Biofilm-Forming by Carbapenem Resistant Enterobacteriaceae May Contribute to the Blood Stream Infection
Abstract
:1. Introduction
2. Results
2.1. Bacterial Strains and Patient Characteristics
2.2. Antimicrobial Susceptibility, Resistance Genes and Transmission Frequencies
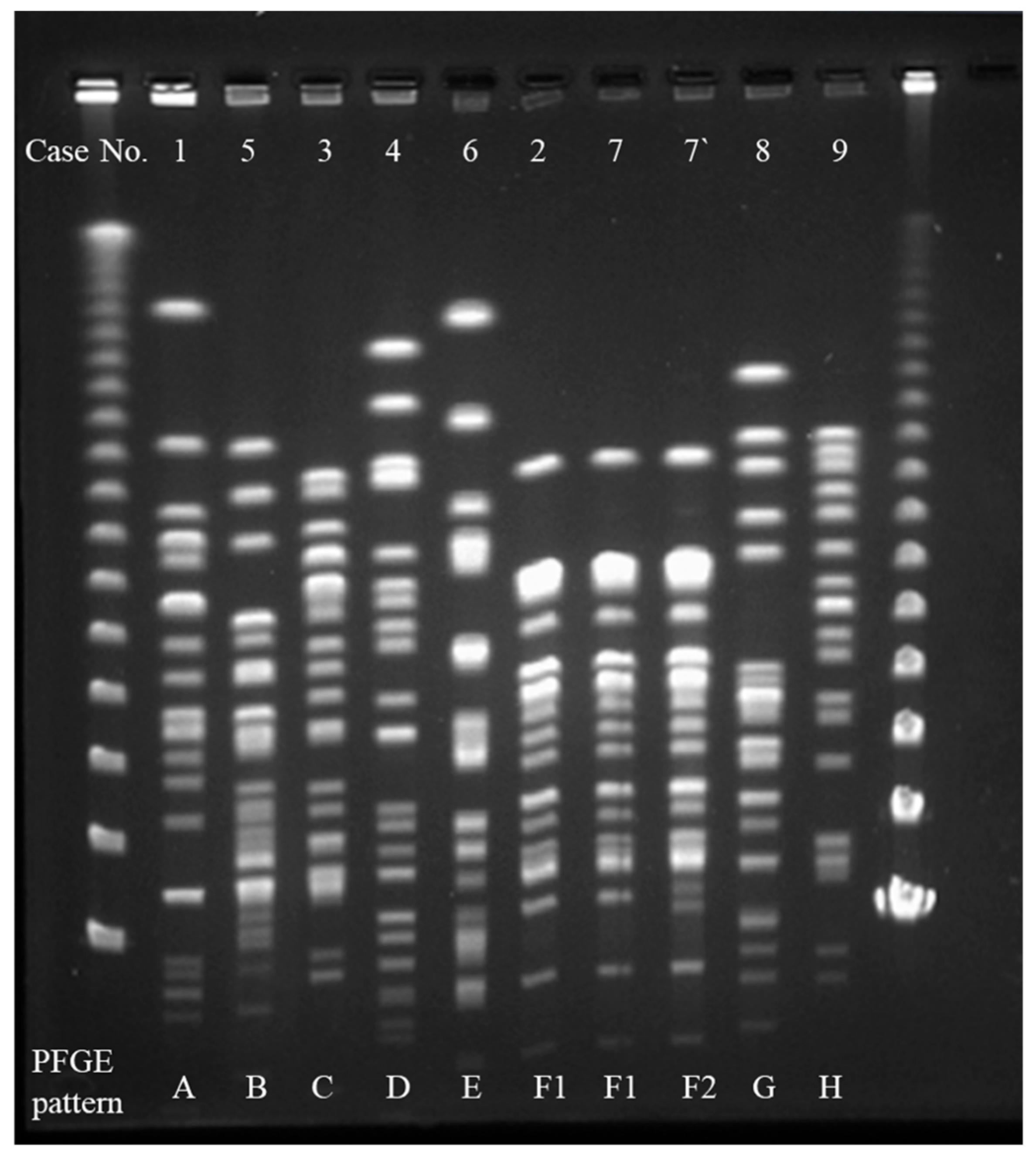
2.3. Interpretation of Pulsed-Field Gel Electrophoresis (PFGE)
2.4. Biofilm Study
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Bacterial Strains and Patients
4.3. MIC
4.4. Molecular Analysis of Antimicrobial Resistance Genes
4.5. Conjugal Transfer of Plasmid
4.6. PFGE
4.7. Microtiter Biofilm Assay
4.8. Biofilm Growth in Continuous Flow Chambers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussein, K.; Rabino, G.; Eluk, O.; Warman, S.; Reisner, S.; Geffen, Y.; Halif, L.; Paul, M. The association between infection control interventions and carbapenem-resistant Enterobacteriaceae incidence in an endemic hospital. J. Hosp. Infect. 2017, 97, 218–225. [Google Scholar] [CrossRef]
- Fernández, J.; Montero, I.; Martínez, Ó.; Fleites, A.; Poirel, L.; Nordmann, P.; Rodicio, M.R. Dissemination of multiresistant Enterobacter cloacae isolates producing OXA-48 and CTX-M-15 in a Spanish hospital. Int. J. Antimicrob. Agents 2015, 46, 469–474. [Google Scholar] [CrossRef]
- Bocanegra-Ibarias, P.; Garza-González, E.; Morfín-Otero, R.; Barrios, H.; Villarreal-Treviño, L.; Rodríguez-Noriega, E.; Garza-Ramos, U.; Petersen-Morfin, S.; Silva-Sanchez, J. Molecular and microbiological report of a hospital outbreak of NDM-1-carrying Enterobacteriaceae in Mexico. PLoS ONE 2017, 12, e0179651. [Google Scholar] [CrossRef]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Satlin, M.J.; Chen, L.; Patel, G.; Gomez-Simmonds, A.; Weston, G.; Kim, A.C.; Seo, S.K.; Rosenthal, M.E.; Sperber, S.J.; Jenkins, S.G.; et al. Multicenter Clinical and Molecular Epidemiological Analysis of Bacteremia Due to Carbapenem-Resistant Enterobacteriaceae (CRE) in the CRE Epicenter of the United States. Antimicrob. Agents Chemother. 2017, 61, e02349-16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, H. Clinical and Mortality Risk Factors in Bloodstream Infections with Carbapenem-Resistant Enterobacteriaceae. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 6212910. [Google Scholar] [CrossRef]
