The Role of EjSVPs in Flower Initiation in Eriobotrya japonica
Abstract
1. Introduction
2. Results
2.1. Cloning and Identification of Loquat EjSVPs
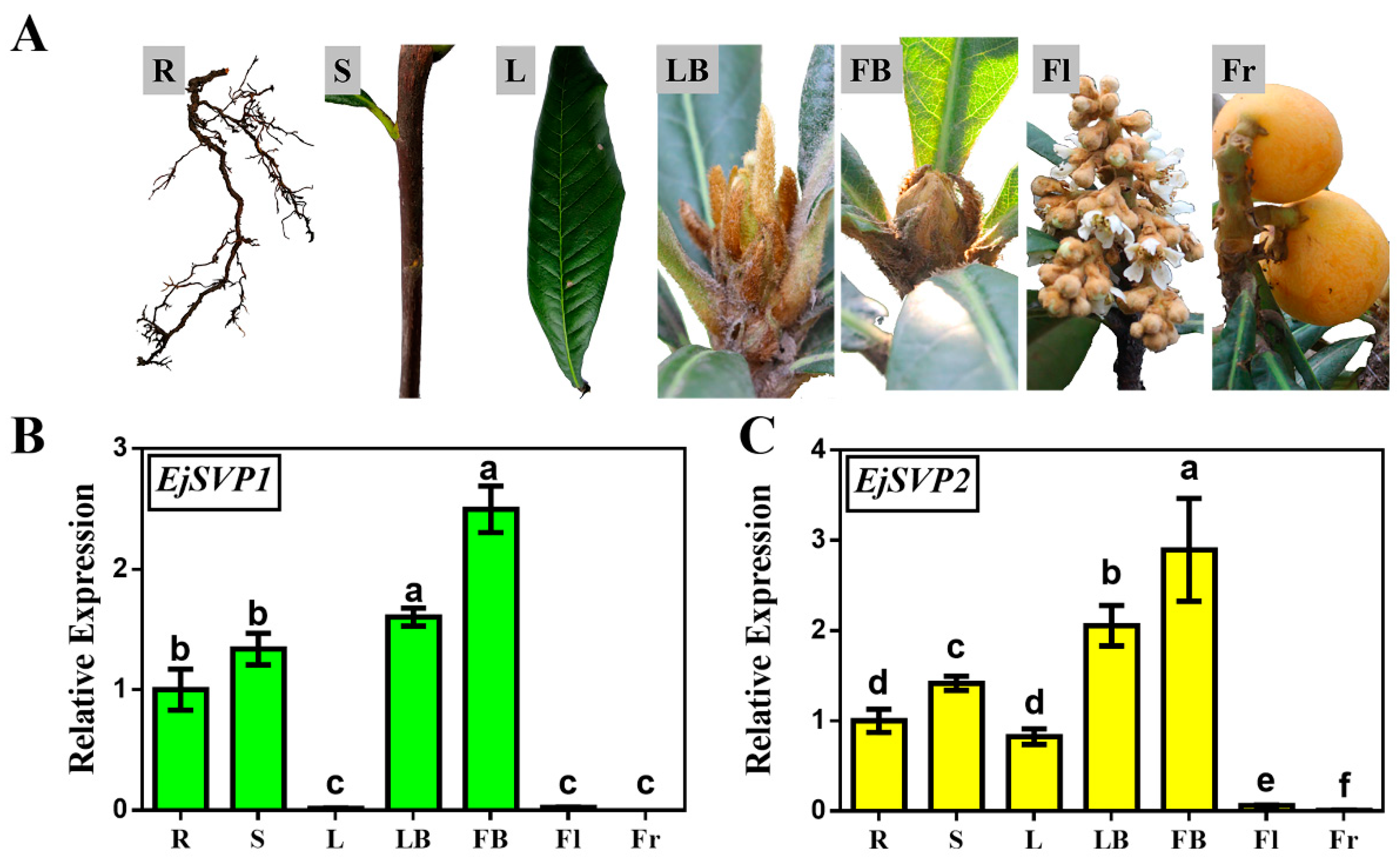
2.2. Tissue-Specific Expression Patterns of EjSVPs in Loquat
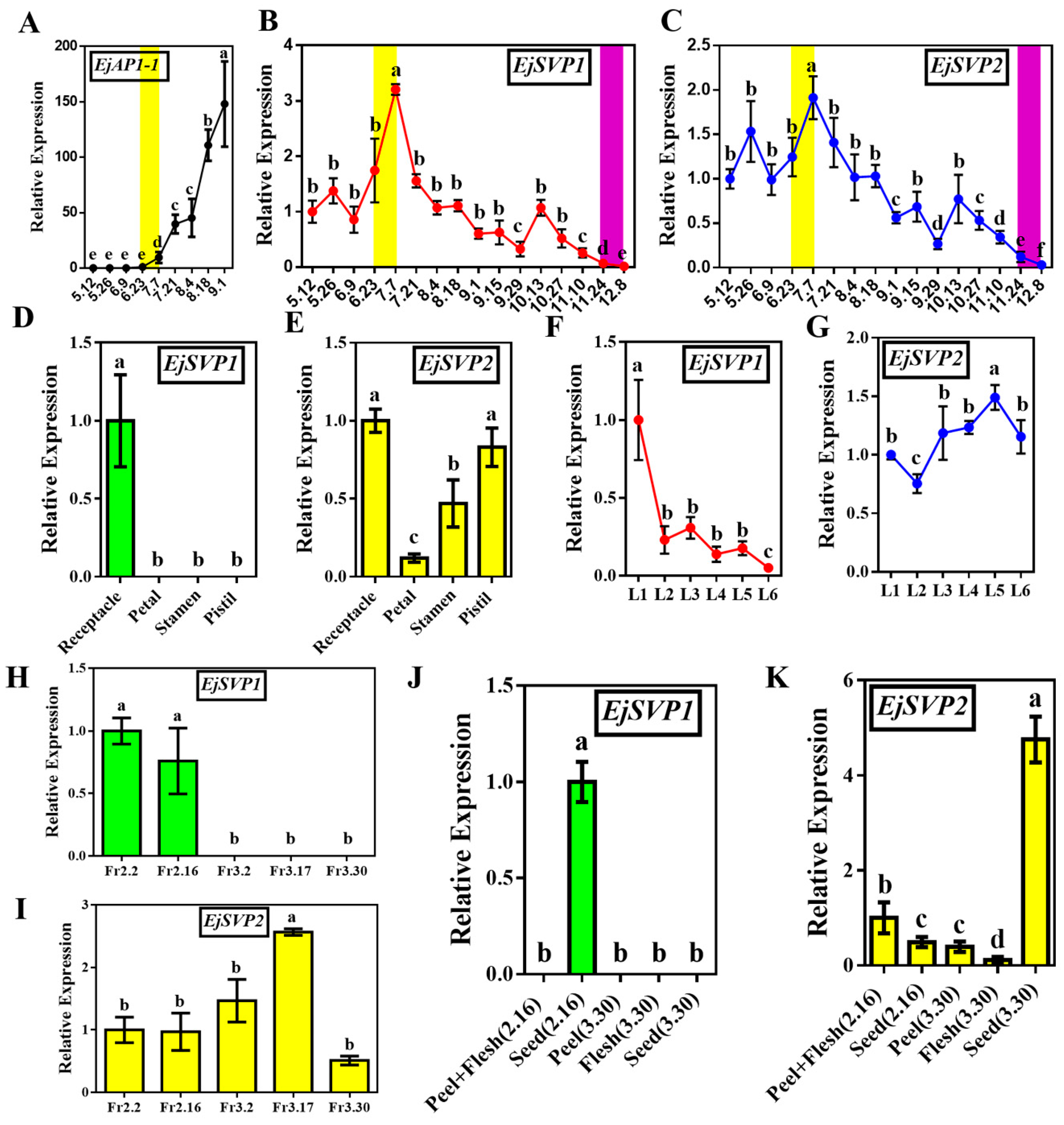
2.3. Temporal and Spatial Expression Patterns of EjSVPs in Loquat
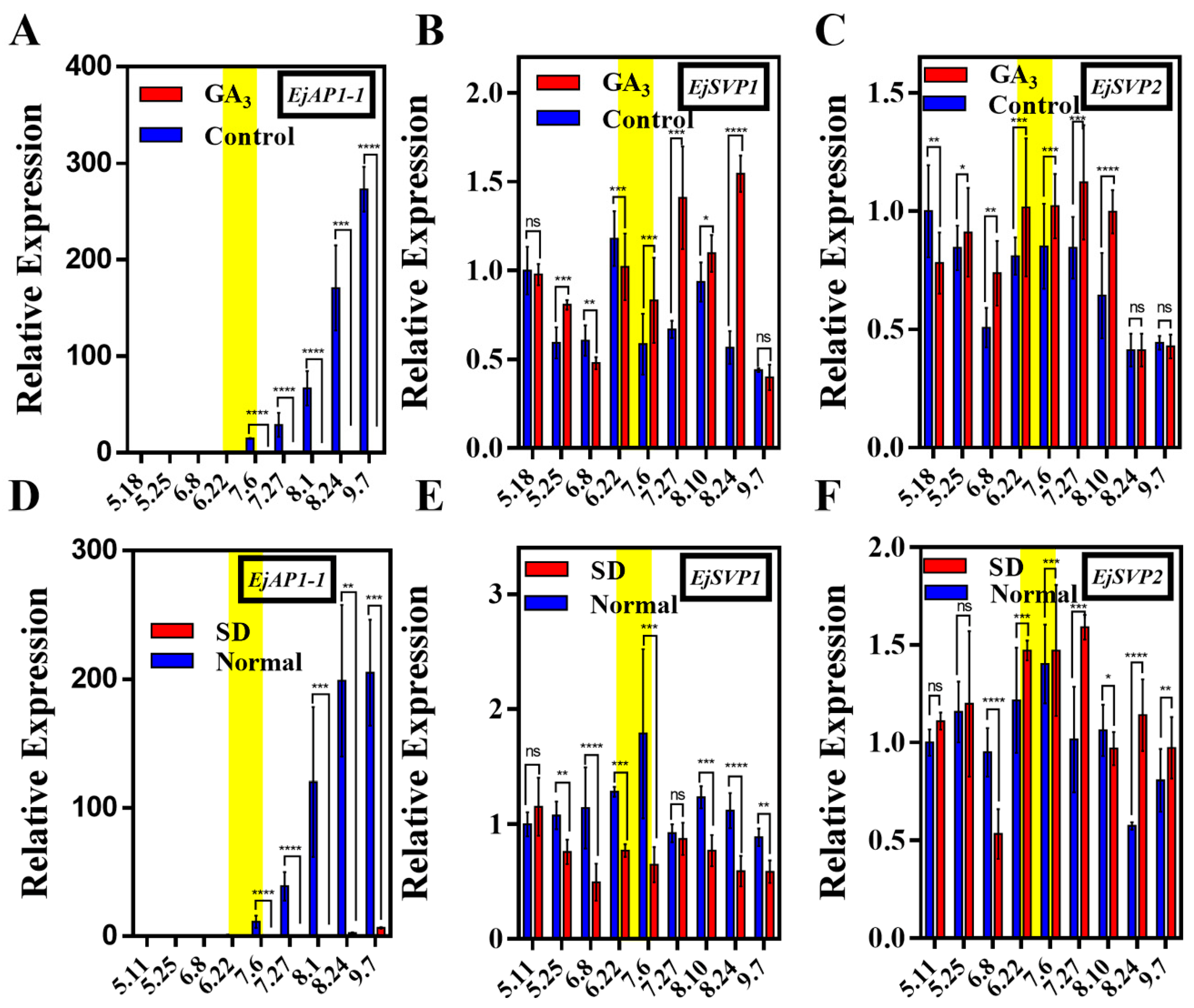
2.4. Effects of Exogenous GA3 Treatment and Short-Day Treatment on the Expression of EjSVPs
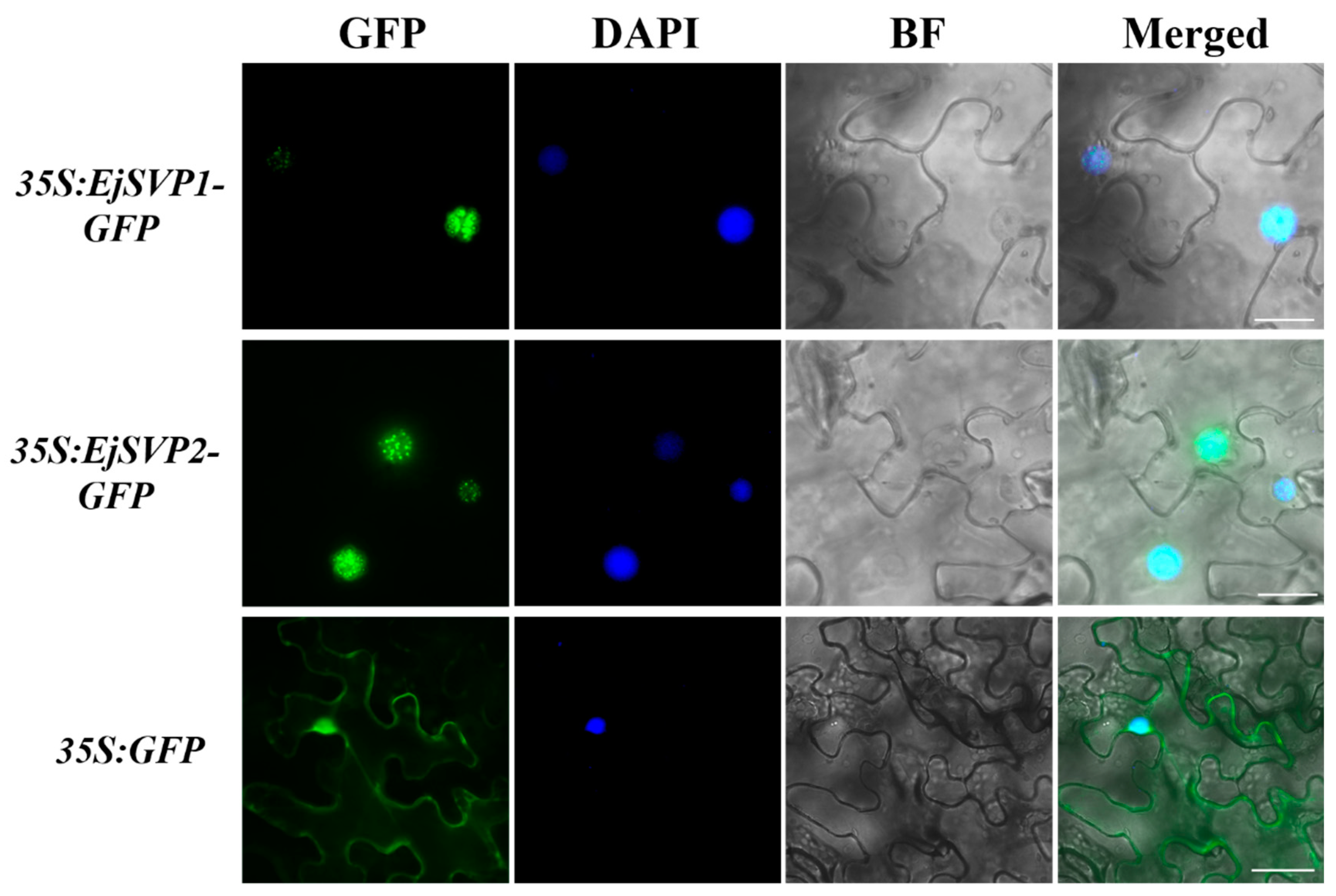
2.5. Subcellular Localization of EjSVPs
2.6. Promoter Analysis of EjSVPs
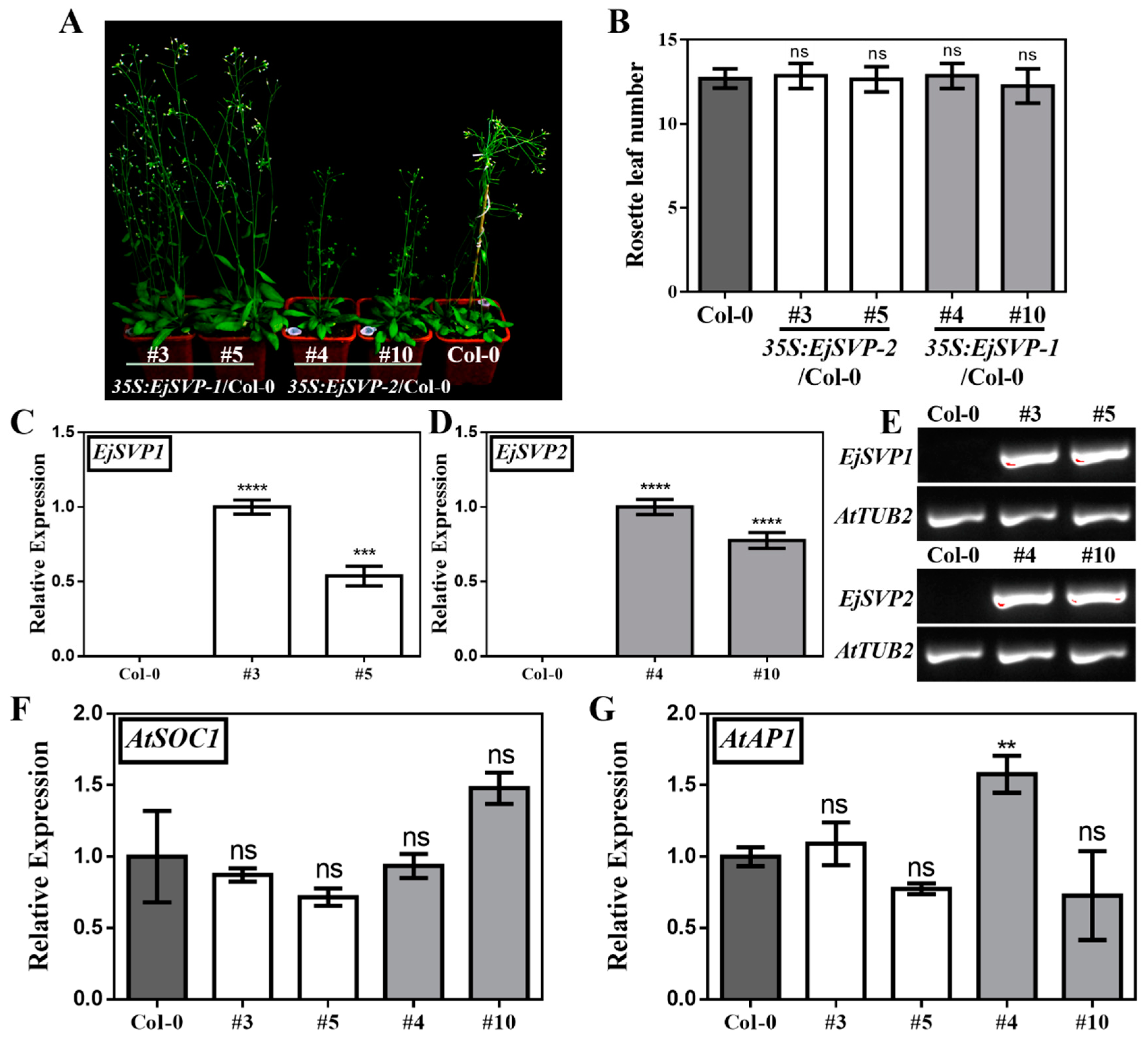
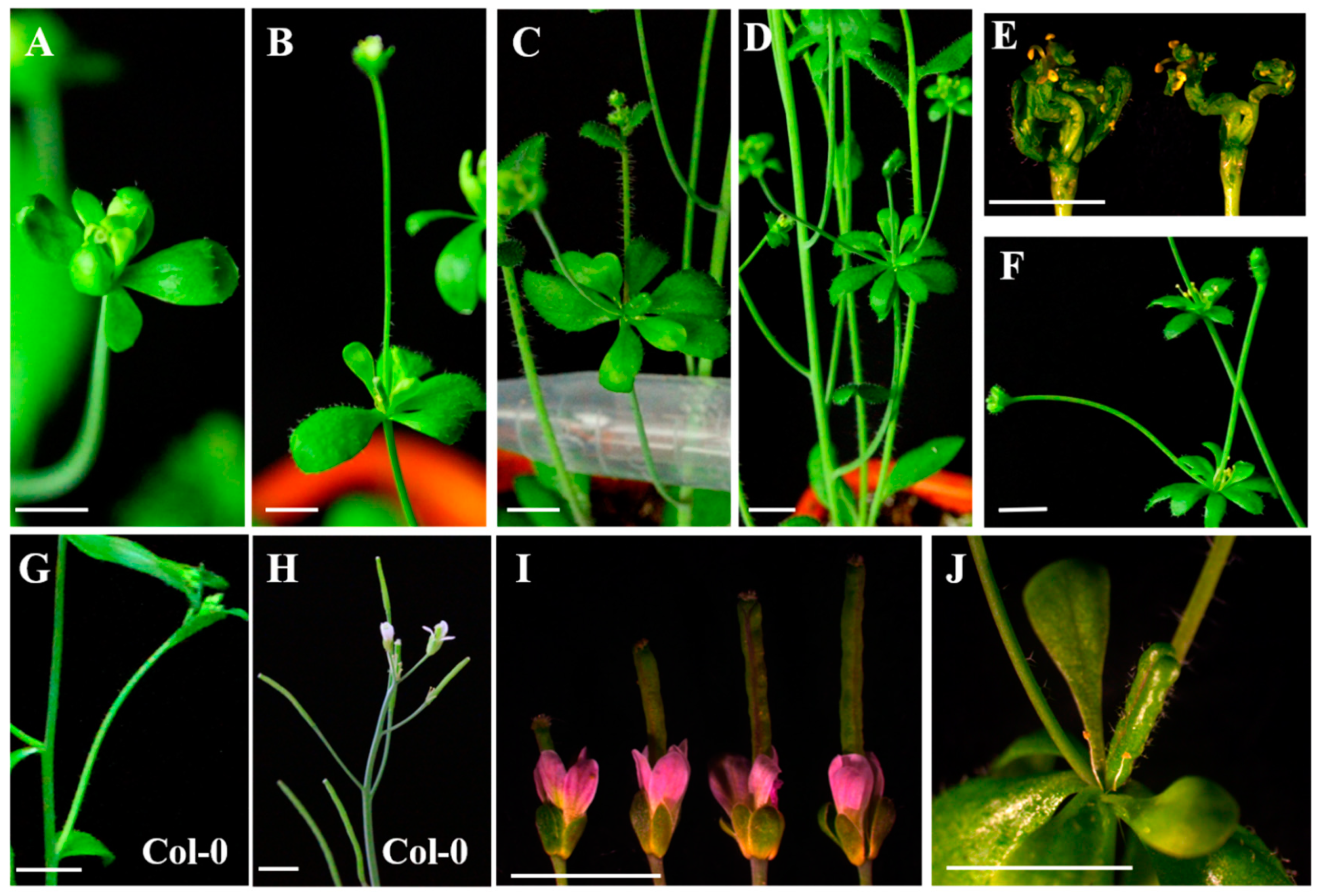
2.7. Functional Analysis of EjSVPs in Arabidopsis thaliana
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. RNA Extraction, Reverse-Transcription, Gene Isolation, and Sequence Analysis
4.3. Gene Expression Analysis
4.4. Short-Day and GA3 Treatments
4.5. Subcellular Localization and Arabidopsis thaliana Transformation
4.6. Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moon, J.; Lee, H.; Kim, M.; Lee, I. Analysis of flowering pathway integrators in Arabidopsis. Plant Cell Physiol. 2005, 46, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Amasino, R.M.; Michaels, S.D. The timing of flowering. Plant Physiol. 2010, 154, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Ito, S.; Imaizumi, T. Flowering time regulation: Photoperiod- and temperature-sensing in leaves. Trends Plant Sci. 2013, 18, 575–583. [Google Scholar] [CrossRef]
- Teotia, S.; Tang, G. To bloom or not to bloom: Role of microRNAs in plant flowering. Mol. Plant 2015, 8, 359–377. [Google Scholar] [CrossRef]
- Theissen, G.; Kim, J.T.; Saedler, H. Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J. Mol. Evol. 1996, 43, 484–516. [Google Scholar] [CrossRef]
- Parenicova, L.; de Folter, S.; Kieffer, M.; Horner, D.S.; Favalli, C.; Busscher, J.; Cook, H.E.; Ingram, R.M.; Kater, M.M.; Davies, B.; et al. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: New openings to the MADS world. Plant Cell 2003, 15, 1538–1551. [Google Scholar] [CrossRef]
