Protein Kinase CK2 Subunits Differentially Perturb the Adhesion and Migration of GN11 Cells: A Model of Immature Migrating Neurons
Abstract
1. Introduction
2. Results
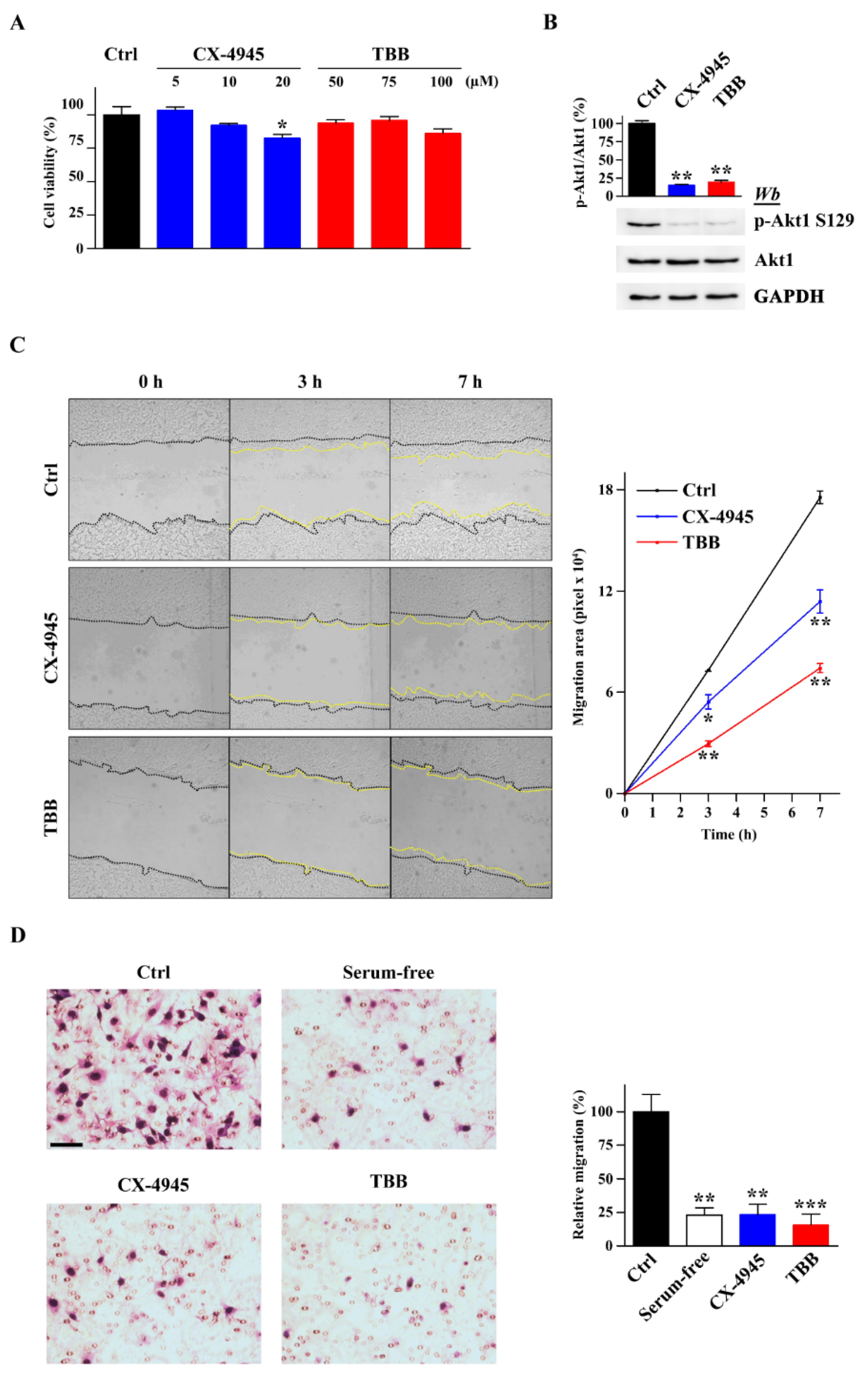
