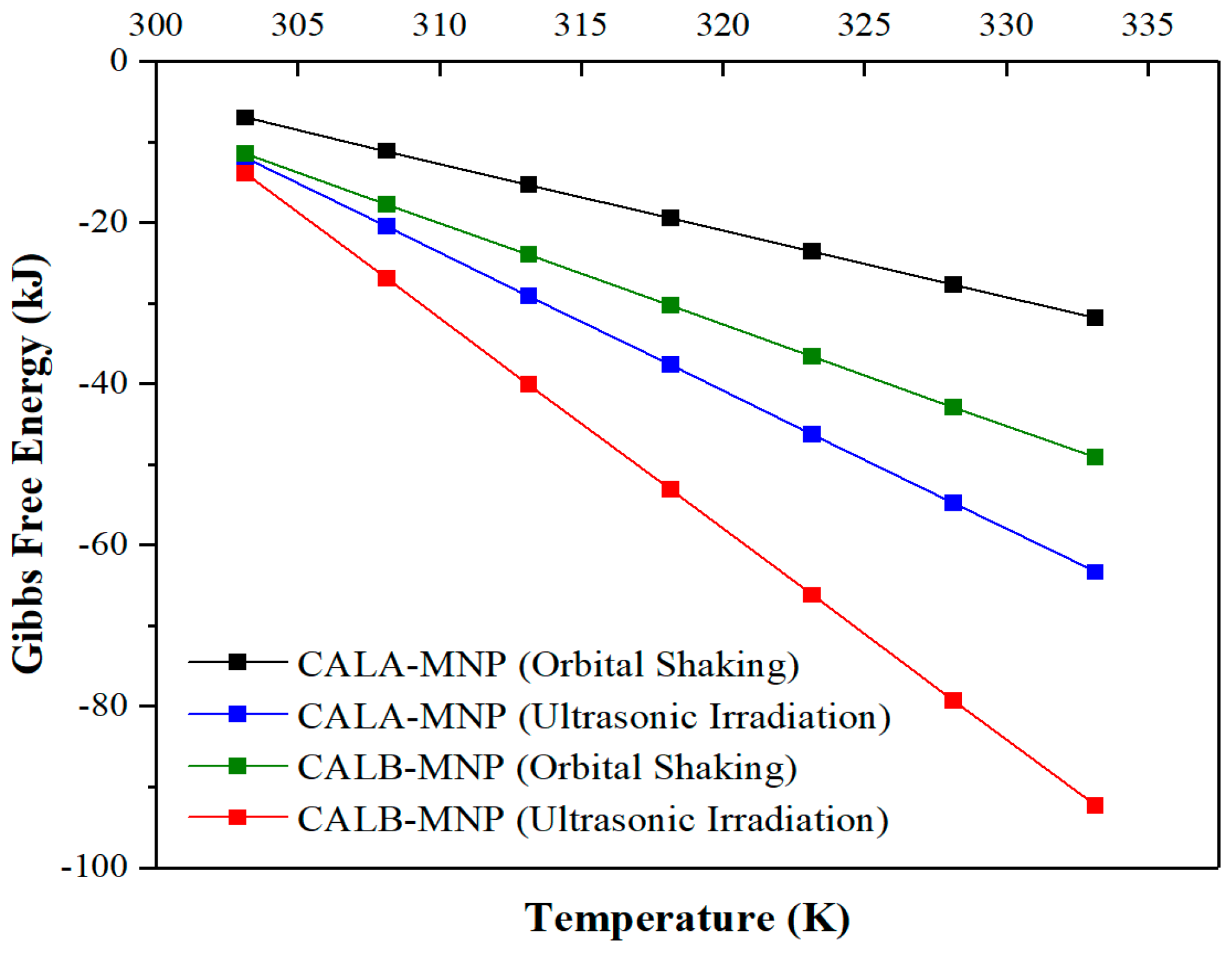
2.6. Thermodynamics of the Enzymatic Esterification
2.7. Operational Stability
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Ultrasound Equipment Setup
3.2.2. Synthesis of Iron Magnetic Nanoparticles (Fe3O4) Functionalized with 3 Aminopropyltriethoxysilane (APTES)
3.2.3. Activation of Fe3O4@APTES with Glutaraldehyde (GLU)
3.2.4. Covalent Immobilization of CALA or CALB onto Fe3O4@APTES-GLU
3.2.5. Adsorption Immobilization of CALA or CALB onto Fe3O4@APTES
3.2.6. Determination of Enzymatic Activity and Protein Concentration
3.2.7. Immobilization Parameters
3.2.8. X-Ray Diffraction (XRD)
3.2.9. Raman Spectroscopy
3.2.10. Fourier-transform Infrared Spectroscopy (FT-IR)
3.2.11. Enzymatic Esterification
3.2.12. Central Composite Design (CCD)
3.2.13. Statistical Analysis
3.2.14. Operational Stability
3.2.15. Thermodynamic Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.B.; Freire, T.M.; Dutra, L.M.U.; Fechine, P.B.A.; Gonçalves, L.R.B.; de Souza, M.C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of lipase A from Candida antarctica onto chitosan-coated magnetic nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.C.M.; dos Santos, K.P.; Freire, R.M.; Barreto, A.C.H.; Fechine, P.B.A.; Gonçalves, L.R.B. Production of flavor esters catalyzed by lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz. J. Chem. Eng. 2017, 34, 681–690. [Google Scholar] [CrossRef]
- Pinheiro, M.P.; Rios, N.S.; Fonseca, T.d.S.; Bezerra, F.d.A.; Rodríguez-Castellón, E.; Fernandez-Lafuente, R.; Carlos de Mattos, M.; dos Santos, J.C.S.; Gonçalves, L.R.B. Kinetic resolution of drug intermediates catalyzed by lipase B from Candida antarctica immobilized on immobead-350. Biotechnol. Prog. 2018, 34, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Arsalan, A.; Younus, H. Enzymes and nanoparticles: Modulation of enzymatic activity via nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzyme Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Riyadi, F.A.; Alam, M.Z.; Moniruzzaman, M. Ionic liquids as a potential solvent for lipase-catalysed reactions: A review. J. Mol. Liq. 2018, 251, 150–166. [Google Scholar] [CrossRef]
- Ghanem, A.; Aboul-Enein, H.Y. Lipase-mediated chiral resolution of racemates in organic solvents. Tetrahedron Asymmetry 2004, 15, 3331–3351. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- Wiseman, A.; Wiseman, A. Handbook of enzyme biotechnology. In Ellis Horwood Series in Biochemistry and Biotechnology Incorporating Molecular Biology, 3rd ed.; Ellis Horwood: New York, NY, USA, 1995. [Google Scholar]
- Høegh, I.; Patkar, S.; Halkier, T.; Hansen, M.T. Two lipases from Candida antarctica: Cloning and expression in Aspergillus oryzae. Can. J. Bot. 1995, 73, 869–875. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Ishii, M.; Kirk, O. Lipases A and B from the yeast Candida antarctica. In Biotechnological Applications of Cold-Adapted Organisms; Margesin, R., Schinner, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 49–61. [Google Scholar]
