Genome-Wide Identification and Analysis of the NPR1-Like Gene Family in Bread Wheat and Its Relatives
Abstract
:1. Introduction
2. Results
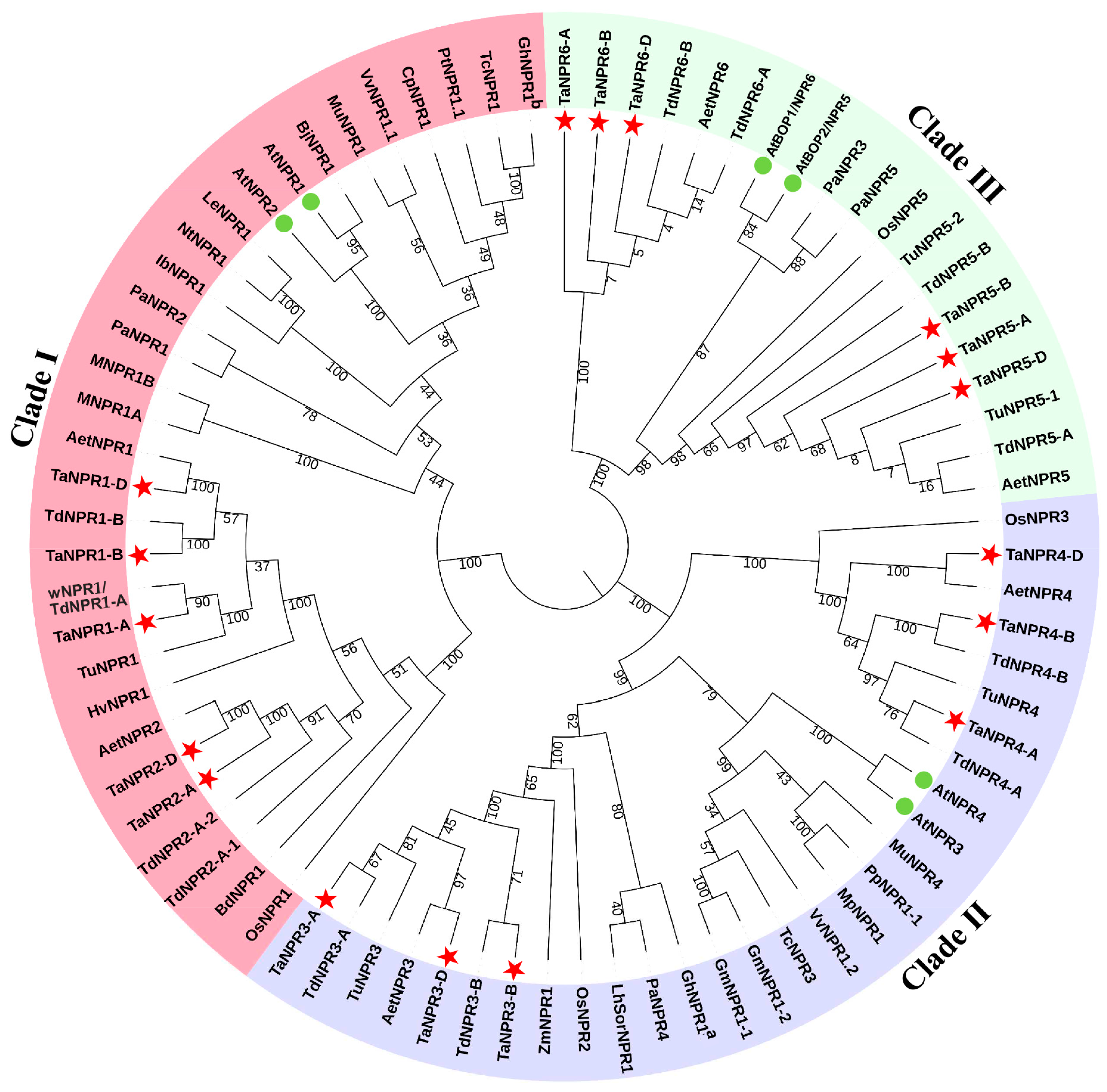
2.1. Identification, Phylogeny, and Characterization of NPR1-Like