2.1. Pharmacological Inhibition of CK2 Impairs GN11 Neuron Migration
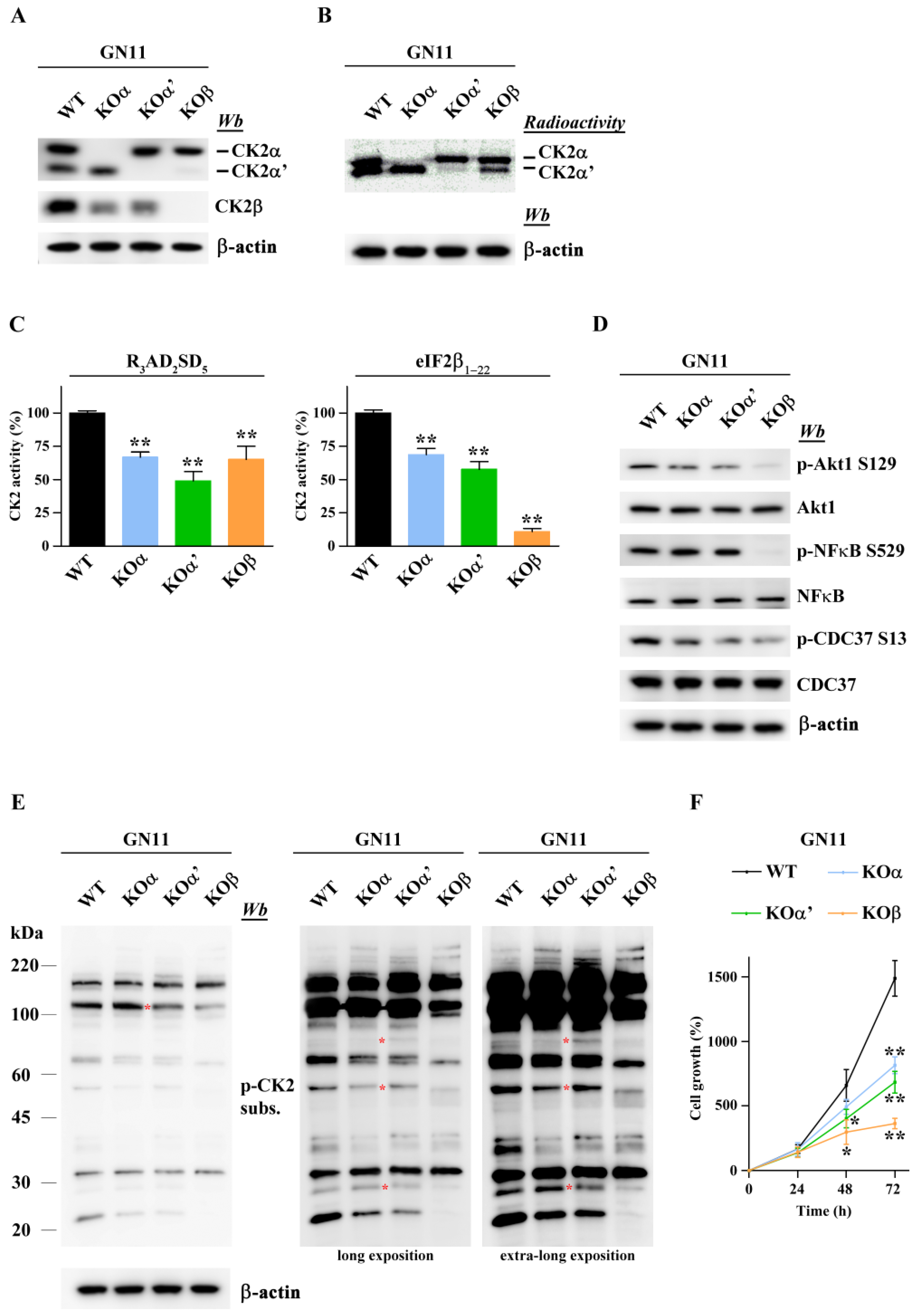
2.2. The Function of Single CK2 Subunits Is Effectively Abolished in GN11 Cells via CRISPR-Cas9 Knockout
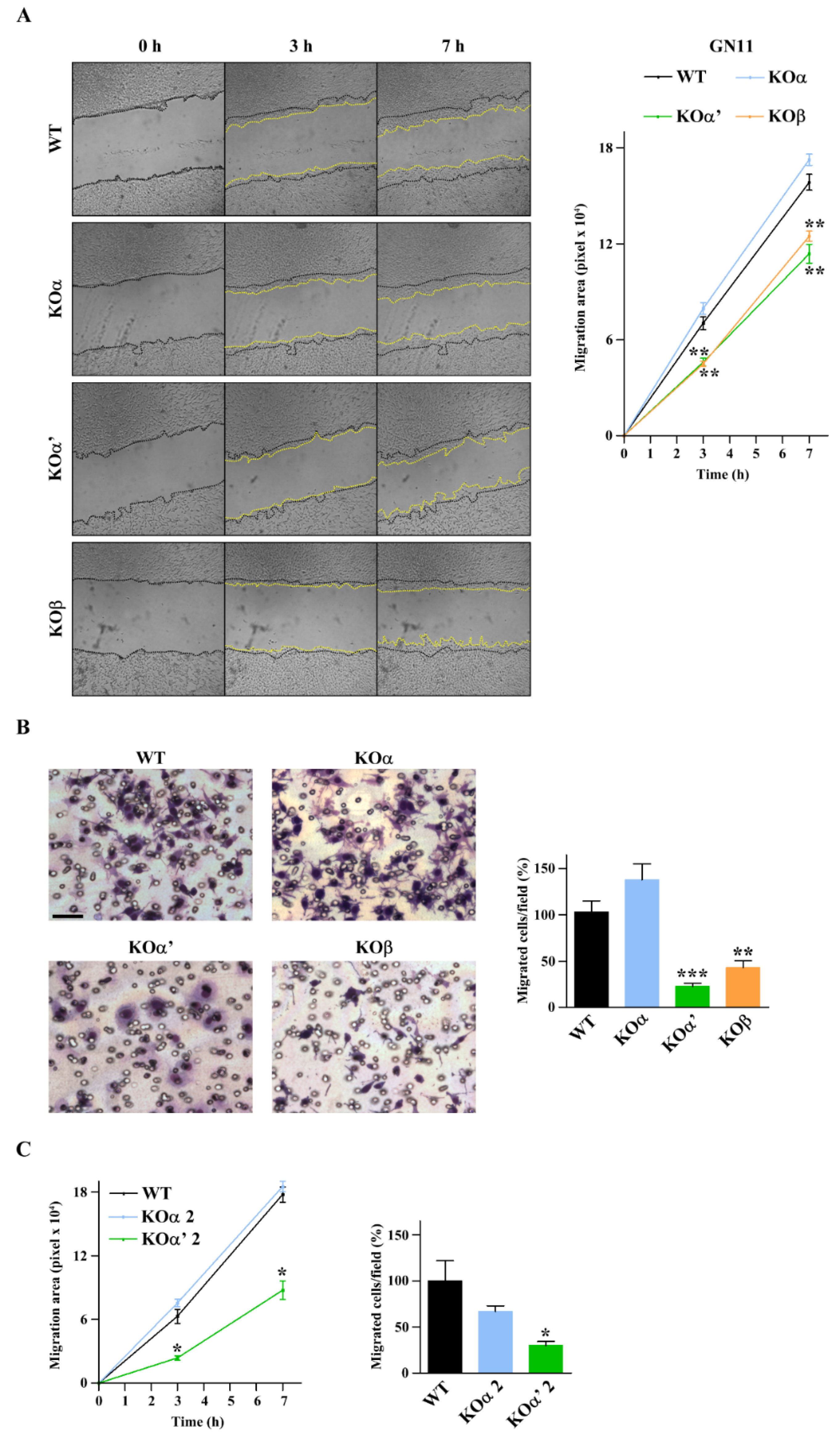
2.3. Differential Inactivation of CK2 Subunits Results in Reduced Proliferation and Different Migratory Activity of GN11 Cells
2.4. CK2 α’ and β Subunit Knockout Cell Lines Display a Different Cytoskeleton Organization Compared to Wild-Type GN11 Cells.