- Lutz, S. Engineering lipase B from Candida antarctica. Tetrahedron Asymmetry 2004, 15, 2743–2748. [Google Scholar] [CrossRef]
- McCabe, R.W.; Rodger, A.; Taylor, A. A study of the secondary structure of Candida antarctica lipase B using synchrotron radiation circular dichroism measurements. Enzyme Microb. Technol. 2005, 36, 70–74. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Mendes, A.A.; Adriano, W.S.; Gonçalves, L.R.B.; Giordano, R.L.C. Multipoint covalent immobilization of microbial lipase on chitosan and agarose activated by different methods. J. Mol. Catal. B Enzym. 2008, 51, 100–109. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Kirk, O.; Christensen, M.W. Lipases from Candida a antarctica: Unique Biocatalysts from a Unique Origin. Org. Process Res. Dev. 2002, 6, 446–451. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.; Mathur, D.; Kumar, R.; Kumar, A.; Prasad, A.K. Biocatalyst CAL-B catalyzed synthesis of modified nucleosides: An overview. Synth. Commun. 2019, 49, 1659–1678. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Carboni-Oerlemans, C.; Tuin, B.; Bargeman, G.; van der Meer, A.; van Gemert, R. Biotechnological applications of Candida antarctica lipase A: State-of-the-art. J. Mol. Catal. B Enzym. 2005, 37, 36–46. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Persson, M.; Bornscheuer, U.T. Enantioselective transesterification of a tertiary alcohol by lipase A from Candida antarctica. Tetrahedron Asymmetry 2002, 13, 2693–2696. [Google Scholar] [CrossRef]
- Sandstrom, A.G.; Wikmark, Y.; Engstrom, K.; Nyhlen, J.; Backvall, J.-E. Combinatorial reshaping of the Candida antarctica lipase A substrate pocket for enantioselectivity using an extremely condensed library. Proc. Natl. Acad. Sci. USA 2012, 109, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.N.; dos Anjos, C.S.; Orozco, E.V.M.; Porto, A.L.M. Versatility of Candida antarctica lipase in the amide bond formation applied in organic synthesis and biotechnological processes. Mol. Catal. 2019, 466, 75–105. [Google Scholar] [CrossRef]
- Ericsson, D.J.; Kasrayan, A.; Johansson, P.; Bergfors, T.; Sandström, A.G.; Bäckvall, J.-E.; Mowbray, S.L. X-ray structure of Candida antarctica lipase A shows a novel lid structure and a likely mode of interfacial activation. J. Mol. Biol. 2008, 376, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Barbosa, O.; Hernandez, K.; Santos, J.; Rodrigues, R.; Fernandez-Lafuente, R. Evaluation of styrene-divinylbenzene beads as a support to immobilize lipases. Molecules 2014, 19, 7629–7645. [Google Scholar] [CrossRef] [PubMed]
- Graebin, N.G.; Martins, A.B.; Lorenzoni, A.S.G.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads improves butyl acetate synthesis. Biotechnol. Prog. 2012, 28, 406–412. [Google Scholar] [CrossRef] [PubMed]
- José, C.; Toledo, M.V.; Nicolás, P.; Lasalle, V.; Ferreira, M.L.; Briand, L.E. Influence of the nature of the support on the catalytic performance of CALB: Experimental and theoretical evidence. Catal. Sci. Technol. 2018, 8, 3513–3526. [Google Scholar] [CrossRef]