- Vandenbussche, M.; Theissen, G.; Van de Peer, Y.; Gerats, T. Structural diversification and neo-functionalization during floral MADS-box gene evolution by C-terminal frameshift mutations. Nucleic. Acids Res. 2003, 31, 4401–4409. [Google Scholar] [CrossRef]
- Smaczniak, C.; Immink, R.G.; Angenent, G.C.; Kaufmann, K. Developmental and evolutionary diversity of plant MADS-domain factors: Insights from recent studies. Development 2012, 139, 3081–3098. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Liu, X.; Zhang, L. Evolutionary Analysis of MIKC(c)-Type MADS-Box Genes in Gymnosperms and Angiosperms. Front. Plant. Sci 2017, 8, 895. [Google Scholar] [CrossRef]
- Hartmann, U.; Hohmann, S.; Nettesheim, K.; Wisman, E.; Saedler, H.; Huijser, P. Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 2000, 21, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Gregis, V.; Sessa, A.; Dorca-Fornell, C.; Kater, M.M. The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. Plant J. 2009, 60, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Thouet, J.; Quinet, M.; Lutts, S.; Kinet, J.M.; Perilleux, C. Repression of floral meristem fate is crucial in shaping tomato inflorescence. PLoS ONE 2012, 7, e31096. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, V.d.S.; Guitton, B.; Costes, E.; Andres, F. I Want to (Bud) Break Free: The Potential Role of DAM and SVP-Like Genes in Regulating Dormancy Cycle in Temperate Fruit Trees. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, V.d.S.; Porto, D.D.; Buffon, V.; Margis-Pinheiro, M.; Pasquali, G.; Revers, L.F. Differential Transcriptional Profiles of Dormancy-Related Genes in Apple Buds. Plant Mol. Biol. Report. 2014, 32, 796–813. [Google Scholar] [CrossRef]
- Mimida, N.; Saito, T.; Moriguchi, T.; Suzuki, A.; Komori, S.; Wada, M. Expression of DORMANCY-ASSOCIATED MADS-BOX (DAM)-like genes in apple. Biol. Plant 2015, 59, 237–244. [Google Scholar] [CrossRef]
- Wisniewski, M.; Norelli, J.; Artlip, T. Overexpression of a peach CBF gene in apple: A model for understanding the integration of growth, dormancy, and cold hardiness in woody plants. Front. Plant Sci. 2015, 6, 85. [Google Scholar] [CrossRef]