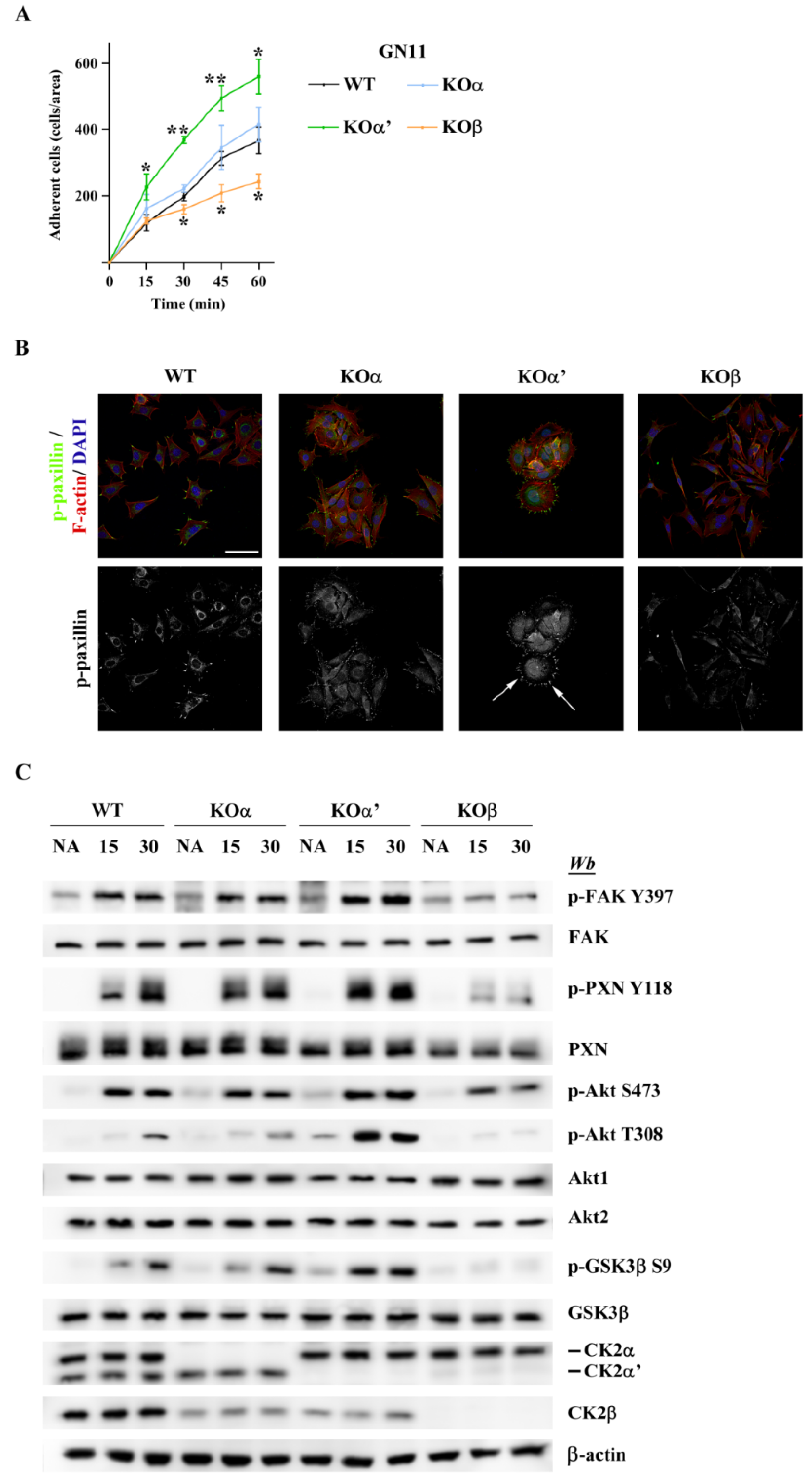
2.5. CK2 Knockout Affects GN11 Adhesion and Signal Transduction
3. Discussion
4. Material and Methods
4.1. Chemicals and Antibodies
4.2. Cell Culture
4.3. Cell lysis and Western Blotting Analysis
4.4. CRISPR/Cas9-Mediated CK2 Knockout
4.5. CK2 Kinase Activity Assay
4.6. In-Gel Kinase Assay of CK2α/α’
4.7. Proliferation Assay
4.8. Immunocytochemistry
4.9. Migration Assays
4.10. Adhesion Assay and Signal Transduction Pathways Analysis
4.11. Quantifications and Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salvi, M.; Sarno, S.; Cesaro, L.; Nakamura, H.; Pinna, L.A. Extraordinary pleiotropy of protein kinase CK2 revealed by weblogo phosphoproteome analysis. Biochim. Biophys. Acta 2009, 1793, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, L.; Salvi, M. CK2 Contribution to the Generation of the Human Phosphoproteome; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 117–128. [Google Scholar]
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, C.; Salizzato, V.; Borgo, C.; Cesaro, L.; Pinna, L.A.; Salvi, M. A Journey through the Cytoskeleton with Protein Kinase CK2. Curr. Protein Pept. Sci. 2019, 20, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Götz, C.; Montenarh, M. Protein kinase CK2 in development and differentiation. Biomed. Rep. 2017, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Pinna, L.A.; Allende, J.E. Protein kinase CK2 in health and disease: Protein kinase CK2: an ugly duckling in the kinome pond. Cell. Mol. Life Sci. 2009, 66, 1795–1799. [Google Scholar] [CrossRef]
- Borgo, C.; Franchin, C.; Scalco, S.; Bosello-Travain, V.; Donella-Deana, A.; Arrigoni, G.; Salvi, M.; Pinna, L.A. Generation and quantitative proteomics analysis of CK2α/α’(-/-) cells. Sci. Rep. 2017, 7, 42409. [Google Scholar] [CrossRef]
- Borgo, C.; Franchin, C.; Cesaro, L.; Zaramella, S.; Arrigoni, G.; Salvi, M.; Pinna, L.A. A proteomics analysis of CK2β(-/-) C2C12 cells provides novel insights into the biological functions of the non-catalytic β subunit. FEBS J. 2019, 286, 1561–1575. [Google Scholar] [CrossRef]
- Seldin, D.C.; Lou, D.Y.; Toselli, P.; Landesman-Bollag, E.; Dominguez, I. Gene targeting of CK2 catalytic subunits. Mol. Cell. Biochem. 2008, 316, 141–147. [Google Scholar] [CrossRef]
- Xu, X.; Toselli, P.A.; Russell, L.D.; Seldin, D.C. Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat. Genet. 1999, 23, 118–121. [Google Scholar] [CrossRef]
- Buchou, T.; Vernet, M.; Blond, O.; Jensen, H.H.; Pointu, H.; Olsen, B.B.; Cochet, C.; Issinger, O.-G.; Boldyreff, B. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol. Cell. Biol. 2003, 23, 908–915. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.; Zhang, S.; Li, Q.; Li, Z.; Zhou, F.; Dong, X.; Liu, L.; Wu, G.; Meng, R. Quinalizarin, a specific CK2 inhibitor, reduces cell viability and suppresses migration and accelerates apoptosis in different human lung cancer cell lines. Indian J. Cancer 2015, 52, 119. [Google Scholar]