- Marty, A.; Dossat, V.; Condoret, J.-S. Continuous operation of lipase-catalyzed reactions in nonaqueous solvents: Influence of the production of hydrophilic compounds. Biotechnol. Bioeng. 1997, 56, 232–237. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Séverac, E.; Galy, O.; Turon, F.; Pantel, C.A.; Condoret, J.-S.; Monsan, P.; Marty, A. Selection of CalB immobilization method to be used in continuous oil transesterification: Analysis of the economical impact. Enzyme Microb. Technol. 2011, 48, 61–70. [Google Scholar] [CrossRef]
- Galvão, W.S.; Neto, D.M.A.; Freire, R.M.; Fechine, P.B.A. Super-paramagnetic nanoparticles with spinel structure: A review of synthesis and biomedical applications. Solid State Phenom. 2015, 241, 139–176. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Li, Y.; Yi, L.; Yang, Y.; Xia, C. Study on immobilization of lipase onto magnetic microspheres with epoxy groups. J. Magn. Magn. Mater. 2009, 321, 252–258. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Feng, J.; Zhang, J.; Deng, X.; Zhou, B.; Zhang, H.; Xue, D.; Li, F.; Mellors, N.J.; et al. One-pot polylol synthesis of graphene decorated with size- and density-tunable Fe3O4 nanoparticles for porcine pancreatic lipase immobilization. Carbon 2013, 60, 488–497. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Bezerra, R.M.; Neto, D.M.A.; Galvão, W.S.; Rios, N.S.; Carvalho, A.C.L.d.M.; Correa, M.A.; Bohn, F.; Fernandez-Lafuente, R.; Fechine, P.B.A.; de Mattos, M.C.; et al. Design of a lipase-nano particle biocatalysts and its use in the kinetic resolution of medicament precursors. Biochem. Eng. J. 2017, 125, 104–115. [Google Scholar] [CrossRef]
- Reis, C.; Sousa, E.; Serpa, J.; Oliveira, R.; Oliveira, R.; Santos, J. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quím. Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Bonazza, H.L.; Manzo, R.M.; dos Santos, J.C.S.; Mammarella, E.J. Operational and thermal stability analysis of Thermomyces lanuginosus lipase covalently immobilized onto modified chitosan supports. Appl. Biochem. Biotechnol. 2018, 184, 182–196. [Google Scholar] [CrossRef]
- Rios, N.S.; Pinheiro, M.P.; dos Santos, J.C.S.; de S. Fonseca, T.; Lima, L.D.; de Mattos, M.C.; Freire, D.M.G.; da Silva, I.J.; Rodríguez-Aguado, E.; Gonçalves, L.R.B. Strategies of covalent immobilization of a recombinant Candida antarctica lipase B on pore-expanded SBA-15 and its application in the kinetic resolution of (R,S)-Phenylethyl acetate. J. Mol. Catal. B Enzym. 2016, 133, 246–258. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Reactivation of lipases by the unfolding and refolding of covalently immobilized biocatalysts. RSC Adv. 2015, 5, 55588–55594. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M. Thermomyces lanuginosus lipase-catalyzed synthesis of natural flavor esters in a continuous flow microreactor. 3 Biotech 2016, 6, 24. [Google Scholar] [CrossRef]