- Kumar, G.; Arya, P.; Gupta, K.; Randhawa, V.; Acharya, V.; Singh, A.K. Comparative phylogenetic analysis and transcriptional profiling of MADS-box gene family identified DAM and FLC-like genes in apple (Malus x domestica). Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Kumar, G.; Gupta, K.; Pathania, S.; Swarnkar, M.K.; Rattan, U.K.; Singh, G.; Sharma, R.K.; Singh, A.K. Chilling Affects Phytohormone and Post-Embryonic Development Pathways during Bud Break and Fruit Set in Apple (Malus domestica Borkh.). Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Porto, D.D.; Falavigna, V.D.; Arenhart, R.A.; Perini, P.; Buffon, V.; Anzanello, R.; dos Santos, H.P.; Fialho, F.B.; de Oliveira, P.R.D.; Revers, L.F. Structural genomics and transcriptional characterization of the Dormancy-Associated MADS-box genes during bud dormancy progression in apple. Tree Genet. Genomes 2016, 12. [Google Scholar] [CrossRef]
- Wu, R.; Tomes, S.; Karunairetnam, S.; Tustin, S.D.; Hellens, R.P.; Allan, A.C.; Macknight, R.C.; Varkonyi-Gasic, E. SVP-like MADS Box Genes Control Dormancy and Budbreak in Apple. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ubi, B.E.; Sakamoto, D.; Ban, Y.; Shimada, T.; Ito, A.; Nakajima, I.; Takemura, Y.; Tamura, F.; Saito, T.; Moriguchi, T. Molecular Cloning of Dormancy-associated MADS-box Gene Homologs and Their Characterization during Seasonal Endodormancy Transitional Phases of Japanese Pear. J. Am. Soc. Hortic. Sci. 2010, 135, 174–182. [Google Scholar] [CrossRef]
- Liu, G.; Li, W.; Zheng, P.; Xu, T.; Chen, L.; Liu, D.; Hussain, S.; Teng, Y. Transcriptomic analysis of ‘Suli’ pear (Pyrus pyrifolia white pear group) buds during the dormancy by RNA-Seq. Bmc Genom. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Saito, T.; Sakamoto, D.; Ito, A.; Fujii, H.; Moriguchi, T. Transcriptome Analysis of Japanese Pear (Pyrus pyrifolia Nakai) Flower Buds Transitioning Through Endodormancy. Plant Cell Physiol. 2013, 54, 1132–1151. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Bai, S.; Ito, A.; Sakamoto, D.; Saito, T.; Ubi, B.E.; Imai, T.; Moriguchi, T. Expression and genomic structure of the dormancy-associated MADS box genes MADS13 in Japanese pears (Pyrus pyrifolia Nakai) that differ in their chilling requirement for endodormancy release. Tree Physiol. 