- Girardi, C.; Ottaviani, D.; Pinna, L.A.; Ruzzene, M. Different Persistence of the Cellular Effects Promoted by Protein Kinase CK2 Inhibitors CX-4945 and TDB. Biomed. Res. Int. 2015, 2015, 185736. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; McFarland, B.C.; Drygin, D.; Yu, H.; Bellis, S.L.; Kim, H.; Bredel, M.; Benveniste, E.N. Targeting protein kinase CK2 suppresses prosurvival signaling pathways and growth of glioblastoma. Clin. Cancer Res. 2013, 19, 6484–6494. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.J.; Park, J.W.; Ryu, B.J.; Son, Y.-J.; Kim, S.H.; Lee, S.Y. CK2 inhibitor CX4945 induces sequential inactivation of proteins in the signaling pathways related with cell migration and suppresses metastasis of A549 human lung cancer cells. Bioorg. Med. Chem. Lett. 2013, 23, 5609–5613. [Google Scholar] [CrossRef]
- Wu, D.; Sui, C.; Meng, F.; Tian, X.; Fu, L.; Li, Y.; Qi, X.; Cui, H.; Liu, Y.; Jiang, Y. Stable knockdown of protein kinase CK2-alpha (CK2α) inhibits migration and invasion and induces inactivation of hedgehog signaling pathway in hepatocellular carcinoma Hep G2 cells. Acta Histochem. 2014, 116, 1501–1508. [Google Scholar] [CrossRef]
- Liu, S.; Gao, Y.; Yu, X.; Zhao, B.; Liu, L.; Zhao, Y.; Luo, Z.; Shi, J. Annexin-1 Mediates Microglial Activation and Migration via the CK2 Pathway during Oxygen-Glucose Deprivation/Reperfusion. Int. J. Mol. Sci. 2016, 17, 1770. [Google Scholar] [CrossRef]
- Brown, M.S.; Diallo, O.T.; Hu, M.; Ehsanian, R.; Yang, X.; Arun, P.; Lu, H.; Korman, V.; Unger, G.; Ahmed, K.; et al. CK2 modulation of NF-kappaB, TP53, and the malignant phenotype in head and neck cancer by anti-CK2 oligonucleotides in vitro or in vivo via sub-50-nm nanocapsules. Clin. Cancer Res. 2010, 16, 2295–2307. [Google Scholar] [CrossRef]
- Blanquet, P.R. Casein kinase 2 as a potentially important enzyme in the nervous system. Prog. Neurobiol. 2000, 60, 211–246. [Google Scholar] [CrossRef]
- Ceglia, I.; Flajolet, M.; Rebholz, H. Predominance of CK2α over CK2α’ in the mammalian brain. Mol. Cell. Biochem. 2011, 356, 169–175. [Google Scholar] [CrossRef]
- Díaz-Nido, J.; Mizuno, K.; Nawa, H.; Marshak, D.R. Regulation of protein kinase CK2 isoform expression during rat brain development. Cell. Mol. Biol. Res. 1994, 40, 581–585. [Google Scholar]
