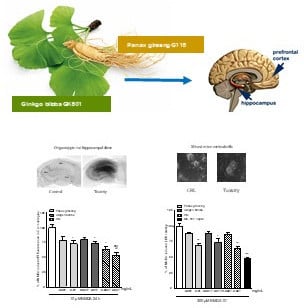
Enhanced Neuroprotective Effects of Panax ginseng G115® and Ginkgo biloba GK501® Combinations In Vitro Models of Excitotoxicity
Abstract
1. Introduction
2. Results
2.1. Safety and Tolerability of Native P. Ginseng and G. Biloba GK501® Extracts Alone or as a Mix in Rat Organotypic Hippocampal Slices
2.2. Neuroprotective Effects of Native P. Ginseng and G. Biloba GK501® Extracts Alone or as a Mix in Rat Organotypic Hippocampal Slices Exposed to Kainic Acid or NMDA-Induced Neurotoxicity
2.3. Safety and Tolerability of Native P. Ginseng and G. Biloba GK501® Extracts Alone or as a Mix in Cortical Mice Cells
2.4. Neuroprotective Effects of Native P. Ginseng and G. Biloba GK501® Extracts Alone or as a Mix in Cortical Mice Cell Cultures Exposed to NMDA-Induced Neurotoxicity
2.5. Effects of Native P. Ginseng and G. Biloba GK501® Extracts Alone or as a Mix on MAP-Kinase/PI-3 Kinase Pathways
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials
4.3. Solubility Tests
4.4. Organotypic Rat Hippocampal Slice Models of Excitotoxicity
4.5. Cortical Mice Cell Cultures Model of Excitotoxicity
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NMDA | N-methyl-d-aspartate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; |
| PI | propidium iodide |
| ERK | extracellular-signal-regulated kinases |
| Akt | protein kinase B |
| mix | combination of Panax ginseng G115® and Ginkgo biloba GK501® |
| LDH | lactate dehydrogenase |
| CNS | central nervous system |
References
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases—What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Brivio, P.; Dell’Agli, M.; Calabrese, F. Botanicals as Modulators of Neuroplasticity: Focus on BDNF. Neural Plast. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Schlaefke, S. Efficacy and tolerability of Ginkgo biloba extract EGb 761® in dementia: A systematic review and meta-analysis of randomized placebo-controlled trials. Clin. Interv. Aging 2014, 28, 2065–2077. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-T.; Chu, K.; Sim, J.-Y.; Heo, J.-H.; Kim, M. Panax Ginseng Enhances Cognitive Performance in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Jeong, J.-H.; Seo, J.-W.; Shin, C.-G.; Kim, Y.-S.; In, J.-G.; Yang, D.-C.; Yi, J.-S.; Choi, Y.-E. Enhanced Triterpene and Phytosterol Biosynthesis in Panax ginseng Overexpressing Squalene Synthase Gene. Plant Cell Physiol. 2004, 45, 976–984. [Google Scholar] [CrossRef]
- Richter, R.; Basar, S.; Koch, A.; König, W.A. Three sesquiterpene hydrocarbons from the roots of Panax ginseng C.A. Meyer (Araliaceae). Phytochemistry 2005, 66, 2708–2713. [Google Scholar] [CrossRef]
- Kim, J.-S. Investigation of Phenolic, Flavonoid, and Vitamin Contents in Different Parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 263–270. [Google Scholar] [CrossRef]
- Han, B.H.; Park, M.H.; Han, Y.N.; Woo, L.K. Alkaloidal components of Panax ginseng. Arch. Pharm. Res. 1986, 9, 21–23. [Google Scholar] [CrossRef]
- Hu, S.; Han, R.; Mak, S.; Han, Y. Protection against 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis by water extract of ginseng (Panax ginseng C.A. Meyer) in SH-SY5Y cells. J. Ethnopharmacol. 2011, 135, 34–42. [Google Scholar] [CrossRef]
- Ahmed, T.; Raza, S.H.; Maryam, A.; Setzer, W.N.; Braidy, N.; Nabavi, S.F.; de Oliveira, M.R.; Nabavi, S.M. Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Res. Bull. 2016, 125, 30–43. [Google Scholar] [CrossRef]
- González-Burgos, E.; Fernandez-Moriano, C.; Gómez-Serranillos, M.P. Potential Neuroprotective Activity of Ginseng in Parkinson’s Disease: A Review. J. Neuroimmune Pharmacol. 2015, 10, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Gille, G.; Moldzio, R.; Saito, H.; Rausch, W.-D. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004, 1021, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front. Pharmacol. 2013, 4, 152. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Shim, J.S.; Song, M.-Y.; Yim, S.-V.; Lee, S.E.; Park, K.-S. Proteomic analysis reveals that the protective effects of ginsenoside Rb1 are associated with the actin cytoskeleton in β-amyloid-treated neuronal cells. J. Ginseng Res. 2016, 40, 278–284. [Google Scholar] [CrossRef]