- Kiss, M.A.; Sefanovits-Bányai, É.; Tóth, Á.; Boross, L. Extractive synthesis of ethyl-oleate using alginate gel co-entrapped yeast cells and lipase enzyme. Eng. Life Sci. 2004, 4, 460–464. [Google Scholar] [CrossRef]
- Gao, W.; Wu, K.; Chen, L.; Fan, H.; Zhao, Z.; Gao, B.; Wang, H.; Wei, D. A novel esterase from a marine mud metagenomic library for biocatalytic synthesis of short-chain flavor esters. Microb. Cell Factories 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Buang, N.A.; Mahat, N.A.; Jamalis, J.; Huyop, F.; Aboul-Enein, H.Y.; Wahab, R.A. Simple adsorption of Candida rugosa lipase onto multi-walled carbon nanotubes for sustainable production of the flavor ester geranyl propionate. J. Ind. Eng. Chem. 2015, 32, 99–108. [Google Scholar] [CrossRef]
- Silva, N.C.A.; Miranda, J.S.; Bolina, I.C.A.; Silva, W.C.; Hirata, D.B.; de Castro, H.F.; Mendes, A.A. Immobilization of porcine pancreatic lipase on poly-hydroxybutyrate particles for the production of ethyl esters from macaw palm oils and pineapple flavor. Biochem. Eng. J. 2014, 82, 139–149. [Google Scholar] [CrossRef]
- Zdarta, J.; Norman, M.; Smułek, W.; Moszyński, D.; Kaczorek, E.; Stelling, A.; Ehrlich, H.; Jesionowski, T. Spongin-based scaffolds from Hippospongia communis demosponge as an effective support for lipase immobilization. Catalysts 2017, 7, 147. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzyme Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef]
- Kasche, V. Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of β-lactam antibiotics, peptides and other condensation products. Enzyme Microb. Technol. 1986, 8, 4–16. [Google Scholar] [CrossRef]
- Roby, M.H.H. Synthesis and characterization of phenolic lipids. In Phenolic Compounds-Natural Sources, Importance and Applications; Soto-Hernández, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; InTech: London, UK, 2017; pp. 89–116. [Google Scholar]
- Fallavena, L.P.; Antunes, F.H.F.; Alves, J.S.; Paludo, N.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Ultrasound technology and molecular sieves improve the thermodynamically controlled esterification of butyric acid mediated by immobilized lipase from Rhizomucor miehei. RSC Adv. 2014, 4, 8675. [Google Scholar] [CrossRef]
- Alves, J.; Garcia-Galan, C.; Schein, M.; Silva, A.; Barbosa, O.; Ayub, M.; Fernandez-Lafuente, R.; Rodrigues, R. Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB. Molecules 2014, 19, 9562–9576. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Zhang, Z.; Sun, R.; Fang, X.; Yu, D. Combination use of ultrasound irradiation and ionic liquid in enzymatic isomerization of glucose to fructose. Process Biochem. 2012, 47, 976–982. [Google Scholar] [CrossRef]
- Chatel, G.; MacFarlane, D.R. Ionic liquids and ultrasound in combination: Synergies and challenges. Chem. Soc. Rev. 2014, 43, 8132–8149. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Miao, M.; Jiang, B.; Cui, S.W.; Jia, X.; Zhang, T. Polysaccharides modification through green technology: Role of ultrasonication towards improving physicochemical properties of (1–3)(1-6)-α-d-glucans. Food Hydrocoll. 2015, 50, 166–173. [Google Scholar] [CrossRef]