- Nabarro, L.E.B.; Shankar, C.; Pragasam, A.K.; Mathew, G.; Jeyaseelan, V.; Veeraraghavan, B.; Verghese, V.P. Clinical and bacterial risk factors for mortality in children with carbapenem-resistant Enterobacteriaceae bloodstream infections in India. Pediatric Infect. Dis. J. 2017, 36, e161–e166. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Miyoshi-Akiyama, T.; Kirikae, T.; Nagamatsu, M.; Shimada, K.; Mezaki, K.; Sugiki, Y.; Kuroda, E.; Kubota, S.; Takeshita, N.; et al. Molecular and epidemiological characterization of IMP-type metallo-β-lactamase-producing Enterobacter cloacae in a Large tertiary care hospital in Japan. Antimicrob. Agents Chemother. 2014, 58, 3441–3450. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Mori, N.; Kagawa, N.; Aoki, K.; Ishi, Y.; Tateda, K.; Aoki, Y. Clinical and molecular analyses of bloodstream infections caused by IMP metallo-β-lactamase-producing Enterobacteriaceae in a tertiary hospital in Japan. J. Infect. Chemother. 2019. [Google Scholar] [CrossRef]
- Yamamoto, N.; Asada, R.; Kawahara, R.; Hagiya, H.; Akeda, Y.; Shanmugakani, R.K.; Yoshida, H.; Yukawa, S.; Yamamoto, K.; Takayama, Y.; et al. Prevalence of, and risk factors for, carriage of carbapenem-resistant Enterobacteriaceae among hospitalized patients in Japan. J. Hosp. Infect. 2017, 97, 212–217. [Google Scholar] [CrossRef]
- Singh, P.K.; Schaefer, A.L.; Parsek, M.R.; Moninger, T.O.; Welsh, M.J.; Greenberg, E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000, 407, 762–764. [Google Scholar] [CrossRef]
- Hashem, A.A.; Abd El Fadeal, N.M.; Shehata, A.S. In vitro activities of vancomycin and linezolid against biofilm-producing methicillin-resistant staphylococci species isolated from catheter-related bloodstream infections from an Egyptian tertiary hospital. J. Med. Microbiol. 2017, 66, 744–752. [Google Scholar] [CrossRef]
- Li, W.S.; Chen, Y.C.; Kuo, S.F.; Chen, F.J.; Lee, C.H. The Impact of Biofilm Formation on the Persistence of Candidemia. Front. Microbiol. 2018, 9, 1196. [Google Scholar] [CrossRef]
- Qin, L.; Sakai, Y.; Bao, R.; Xie, H.; Masunaga, K.; Miura, M.; Hashimoto, K.; Tanamachi, C.; Hu, B.; Watanabe, H. Characteristics of multidrug-resistant Corynebacterium spp. isolated from blood cultures from hospitalized patients in Japan. Jpn. J. Infect. Dis. 2017, 70, 152–157. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; CLSI Document M100-S22; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Nakano, A.; Nakano, R.; Suzuki, Y.; Saito, K.; Kasahara, K.; Endo, S.; Yano, H. Rapid Identification of bla(IMP-1) and bla(IMP-6) by Multiplex Amplification Refractory Mutation System PCR. Ann. Lab. Med. 2018, 38, 378–380. [Google Scholar] [CrossRef]
- Yan, J.J.; Hsueh, P.R.; Ko, W.C.; Luh, K.T.; Tsai, S.H.; Wu, H.M.; Wu, J.J. Metallo-beta-lactamases in clinical Pseudomonas isolates in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob. Agents Chemother. 2001, 45, 2224–2228. [Google Scholar] [CrossRef]