- Tan, X.; Gu, J.; Zhao, B.; Wang, S.; Yuan, J.; Wang, C.; Chen, J.; Liu, J.; Feng, L.; Jia, X. Ginseng improves cognitive deficit via the RAGE/NF-κB pathway in advanced glycation end product-induced rats. J. Ginseng Res. 2015, 39, 116–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Pi, Z.; Song, F.; Liu, Z. Ginsenosides attenuate d-galactose- and AlCl3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 194, 188–195. [Google Scholar] [CrossRef]
- Wu, J.; Jeong, H.K.; Bulin, S.E.; Kwon, S.W.; Park, J.H.; Bezprozvanny, I. Ginsenosides protect striatal neurons in a cellular model of Huntington’s disease. J. Neurosci. Res. 2009, 87, 1904–1912. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yang, B.; Zheng, Y.; Yao, M.; Sun, M.; Xu, L.; Lin, C.; Chang, D.; Tian, F. Ginkgo biloba Extract Inhibits Astrocytic Lipocalin-2 Expression and Alleviates Neuroinflammatory Injury via the JAK2/STAT3 Pathway After Ischemic Brain Stroke. Front. Pharmacol. 2018, 9, 518. [Google Scholar] [CrossRef]
- Longpre, F.; Garneau, P.; Christen, Y.; Ramassamy, C. Protection by EGb 761 against β-amyloid-induced neurotoxicity: Involvement of NF-κB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic. Biol. Med. 2006, 41, 1781–1794. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, J.-I.; Lee, W.-Y.; Kim, S.-E. Neuroprotective effect of Ginkgo biloba L. extract in a rat model of Parkinson’s disease. Phyther. Res. 2004, 18, 663–666. [Google Scholar] [CrossRef]
- Rojas, P.; Montes, S.; Serrano-García, N.; Rojas-Castañeda, J. Effect of EGb761 supplementation on the content of copper in mouse brain in an animal model of Parkinson’s disease. Nutrition 2009, 25, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Drieu, K.; Papadopoulos, V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from β-amyloid-induced cell death by inhibiting the formation of β-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001, 889, 181–190. [Google Scholar] [CrossRef]
- Wu, R.; Shui, L.; Wang, S.; Song, Z.; Tai, F. Bilobalide alleviates depression-like behavior and cognitive deficit induced by chronic unpredictable mild stress in mice. Behav. Pharmacol. 2016, 27, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Vining Smith, J.; Luo, Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J. Alzheimer’s Dis. 2003, 5, 287–300. [Google Scholar] [CrossRef]
- Liu, X.; Hao, W.; Qin, Y.; Decker, Y.; Wang, X.; Burkart, M.; Schötz, K.; Menger, M.D.; Fassbender, K.; Liu, Y. Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain. Behav. Immun. 2015, 46, 121–131. [Google Scholar] [CrossRef]
- Reay, J.L.; Schaik, P.; Wilson, C.J. A systematic review of research investigating the physiological and psychological effects of combining Ginkgo biloba and Panax ginseng into a single treatment in humans: Implications for research design and analysis. Brain Behav. 2019, 9, e01217. [Google Scholar] [CrossRef]
- Scartabelli, T.; Gerace, E.; Landucci, E.; Moroni, F.; Pellegrini-Giampietro, D.E. Neuroprotection by group I mGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3K–Akt signaling pathway: A novel postconditioning strategy? Neuropharmacology 2008, 55, 509–516. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Matsuda, S.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Implications of PI3K/AKT/PTEN Signaling on Superoxide Dismutases Expression and in the Pathogenesis of Alzheimer’s Disease. Diseases 2018, 6, 28. [Google Scholar] [CrossRef]
- Chen, X.; Ren, S.; Dong, J.; Qiu, C.; Chen, Y.; Tao, H. Ginkgo biloba extract-761 protects myocardium by regulating Akt/Nrf2 signal pathway. Drug Des. Dev. Ther. 2019, 13, 647–655. [Google Scholar] [CrossRef]