- Bansode, S.R.; Rathod, V.K. Ultrasound assisted lipase catalysed synthesis of isoamyl butyrate. Process Biochem. 2014, 49, 1297–1303. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z.; Bhatia, S. Review on sonochemical methods in the presence of catalysts and chemical additives for treatment of organic pollutants in wastewater. Desalination 2011, 277, 1–14. [Google Scholar] [CrossRef]
- Patience, G.S.; Bérard, A. Experimental Planning. In Experimental Methods and Instrumentation for Chemical Engineers; Elsevier: New York, NY, USA, 2018; pp. 65–106. [Google Scholar]
- Getachew, A.T.; Chun, B.-S. Optimization of coffee oil flavor encapsulation using response surface methodology. LWT Food Sci. Technol. 2016, 70, 126–134. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. An online immobilized α-glucosidase microreactor for enzyme kinetics and inhibition assays. RSC Adv. 2015, 5, 56841–56847. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Mateo, C.; Godoy, C.; Pessela, B.C.C.; Rodrigues, D.S.; Giordano, R.L.C.; Fernandez-Lafuente, R.; Guisan, J.M. The co-operative effect of physical and covalent protein adsorption on heterofunctional supports. Process Biochem. 2009, 44, 757–763. [Google Scholar] [CrossRef]
- Daou, T.J.; Pourroy, G.; Bégin-Colin, S.; Grenèche, J.M.; Ulhaq-Bouillet, C.; Legaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic iron oxide nanoparticles: Reproducible tuning of the size and nanosized-dependent composition, defects, and spin canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Jubb, A.M.; Allen, H.C. Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Kolen’ko, Y.V.; Bañobre-López, M.; Rodríguez-Abreu, C.; Carbó-Argibay, E.; Sailsman, A.; Piñeiro-Redondo, Y.; Cerqueira, M.F.; Petrovykh, D.Y.; Kovnir, K.; Lebedev, O.I.; et al. Large-Scale Synthesis of Colloidal Fe3O4 Nanoparticles Exhibiting High Heating Efficiency in Magnetic Hyperthermia. J. Phys. Chem. C 2014, 118, 8691–8701. [Google Scholar] [CrossRef]
- Feng, B.; Hong, R.Y.; Wang, L.S.; Guo, L.; Li, H.Z.; Ding, J.; Zheng, Y.; Wei, D.G. Synthesis of Fe3O4/APTES/PEG diacid functionalized magnetic nanoparticles for MR imaging. Colloids Surf. Physicochem. Eng. Asp. 2008, 328, 52–59. [Google Scholar] [CrossRef]
- Andrade, L.H.; Rebelo, L.P.; Netto, C.G.C.M.; Toma, H.E. Kinetic resolution of a drug precursor by Burkholderia cepacia lipase immobilized by different methodologies on superparamagnetic nanoparticles. J. Mol. Catal. B Enzym. 2010, 66, 55–62. [Google Scholar] [CrossRef]
- Azami, M.; Rabiee, M.; Moztarzadeh, F. Glutaraldehyde crosslinked gelatin/hydroxyapatite nanocomposite scaffold, engineered via compound techniques. Polym. Compos. 2010, 31, 2112–2120. [Google Scholar] [CrossRef]