2013, 33, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Saito, T.; Sakamoto, D.; Sugiura, T.; Bai, S.; Moriguchi, T. Physiological differences between bud breaking and flowering after dormancy completion revealed by DAM and FT/TFL1 expression in Japanese pear (Pyrus pyrifolia). Tree Physiol. 2016, 36, 109–120. [Google Scholar] [CrossRef]
- Niu, Q.; Li, J.; Cai, D.; Qian, M.; Jia, H.; Bai, S.; Hussain, S.; Liu, G.; Teng, Y.; Zheng, X. Dormancy-associated MADS-box genes and microRNAs jointly control dormancy transition in pear (Pyrus pyrifolia white pear group) flower bud. J. Exp. Bot. 2016, 67, 239–257. [Google Scholar] [CrossRef]
- Li, Z.; Reighard, G.L.; Abbott, A.G.; Bielenberg, D.G. Dormancy-associated MADS genes from the EVG locus of peach Prunus persica (L.) Batsch have distinct seasonal and photoperiodic expression patterns. J. Exp. Bot. 2009, 60, 3521–3530. [Google Scholar] [CrossRef]
- Jimenez, S.; Reighard, G.L.; Bielenberg, D.G. Gene expression of DAM5 and DAM6 is suppressed by chilling temperatures and inversely correlated with bud break rate. Plant Mol. Biol. 2010, 73, 157–167. [Google Scholar] [CrossRef]
- Yamane, H.; Ooka, T.; Jotatsu, H.; Hosaka, Y.; Sasaki, R.; Tao, R. Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS-box genes, by low temperature and dormancy-breaking reagent treatment. J. Exp. Bot. 2011, 62, 3481–3488. [Google Scholar] [CrossRef]
- Yamane, H.; Tao, R.; Ooka, T.; Jotatsu, H.; Sasaki, R.; Yonemori, K. Comparative Analyses of Dormancy-associated MADS-box Genes, PpDAM5 and PpDAM6, in Low- and High-chill Peaches (Prunus persica L.). J. Jpn. Soc. Hortic. Sci. 2011, 80, 276–283. [Google Scholar] [CrossRef]
- Yamane, H.; Ooka, T.; Jotatsu, H.; Sasaki, R.; Tao, R. Expression analysis of PpDAM5 and PpDAM6 during flower bud development in peach (Prunus persica). Sci Hortic. (Amst.) 2011, 129, 844–848. [Google Scholar] [CrossRef]