- Okur, V.; Cho, M.T.; Henderson, L.; Retterer, K.; Schneider, M.; Sattler, S.; Niyazov, D.; Azage, M.; Smith, S.; Picker, J.; et al. De novo mutations in CSNK2A1 are associated with neurodevelopmental abnormalities and dysmorphic features. Hum. Genet. 2016, 135, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Poirier, K.; Hubert, L.; Viot, G.; Rio, M.; Billuart, P.; Besmond, C.; Bienvenu, T. CSNK2B splice site mutations in patients cause intellectual disability with or without myoclonic epilepsy. Hum. Mutat. 2017, 38, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.I.; Bowden, R.; Parker, M.J.; Patterson, J.; Patterson, J.; Price, S.; Sarkar, A.; Castle, B.; Deshpande, C.; Splitt, M.; et al. Extending the phenotype associated with the CSNK2A1-related Okur-Chung syndrome-A clinical study of 11 individuals. Am. J. Med. Genet. A 2018, 176, 1108–1114. [Google Scholar] [CrossRef]
- Nakashima, M.; Tohyama, J.; Nakagawa, E.; Watanabe, Y.; Siew, C.G.; Kwong, C.S.; Yamoto, K.; Hiraide, T.; Fukuda, T.; Kaname, T.; et al. Identification of de novo CSNK2A1 and CSNK2B variants in cases of global developmental delay with seizures. J. Hum. Genet. 2019, 64, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Trinh, J.; Hüning, I.; Budler, N.; Hingst, V.; Lohmann, K.; Gillessen-Kaesbach, G. A novel de novo mutation in CSNK2A1: Reinforcing the link to neurodevelopmental abnormalities and dysmorphic features. J. Hum. Genet. 2017, 62, 1005–1006. [Google Scholar] [CrossRef]
- Guerrini, R.; Dobyns, W.B. Malformations of cortical development: clinical features and genetic causes. Lancet Neurol. 2014, 13, 710–726. [Google Scholar] [CrossRef]
- Radovick, S.; Wray, S.; Lee, E.; Nicols, D.K.; Nakayama, Y.; Weintraub, B.D.; Westphal, H.; Cutler, G.B.; Wondisford, F.E. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc. Natl. Acad. Sci. USA 1991, 88, 3402–3406. [Google Scholar] [CrossRef]
- Di Maira, G.; Salvi, M.; Arrigoni, G.; Marin, O.; Sarno, S.; Brustolon, F.; Pinna, L.A.; Ruzzene, M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005, 12, 668–677. [Google Scholar] [CrossRef]
- Salvi, M.; Sarno, S.; Marin, O.; Meggio, F.; Itarte, E.; Pinna, L.A. Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett. 2006, 580, 3948–3952. [Google Scholar] [CrossRef]
- Franchin, C.; Borgo, C.; Cesaro, L.; Zaramella, S.; Vilardell, J.; Salvi, M.; Arrigoni, G.; Pinna, L.A. Re-evaluation of protein kinase CK2 pleiotropy: New insights provided by a phosphoproteomics analysis of CK2 knockout cells. Cell. Mol. Life Sci. 2018, 75, 2011–2026. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Reece, J.; Putney, J.W. Role of the cytoskeleton in calcium signaling in NIH 3T3 cells. An intact cytoskeleton is required for agonist-induced [Ca2+]i signaling, but not for capacitative calcium entry. J. Biol. Chem. 1997, 272, 26555–26561. [Google Scholar] [CrossRef] [PubMed]