- Ohnishi, M.; Okatani, A.T.; Harada, K.; Sawada, T.; Marumo, K.; Murakami, M.; Sato, R.; Esaki, H.; Shimura, K.; Kato, H.; et al. Genetic characteristics of CTX-M-type extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae involved in mastitis cases on Japanese dairy farms, 2007 to 2011. J. Clin. Microbiol. 2013, 51, 3117–3122. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Nakano, R.; Nakano, A.; Hikosaka, K.; Kawakami, S.; Matsunaga, N.; Asahara, M.; Ishigaki, S.; Furukawa, T.; Suzuki, M.; Shibayama, K.; et al. First report of metallo-β-lactamase NDM-5-producing Escherichia coli in Japan. Antimicrob. Agents Chemother. 2014, 58, 7611–7612. [Google Scholar] [CrossRef] [PubMed]
- Ribot, E.M.; Fair, M.A.; Gautom, R.; Cameron, D.N.; Hunter, S.B.; Swaminathan, B.; Barrett, T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar]
- Murphy, T.F.; Kirkham, C. Biofilm formation by nontypeable Haemophilus influenzae: Strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2002, 2, 7. [Google Scholar] [CrossRef]
- Schwartz, K.; Stephenson, R.; Hernandez, M.; Jambang, N.; Boles, B.R. The use of drip flow and rotating disk reactors for Staphylococcus aureus biofilm analysis. J. Vis. Exp. 2010, 27, 2470. [Google Scholar] [CrossRef] [Green Version]


| Case No. | The Month of Detection CRE | Age, Sex | Primary Infection | Underlying Disease | Indwelling Devices | Antibiotic Therapy | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 2014.11 | 40, Male | Acute pyelonephritis | Chronic kidney disease, Hemodialysis | Urinary tract stent | Meropenem+Ciprofloxacin | Survive |
| 2 | 2015.2 | 62, Male | Febrile neutroenia | Diffuse large B-cell lymphoma, Bone marrow transplantation | Central venous catheter | Meropenem | Death |
| 3 | 2015.2 | 21, Female | Catheter-related blood stream infection | Mixed connective tissue disease, Acute kidney injury, Hemodialysis | Central venous catheter, Urethral catheter | Piperacillin/tazobactam+Tigecycline | Death |
| 4 | 2015.7 | 71, Male | Acute pyelonephritis | Renal cell carcinoma | Urinary tract stent, Urethral catheter | Tigecycline+Amikacin | Survive |
| 5 | 2015.8 | 79, Female | Obstructive cholangitis | Papilla cancer, Diabetes mellitus | Retrograde transhepatic biliary drainage tube | Levofloxacin | Survive |
| 6 | 2015.8 | 56, Male | Acute pyelonephritis | Urinary tract stone, Diabetes mellitus | Urinary tract stent, Urethral catheter | Meropenem | Survive |