- Esmaeilnejad-Ahranjani, P.; Kazemeini, M.; Singh, G.; Arpanaei, A. Amine-functionalized magnetic nanocomposite particles for efficient immobilization of lipase: Effects of functional molecule size on properties of the immobilized lipase. RSC Adv. 2015, 5, 33313–33327. [Google Scholar] [CrossRef]
- de Oliveira, U.M.F.; Lima de Matos, L.J.B.; de Souza, M.C.M.; Pinheiro, B.B.; dos Santos, J.C.S.; Gonçalves, L.R.B. Efficient biotechnological synthesis of flavor esters using a low-cost biocatalyst with immobilized Rhizomucor miehei lipase. Mol. Biol. Rep. 2019, 46, 597–608. [Google Scholar] [CrossRef]
- Manan, F.M.A.; Rahman, I.N.A.; Marzuki, N.H.C.; Mahat, N.A.; Huyop, F.; Wahab, R.A. Statistical modelling of eugenol benzoate synthesis using Rhizomucor miehei lipase reinforced nanobioconjugates. Process Biochem. 2016, 51, 249–262. [Google Scholar] [CrossRef]
- Ozyilmaz, G.; Gezer, E. Production of aroma esters by immobilized Candida rugosa and porcine pancreatic lipase into calcium alginate gel. J. Mol. Catal. B Enzym. 2010, 64, 140–145. [Google Scholar] [CrossRef]
- Foresti, M.L.; Ferreira, M.L. Chitosan-immobilized lipases for the catalysis of fatty acid esterifications. Enzyme Microb. Technol. 2007, 40, 769–777. [Google Scholar] [CrossRef]
- Wang, D.; Yan, L.; Ma, X.; Wang, W.; Zou, M.; Zhong, J.; Ding, T.; Ye, X.; Liu, D. Ultrasound promotes enzymatic reactions by acting on different targets: Enzymes, substrates and enzymatic reaction systems. Int. J. Biol. Macromol. 2018, 119, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, K.C.; Bhanage, B.M. The combine use of ultrasound and lipase immobilized on co-polymer matrix for efficient biocatalytic application studies. J. Mol. Catal. B Enzym. 2015, 122, 255–264. [Google Scholar] [CrossRef]
- Pires-Cabral, P.; da Fonseca, M.M.R.; Ferreira-Dias, S. Synthesis of ethyl butyrate in organic media catalyzed by Candida rugosa lipase immobilized in polyurethane foams: A kinetic study. Biochem. Eng. J. 2009, 43, 327–332. [Google Scholar] [CrossRef]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and Engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef]
- Bansode, S.R.; Rathod, V.K. An investigation of lipase catalysed sonochemical synthesis: A review. Ultrason. Sonochem. 2017, 38, 503–529. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Li, C.; Zhi, G.-Y. Kinetic and thermodynamic investigation of enzymatic l-ascorbyl acetate synthesis. J. Biotechnol. 2013, 168, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Segel, I.H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems; Wiley Classics Library, Ed.; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Razak, N.N.A.; Annuar, M.S.M. Enzymatic synthesis of flavonoid ester: Elucidation of its kinetic mechanism and equilibrium thermodynamic behavior. Ind. Eng. Chem. Res. 2015, 54, 5604–5612. [Google Scholar] [CrossRef]
- Zhou, Z.; Shao, H.; Han, X.; Wang, K.; Gong, C.; Yang, X. The extraction efficiency enhancement of polyphenols from Ulmus pumila L. barks by trienzyme-assisted extraction. Ind. Crops Prod. 2017, 97, 401–408. [Google Scholar] [CrossRef]