- Defilippi, P.; Olivo, C.; Tarone, G.; Mancini, P.; Torrisi, M.R.; Eva, A. Actin cytoskeleton polymerization in Dbl-transformed NIH3T3 fibroblasts is dependent on cell adhesion to specific extracellular matrix proteins. Oncogene 1997, 14, 1933–1943. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Guilluy, C. Focal adhesions, stress fibers and mechanical tension. Exp. Cell Res. 2016, 343, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Lu, S.; Szeto, K.W.; Sun, J.; Wang, Y.; Lasheras, J.C.; Chien, S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci. Rep. 2014, 4, 6024. [Google Scholar] [CrossRef]
- Xue, G.; Hemmings, B.A. PKB/Akt–Dependent Regulation of Cell Motility. J. Natl. Cancer Inst. 2013, 105, 393–404. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Sun, T.; Rodriguez, M.; Kim, L. Glycogen synthase kinase 3 in the world of cell migration. Dev. Growth Differ. 2009, 51, 735–742. [Google Scholar] [CrossRef]
- To, C.; Roy, A.; Chan, E.; Prado, M.A.M.; Di Guglielmo, G.M. Synthetic triterpenoids inhibit GSK3β activity and localization and affect focal adhesions and cell migration. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1274–1284. [Google Scholar] [CrossRef]
- Castello, J.; Ragnauth, A.; Friedman, E.; Rebholz, H. CK2-An Emerging Target for Neurological and Psychiatric Disorders. Pharmaceuticals 2017, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Soden, S.E.; Saunders, C.J.; Willig, L.K.; Farrow, E.G.; Smith, L.D.; Petrikin, J.E.; LePichon, J.-B.; Miller, N.A.; Thiffault, I.; Dinwiddie, D.L.; et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci. Transl. Med. 2014, 6, 265ra168. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef]
- Ku, C.S.; Polychronakos, C.; Tan, E.K.; Naidoo, N.; Pawitan, Y.; Roukos, D.H.; Mort, M.; Cooper, D.N. A new paradigm emerges from the study of de novo mutations in the context of neurodevelopmental disease. Mol. Psychiatry 2013, 18, 141–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stouffer, M.A.; Golden, J.A.; Francis, F. Neuronal migration disorders: Focus on the cytoskeleton and epilepsy. Neurobiol. Dis. 2016, 92, 18–45. [Google Scholar] [CrossRef]
- Maggi, R.; Pimpinelli, F.; Molteni, L.; Milani, M.; Martini, L.; Piva, F. Immortalized luteinizing hormone-releasing hormone neurons show a different migratory activity in vitro. Endocrinology 2000, 141, 2105–2112. [Google Scholar] [CrossRef][Green Version]
- Cariboni, A.; Hickok, J.; Rakic, S.; Andrews, W.; Maggi, R.; Tischkau, S.; Parnavelas, J.G. Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons. J. Neurosci. 2007, 27, 2387–2395. [Google Scholar] [CrossRef]
- Cariboni, A.; Rakic, S.; Liapi, A.; Maggi, R.; Goffinet, A.; Parnavelas, J.G. Reelin provides an inhibitory signal in the migration of gonadotropin-releasing hormone neurons. Development 2005, 132, 4709–4718. [Google Scholar] [CrossRef]