- Sasaki, R.; Yamane, H.; Ooka, T.; Jotatsu, H.; Kitamura, Y.; Akagi, T.; Tao, R. Functional and Expressional Analyses of PmDAM Genes Associated with Endodormancy in Japanese Apricot. Plant Physiol. 2011, 157, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhou, Y.; Li, Y.; Zhuo, X.; Ahmad, S.; Han, Y.; Yong, X.; Zhang, Q. Crosstalk of PmCBFs and PmDAMs Based on the Changes of Phytohormones under Seasonal Cold Stress in the Stem of Prunus mume. Int. J. Mol. Sci. 2018, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhou, Y.; Ahmad, S.; Yong, X.; Xie, X.; Han, Y.; Li, Y.; Sun, L.; Zhang, Q. PmCBFs synthetically affect PmDAM6 by alternative promoter binding and protein complexes towards the dormancy of bud for Prunus mume. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Xin, D.; Chen, W.; Shao, X.; Wang, Y.; Guo, W. RNA-Seq-based transcriptome analysis of dormant flower buds of Chinese cherry (Prunus pseudocerasus). Gene 2015, 555, 362–376. [Google Scholar] [CrossRef]
- Rothkegel, K.; Sanchez, E.; Montes, C.; Greve, M.; Tapia, S.; Bravo, S.; Prieto, H.; Miyasaka Almeida, A. DNA methylation and small interference RNAs participate in the regulation of MADS-box genes involved in dormancy in sweet cherry (Prunus avium L.). Tree Physiol. 2017, 37, 1739–1751. [Google Scholar] [CrossRef]
- Horvath, D.P.; Chao, W.S.; Suttle, J.C.; Thimmapuram, J.; Anderson, J.V. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). Bmc Genom. 2008, 9, 536. [Google Scholar] [CrossRef]
- Horvath, D.P.; Sung, S.; Kim, D.; Chao, W.; Anderson, J. Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Mol. Biol. 2010, 73, 169–179. [Google Scholar] [CrossRef]
- Wu, R.-M.; Walton, E.F.; Richardson, A.C.; Wood, M.; Hellens, R.P.; Varkonyi-Gasic, E. Conservation and divergence of four kiwifruit SVP-like MADS-box genes suggest distinct roles in kiwifruit bud dormancy and flowering. J. Exp. Bot. 2012, 63, 797–807. [Google Scholar] [CrossRef]
- Singh, R.K.; Maurya, J.P.; Azeez, A.; Miskolczi, P.; Tylewicz, S.; Stojkovic, K.; Delhomme, N.; Busov, V.; Bhalerao, R.P. A genetic network mediating the control of bud break in hybrid aspen. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kurokura, T.; Mimida, N.; Battey, N.H.; Hytonen, T. The regulation of seasonal flowering in the Rosaceae. J. Exp. Bot. 2013, 64, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Peng, J.; Zhu, Y.; Su, W.; Zhang, L.; Jing, Y.; Lin, S.; Gao, Y. The Role of EjSOC1s in Flower Initiation in Eriobotrya japonica. Front. Plant Sci 2019, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Esumi, T.; Tao, R.; Yonemori, K. Isolation of LEAFY and TERMINAL FLOWER 1 homologues from six fruit tree species in the subfamily Maloideae of the Rosaceae. Sex. Plant Reprod. 2005, 17, 277–287. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Q.; Meng, N.; Song, H.; Li, C.; Hu, G.; Wu, J.; Lin, S.; Zhang, Z. Over-expression of EjLFY-1 Leads to an Early Flowering Habit in Strawberry (Fragaria x ananassa) and Its Asexual Progeny. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, H.; Liu, Z.; Hu, G.; Lin, S. Molecular characterization of loquat EjAP1 gene in relation to flowering. Plant Growth Regul. 2013, 70, 287–296. [Google Scholar] [CrossRef]
- Reig, C.; Gil-Munoz, F.; Vera-Sirera, F.; Garcia-Lorca, A.; Martinez-Fuentes, A.; Mesejo, C.; Perez-Amador, M.A.; Agusti, M. Bud sprouting and floral induction and expression of FT in loquat [Eriobotrya japonica (Thunb.) Lindl.]. Planta 2017, 246, 915–925. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Lin, S.; Gao, Y. Molecular Characterization of FT and FD Homologs from Eriobotrya deflexa Nakai forma koshunensis. Front. Plant Sci. 2016, 7, 8. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Zhu, Y.; Su, W.; Long, T.; Huang, T.; Peng, J.; Yu, H.; Lin, S.; Gao, Y. Functional characterization of GI and CO homologs from Eriobotrya deflexa Nakai forma koshunensis. Plant Cell Rep. 2019. [Google Scholar] [CrossRef]
- Gonzalez, N.; Vanhaeren, H.; Inze, D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 2012, 17, 332–340. [Google Scholar] [CrossRef]
- Nelissen, H.; Gonzalez, N.; Inze, D. Leaf growth in dicots and monocots: So different yet so alike. Curr Opin Plant Biol. 2016, 33, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Bai, S.; Imai, T.; Ito, A.; Nakajima, I.; Moriguchi, T. Histone modification and signalling cascade of the dormancy-associated MADS-box gene, PpMADS13-1, in Japanese pear (Pyrus pyrifolia) during endodormancy. Plant Cell Environ. 