- Rojas, M.L.; Trevilin, J.H.; Funcia, E.d.S.; Gut, J.A.W.; Augusto, P.E.D. Using ultrasound technology for the inactivation and thermal sensitization of peroxidase in green coconut water. Ultrason. Sonochem. 2017, 36, 173–181. [Google Scholar] [CrossRef]
- Dalagnol, L.M.G.; Silveira, V.C.C.; da Silva, H.B.; Manfroi, V.; Rodrigues, R.C. Improvement of pectinase, xylanase and cellulase activities by ultrasound: Effects on enzymes and substrates, kinetics and thermodynamic parameters. Process Biochem. 2017, 61, 80–87. [Google Scholar] [CrossRef]
- Martins, A.B.; Schein, M.F.; Friedrich, J.L.R.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Ultrasound-assisted butyl acetate synthesis catalyzed by Novozym 435: Enhanced activity and operational stability. Ultrason. Sonochem. 2013, 20, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Blanco, G. Elements of Thermodynamics and Biochemical Kinetics. In Medical Biochemistry; Elsevier: New York, NY, USA, 2017; pp. 141–152. [Google Scholar]
- Zaak, H.; Peirce, S.; de Albuquerque, T.; Sassi, M.; Fernandez-Lafuente, R. Exploiting the versatility of aminated supports activated with glutaraldehyde to immobilize β-galactosidase from aspergillus oryzae. Catalysts 2017, 7, 250. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Immobilized Lipase on Fe3O4 Nanoparticles as Biocatalyst for Biodiesel Production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- de Souza, T.C.; de S. Fonseca, T.; da Costa, J.A.; Rocha, M.V.P.; de Mattos, M.C.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C.S. Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: Application to the chemoenzymatic production of (R)-Indanol. J. Mol. Catal. B Enzym. 2016, 130, 58–69. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Silva, J.A.; Macedo, G.P.; Rodrigues, D.S.; Giordano, R.L.C.; Gonçalves, L.R.B. Immobilization of Candida antarctica lipase B by covalent attachment on chitosan-based hydrogels using different support activation strategies. Biochem. Eng. J. 2012, 60, 16–24. [Google Scholar] [CrossRef]
- Bleicher, L.; Sasaki, J.M.; Paiva Santos, C.O. Development of a graphical interface for the Rietveld refinement program DBWS. J. Appl. Crystallogr. 2000, 33, 1189. [Google Scholar] [CrossRef]
- Freire, R.M.; Ribeiro, T.S.; Vasconcelos, I.F.; Denardin, J.C.; Barros, E.B.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Fechine, P.B.A. MZnFe2O4 (M = Ni, Mn) cubic superparamagnetic nanoparticles obtained by hydrothermal synthesis. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]


| Biocatalyst | IY (%) | AtT (U/g) | AtD (U/g) | AtR (%) |
|---|---|---|---|---|
| Fe3O4@APTES–GLU–CALA | 100 ± 1.2 | 203.3 ± 1.2 | 198.3 ± 2.7 | 97.5 ± 1.9 |
| Fe3O4@APTES-CALA | 80 ± 3.1 | 110.7 ± 3.1 | 46.2 ± 3.3 | 41.7 ± 3.2 |
| Fe3O4@APTES–GLU–CALB | 57.6 ± 3.8 | 65.7 ± 3.8 | 52.9 ± 1.7 | 80.5 ± 3.2 |
| Fe3O4@APTES-CALB | 38.2 ± 3.3 | 43.6 ± 3.3 | 31.3 ± 1.2 | 71.3 ± 2.2 |