| 7 | 2015.9 | 59, Female | Vertebral osteomyelitis | Diffuse large B-cell lymphoma, Lung cancer | Central venous catheter | Tigecycline+Amikacin | Survive |
| 8 | 2015.11 | 84, Male | Acute pyelonephritis | Benign prostatic hyperplasia | None | Cefepime | Survive |
| 9 | 2015.12 | 31, Male | Primary bacteremia | Severe burn, acute respiratory distress syndrome, Diabetes mellitus | Central venous catheter, Urethral catheter | Levofloxacin | Survive |
| Case No. | Species | CFPM | CMZ | IPM | MEPM | P/T | AMK | LVFX | Resistant Genes | Transfer Frequency |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Klebsiella pneumoniae | >16 | >32 | 2 | >2 | 64 | <4 | <0.5 | blaIMP-1, blaTEM-1, blaSHV-12 | not transferred |
| 2 | Enterobacter cloacae | 16 | >32 | <1 | 2 | 64 | <4 | 2 | blaIMP-1, blaSHV-12 | not transferred |
| 3 | Enterobacter cloacae | 16 | >32 | <1 | >2 | <16 | <4 | >4 | blaIMP-1 | 4.0 × 10−7 |
| 4 | Enterobacter cloacae | >16 | >32 | 2 | 2 | >64 | 16 | >4 | blaTEM-1, blaSHV-12 | not determined |
| 5 | Citrobacter freundii | <2 | >32 | 2 | >2 | <16 | <4 | <0.5 | blaIMP-1 | 1.8 × 10−6 |
| 6 | Enterobacter cloacae | >16 | >32 | 2 | <1 | 64 | <4 | >4 | blaCTX-M-27 | not determined |
| 7 | Enterobacter cloacae | 16 | >32 | >2 | >2 | >64 | <4 | >4 | blaIMP-1, blaTEM-1, blaSHV-12 | not transferred |
| 7′ | Enterobacter cloacae | >16 | >32 | >2 | >2 | 64 | <4 | >4 | blaIMP-1, blaTEM-1 | not transferred |
| 8 | Enterobacter cloacae | <2 | >32 | 2 | <1 | <16 | <4 | <0.5 | not detected | not determined |
| 9 | Enterobacter cloacae | <2 | >32 | 2 | <1 | <16 | <4 | <0.5 | not detected | not determined |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaita, K.; Gotoh, K.; Nakano, R.; Iwahashi, J.; Sakai, Y.; Horita, R.; Yano, H.; Watanabe, H. Biofilm-Forming by Carbapenem Resistant Enterobacteriaceae May Contribute to the Blood Stream Infection. Int. J. Mol. Sci. 2019, 20, 5954. https://doi.org/10.3390/ijms20235954
Yaita K, Gotoh K, Nakano R, Iwahashi J, Sakai Y, Horita R, Yano H, Watanabe H. Biofilm-Forming by Carbapenem Resistant Enterobacteriaceae May Contribute to the Blood Stream Infection. International Journal of Molecular Sciences. 2019; 20(23):5954. https://doi.org/10.3390/ijms20235954
Chicago/Turabian StyleYaita, Kenichiro, Kenji Gotoh, Ryuichi Nakano, Jun Iwahashi, Yoshiro Sakai, Rie Horita, Hisakazu Yano, and Hiroshi Watanabe. 2019. "Biofilm-Forming by Carbapenem Resistant Enterobacteriaceae May Contribute to the Blood Stream Infection" International Journal of Molecular Sciences 20, no. 23: 5954. https://doi.org/10.3390/ijms20235954