2015, 38, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Jaiswal, A.; Taj, G.; Jaiswal, J.P.; Qureshi, M.I.; Singh, N.K. DREB1/CBF transcription factors: Their structure, function and role in abiotic stress tolerance in plants. J. Genet. 2012, 91, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lang, Z.; Zhu, J.-K. Cold responsive gene transcription becomes more complex. Trends Plant Sci. 2015, 20, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Shen, Y.; Chang, H.C.; Hou, Y.; Harris, A.; Ma, S.F.; McPartland, M.; Hymus, G.J.; Adam, L.; Marion, C.; et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol 2010, 187, 57–66. [Google Scholar] [CrossRef]
- Adrian, J.; Farrona, S.; Reimer, J.J.; Albani, M.C.; Coupland, G.; Turck, F. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell 2010, 22, 1425–1440. [Google Scholar] [CrossRef]
- Lin, S.Q. A Review on Research of the Wild Species in Genus Eriobotrya Germplasm and Their Innovative Utilization. Acta Hortic. Sin. 2017, 44, 1704–1716. [Google Scholar] [CrossRef]
- Gu, C.; Spongberg, S.A. ERIOBOTRYA Lindley. In Flora of China; Zhengyi, W., Raven, P.H., Hong, D.Y., Eds.; Missouri Botanical Garden Press: Jefferson, MO, USA, 2003; Volume 9, pp. 138–140. [Google Scholar]
- Yu, H.; Ito, T.; Wellmer, F.; Meyerowitz, E.M. Repression of AGAMOUS-LIKE 24 is a crucial step in promoting flower development. Nat. Genet. 2004, 36, 157–161. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Er, H.L.; Soo, H.M.; Kumar, P.P.; Han, J.H.; Liou, Y.C.; Yu, H. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 2008, 135, 1481–1491. [Google Scholar] [CrossRef]
- Lee, J.; Oh, M.; Park, H.; Lee, I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J. 2008, 55, 832–843. [Google Scholar] [CrossRef]
- Kitamura, Y.; Takanori, T.; Yamane, H.; Tao, R. Simultaneous down-regulation of DORMANCY-ASSOCIATED MADS-box6 and SOC1 during dormancy release in Japanese apricot (Prunus mume) flower buds. Hortic. Sci. Biotechnol. 2016, 91, 476–482. [Google Scholar] [CrossRef]