- Xu, Y.; Tan, H.-Y.; Li, S.; Wang, N.; Feng, Y. Panax notoginseng for Inflammation-Related Chronic Diseases: A Review on the Modulations of Multiple Pathways. Am. J. Chin. Med. 2018, 46, 971–996. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Gencarelli, M.; Mazzantini, C.; Laurino, A.; Pellegrini-Giampietro, D.E.; Raimondi, L. N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoromethyl-benzamide (EPPTB) prevents 3-iodothyronamine (T1AM)-induced neuroprotection against kainic acid toxicity. Neurochem. Int. 2019, 129, 104460. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Wang, Y.; Mu, P.; Chen, C.; Huang, P.; Liu, D. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion-induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol. Med. Rep. 2019, 13, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Filippi, L.; Gerace, E.; Catarzi, S.; Guerrini, R.; Pellegrini-Giampietro, D.E. Neuroprotective effects of topiramate and memantine in combination with hypothermia in hypoxic-ischemic brain injury in vitro and in vivo. Neurosci. Lett. 2018, 668, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Gerace, E.; Resta, F.; Landucci, E.; Renzi, D.; Masi, A.; Pellegrini-Giampietro, D.E.; Calabrò, A.; Mannaioni, G. The gliadin peptide 31–43 exacerbates kainate neurotoxicity in epilepsy models. Sci. Rep. 2017, 7, 15146. [Google Scholar] [CrossRef]
- Laurino, A.; Landucci, E.; Resta, F.; De Siena, G.; Pellegrini-Giampietro, D.E.; Masi, A.; Mannaioni, G.; Raimondi, L. Anticonvulsant and Neuroprotective Effects of the Thyroid Hormone Metabolite 3-Iodothyroacetic acid. Thyroid 2018, 28, 1387–1397. [Google Scholar] [CrossRef]
- Landucci, E.; Lattanzi, R.; Gerace, E.; Scartabelli, T.; Balboni, G.; Negri, L.; Pellegrini-Giampietro, D.E. Prokineticins are neuroprotective in models of cerebral ischemia and ischemic tolerance in vitro. Neuropharmacology 2016, 108, 39–48. [Google Scholar] [CrossRef]
- Meli, E.; Pangallo, M.; Picca, R.; Baronti, R.; Moroni, F.; Pellegrini-Giampietro, D.E. Differential role of poly(ADP-ribose) polymerase-1in apoptotic and necrotic neuronal death induced by mild or intense NMDA exposure in vitro. Mol. Cell. Neurosci. 2004, 25, 172–180. [Google Scholar] [CrossRef]
- Conti, P.; Pinto, A.; Tamborini, L.; Madsen, U.; Nielsen, B.; Bräuner-Osborne, H.; Hansen, K.B.; Landucci, E.; Pellegrini-Giampietro, D.E.; De Sarro, G.; et al. Novel 3-Carboxy- and 3-Phosphonopyrazoline Amino Acids as Potent and Selective NMDA Receptor Antagonists: Design, Synthesis, and Pharmacological Characterization. ChemMedChem 2010, 5, 1465–1475. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landucci, E.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Enhanced Neuroprotective Effects of Panax ginseng G115® and Ginkgo biloba GK501® Combinations In Vitro Models of Excitotoxicity. Int. J. Mol. Sci. 2019, 20, 5872. https://doi.org/10.3390/ijms20235872
Landucci E, Pellegrini-Giampietro DE, Bilia AR, Bergonzi MC. Enhanced Neuroprotective Effects of Panax ginseng G115® and Ginkgo biloba GK501® Combinations In Vitro Models of Excitotoxicity. International Journal of Molecular Sciences. 2019; 20(23):5872. https://doi.org/10.3390/ijms20235872
Chicago/Turabian StyleLanducci, Elisa, Domenico E. Pellegrini-Giampietro, Anna Rita Bilia, and Maria Camilla Bergonzi. 2019. "Enhanced Neuroprotective Effects of Panax ginseng G115® and Ginkgo biloba GK501® Combinations In Vitro Models of Excitotoxicity" International Journal of Molecular Sciences 20, no. 23: 5872. https://doi.org/10.3390/ijms20235872
APA StyleLanducci, E., Pellegrini-Giampietro, D. E., Bilia, A. R., & Bergonzi, M. C. (2019). Enhanced Neuroprotective Effects of Panax ginseng G115® and Ginkgo biloba GK501® Combinations In Vitro Models of Excitotoxicity. International Journal of Molecular Sciences, 20(23), 5872. https://doi.org/10.3390/ijms20235872