- Bibby, A.C.; Litchfield, D.W. The multiple personalities of the regulatory subunit of protein kinase CK2: CK2 dependent and CK2 independent roles reveal a secret identity for CK2beta. Int. J. Biol. Sci. 2005, 1, 67–79. [Google Scholar] [CrossRef]
- Salizzato, V.; Zanin, S.; Borgo, C.; Lidron, E.; Salvi, M.; Rizzuto, R.; Pallafacchina, G.; Donella-Deana, A. Protein kinase CK2 subunits exert specific and coordinated functions in skeletal muscle differentiation and fusogenic activity. FASEB J. 2019, 33, 10648–10667. [Google Scholar] [CrossRef]
- Ruzzene, M.; Di Maira, G.; Tosoni, K.; Pinna, L.A. Assessment of CK2 constitutive activity in cancer cells. Meth. Enzymol. 2010, 484, 495–514. [Google Scholar] [PubMed]
- Cariboni, A.; Pimpinelli, F.; Colamarino, S.; Zaninetti, R.; Piccolella, M.; Rumio, C.; Piva, F.; Rugarli, E.I.; Maggi, R. The product of X-linked Kallmann’s syndrome gene (KAL1) affects the migratory activity of gonadotropin-releasing hormone (GnRH)-producing neurons. Hum. Mol. Genet. 2004, 13, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Cariboni, A.; Davidson, K.; Rakic, S.; Maggi, R.; Parnavelas, J.G.; Ruhrberg, C. Defective gonadotropin-releasing hormone neuron migration in mice lacking SEMA3A signalling through NRP1 and NRP2: Implications for the aetiology of hypogonadotropic hypogonadism. Hum. Mol. Genet. 2011, 20, 336–344. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettieri, A.; Borgo, C.; Zanieri, L.; D’Amore, C.; Oleari, R.; Paganoni, A.; Pinna, L.A.; Cariboni, A.; Salvi, M. Protein Kinase CK2 Subunits Differentially Perturb the Adhesion and Migration of GN11 Cells: A Model of Immature Migrating Neurons. Int. J. Mol. Sci. 2019, 20, 5951. https://doi.org/10.3390/ijms20235951
Lettieri A, Borgo C, Zanieri L, D’Amore C, Oleari R, Paganoni A, Pinna LA, Cariboni A, Salvi M. Protein Kinase CK2 Subunits Differentially Perturb the Adhesion and Migration of GN11 Cells: A Model of Immature Migrating Neurons. International Journal of Molecular Sciences. 2019; 20(23):5951. https://doi.org/10.3390/ijms20235951
Chicago/Turabian StyleLettieri, Antonella, Christian Borgo, Luca Zanieri, Claudio D’Amore, Roberto Oleari, Alyssa Paganoni, Lorenzo A. Pinna, Anna Cariboni, and Mauro Salvi. 2019. "Protein Kinase CK2 Subunits Differentially Perturb the Adhesion and Migration of GN11 Cells: A Model of Immature Migrating Neurons" International Journal of Molecular Sciences 20, no. 23: 5951. https://doi.org/10.3390/ijms20235951
APA StyleLettieri, A., Borgo, C., Zanieri, L., D’Amore, C., Oleari, R., Paganoni, A., Pinna, L. A., Cariboni, A., & Salvi, M. (2019). Protein Kinase CK2 Subunits Differentially Perturb the Adhesion and Migration of GN11 Cells: A Model of Immature Migrating Neurons. International Journal of Molecular Sciences, 20(23), 5951. https://doi.org/10.3390/ijms20235951