- Jeong Hwan, L.; Seong Jeon, Y.; Soo Hyun, P.; Ildoo, H.; Jong Seob, L.; Hoon, A.J.J.G.D. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007, 21, 397–402. [Google Scholar]
- Yan, Y.; Shen, L.; Chen, Y.; Bao, S.; Thong, Z.; Yu, H. A MYB-domain protein EFM mediates flowering responses to environmental cues in Arabidopsis. Dev. Cell 2014, 30, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Jeong Hwan, L.; Hak-Seung, R.; Kyung Sook, C.; David, P.; Soonkap, K.; Markus, S.; Hoon, A.J.J.S. Regulation of temperature-responsive flowering by MADS-box transcription factor repressors. Science 2013, 342, 628–632. [Google Scholar]
- Riboni, M.; Galbiati, M.; Tonelli, C.; Conti, L. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and suppressor of overexpression of constans. Plant Physiol. 2013, 162, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shan, L.L.; Li, X.; Wang, P.; Cai, C.; Zhang, B.; Sun, C.D.; Zhang, W.S.; Xu, C.J.; Ferguson, I.; Chen, K.S. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation. Planta 2008, 227, 1243–1254. [Google Scholar] [CrossRef]
- Lee, L.Y.; Hou, X.; Fang, L.; Fan, S.; Kumar, P.P.; Yu, H. STUNTED mediates the control of cell proliferation by GA in Arabidopsis. Development 2012, 139, 1568–1576. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Peng, J.; Zhang, Z.; Lin, S.; Lin, S.; Yang, X. The Role of EjSVPs in Flower Initiation in Eriobotrya japonica. Int. J. Mol. Sci. 2019, 20, 5933. https://doi.org/10.3390/ijms20235933
Jiang Y, Peng J, Zhang Z, Lin S, Lin S, Yang X. The Role of EjSVPs in Flower Initiation in Eriobotrya japonica. International Journal of Molecular Sciences. 2019; 20(23):5933. https://doi.org/10.3390/ijms20235933
Chicago/Turabian StyleJiang, Yuanyuan, Jiangrong Peng, Zhike Zhang, Shoukai Lin, Shunquan Lin, and Xianghui Yang. 2019. "The Role of EjSVPs in Flower Initiation in Eriobotrya japonica" International Journal of Molecular Sciences 20, no. 23: 5933. https://doi.org/10.3390/ijms20235933
APA StyleJiang, Y., Peng, J., Zhang, Z., Lin, S., Lin, S., & Yang, X. (2019). The Role of EjSVPs in Flower Initiation in Eriobotrya japonica. International Journal of Molecular Sciences, 20(23), 5933. https://doi.org/10.3390/ijms20235933