| Run | X1 | X2 | X3 | X4 | Conversion (%) | |
|---|---|---|---|---|---|---|
| CALA-MNP | CALB-MNP | |||||
| 1 | −1 | −1 | −1 | −1 | 65.4 ± 1.4 | 70.5 ± 2.6 |
| 2 | −1 | −1 | −1 | 1 | 72.3 ± 1.0 | 78.3 ± 2.0 |
| 3 | −1 | −1 | 1 | −1 | 70.5 ± 0.3 | 63.3 ± 1.8 |
| 4 | −1 | −1 | 1 | 1 | 75.4 ± 0.4 | 67.9 ± 0.1 |
| 5 | −1 | 1 | −1 | −1 | 40.0 ± 1.2 | 42.8 ± 0.2 |
| 6 | −1 | 1 | −1 | 1 | 35.5 ± 1.0 | 40.7 ± 2.0 |
| 7 | −1 | 1 | 1 | −1 | 44.2 ± 0.7 | 31.1 ± 3.3 |
| 8 | −1 | 1 | 1 | 1 | 38.8 ± 0.2 | 34.7 ± 0.0 |
| 9 | 1 | −1 | −1 | −1 | 63.8 ± 0.4 | 73.5 ± 1.0 |
| 10 | 1 | −1 | −1 | 1 | 76.9 ± 1.0 | 83.3 ± 2.7 |
| 11 | 1 | −1 | 1 | −1 | 75.8 ± 1.9 | 69.1 ± 1.7 |
| 12 | 1 | −1 | 1 | 1 | 77.1 ± 1.7 | 70.0 ± 2.9 |
| 13 | 1 | 1 | −1 | −1 | 40.8 ± 0.3 | 43.1 ± 0.9 |
| 14 | 1 | 1 | −1 | 1 | 38.6 ± 0.2 | 43.8 ± 1.1 |
| 15 | 1 | 1 | 1 | −1 | 45.2 ± 1.4 | 39.8 ± 1.6 |
| 16 | 1 | 1 | 1 | 1 | 40.2 ± 0.2 | 36.6 ± 2.8 |
| 17 | −1 | 0 | 0 | 0 | 77.3 ± 2.0 | 71.2 ± 2.5 |
| 18 | 1 | 0 | 0 | 0 | 84.0 ± 1.6 | 77.7 ± 1.4 |
| 19 | 0 | −1 | 0 | 0 | 96.3 ± 2.1 | 92.3 ± 2.7 |
| 20 | 0 | 1 | 0 | 0 | 73.8 ± 2.4 | 74.7 ± 2.9 |
| 21 | 0 | 0 | −1 | 0 | 75.4 ± 2.0 | 65.3 ± 2.4 |
| 22 | 0 | 0 | 1 | 0 | 85.6 ± 2.0 | 73.4 ± 2.9 |
| 23 | 0 | 0 | 0 | −1 | 76.1 ± 1.2 | 70.0 ± 2.0 |
| 24 | 0 | 0 | 0 | 1 | 80.2 ± 1.5 | 77.4 ± 2.2 |
| 25(C) | 0 | 0 | 0 | 0 | 88.1 ± 1.0 | 83.2 ± 1.0 |
| 26(C) | 0 | 0 | 0 | 0 | 89.1 ± 0.0 | 83.8 ± 1.4 |
| 27(C) | 0 | 0 | 0 | 0 | 89.6 ± 0.1 | 83.3 ± 0.1 |
| CALA-MNP | CALB-MNP | |||
|---|---|---|---|---|
| Orbital Shaking | Ultrasonic Irradiation | Orbital Shaking | Ultrasonic Irradiation | |
| 223.1 | 507.8 | 369.9 | 779.1 | |
| 0.8 | 1.7 | 1.3 | 2.6 | |
| Variable | Name | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | Enzyme Content (mg) | 5 | 10 | 15 |
| X2 | Molar Ratio (acid/alcohol) | 1:1 | 1:3 | 1:5 |
| X3 | Temperature (°C) | 30 | 45 | 60 |
| X4 | Time (h) | 4 | 6 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. Int. J. Mol. Sci. 2019, 20, 5807. https://doi.org/10.3390/ijms20225807
Monteiro RRC, Neto DMA, Fechine PBA, Lopes AAS, Gonçalves LRB, dos Santos JCS, de Souza MCM, Fernandez-Lafuente R. Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. International Journal of Molecular Sciences. 2019; 20(22):5807. https://doi.org/10.3390/ijms20225807
Chicago/Turabian StyleMonteiro, Rodolpho R. C., Davino M. Andrade Neto, Pierre B. A. Fechine, Ada A. S. Lopes, Luciana R. B. Gonçalves, José C. S. dos Santos, Maria C. M. de Souza, and Roberto Fernandez-Lafuente. 2019. "Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation" International Journal of Molecular Sciences 20, no. 22: 5807. https://doi.org/10.3390/ijms20225807
APA StyleMonteiro, R. R. C., Neto, D. M. A., Fechine, P. B. A., Lopes, A. A. S., Gonçalves, L. R. B., dos Santos, J. C. S., de Souza, M. C. M., & Fernandez-Lafuente, R. (2019). Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. International Journal of Molecular Sciences, 20(22), 5807. https://doi.org/10.3390/ijms20225807