Genes in Bread Wheat and its Relatives
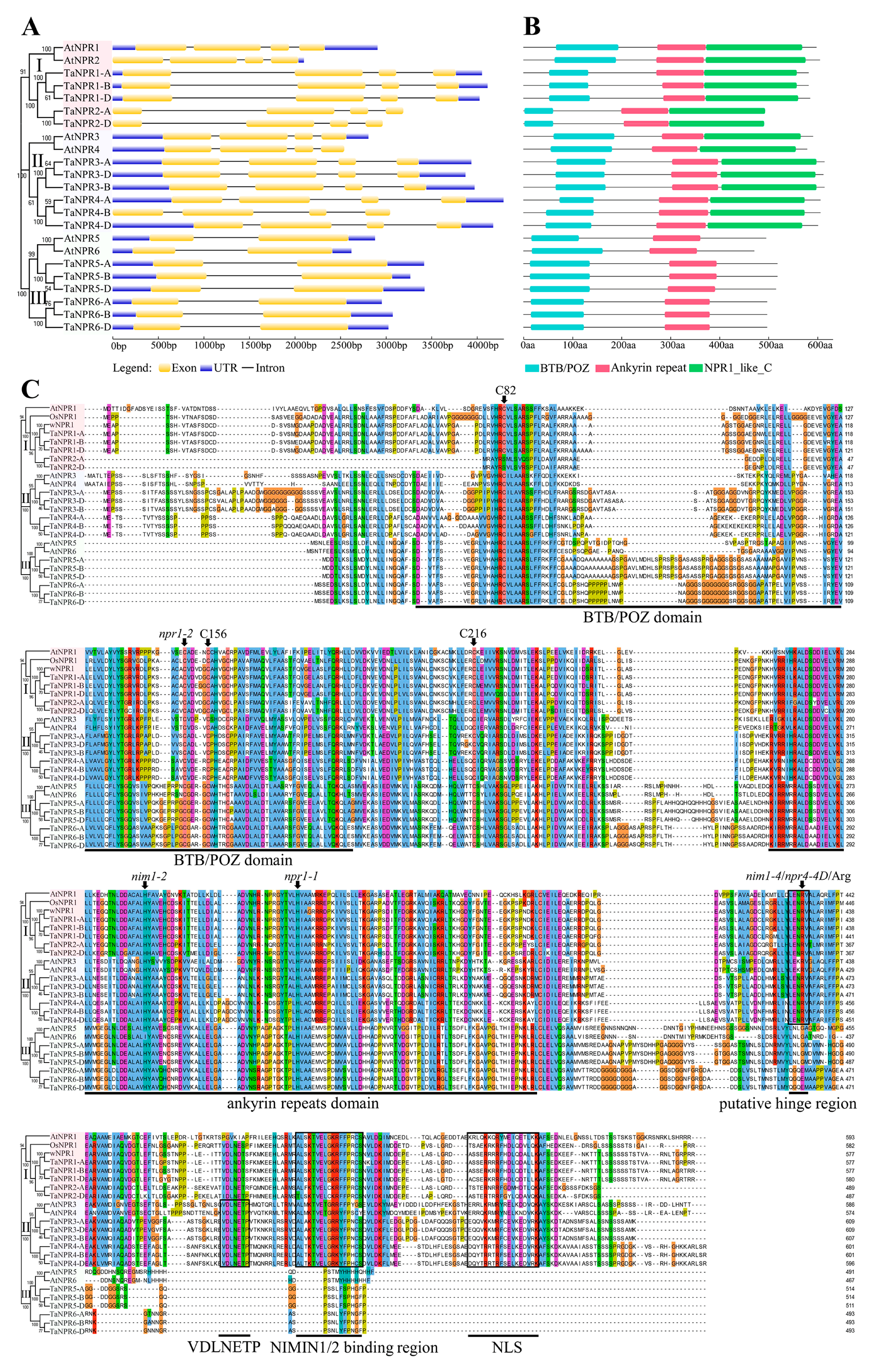
2.2. Sequence and Structural Analysis of TaNPR1-Like Genes and Proteins
2.3. Analysis of cis-Regulatory Elements in the Promoter Regions of TaNPR1-like Genes
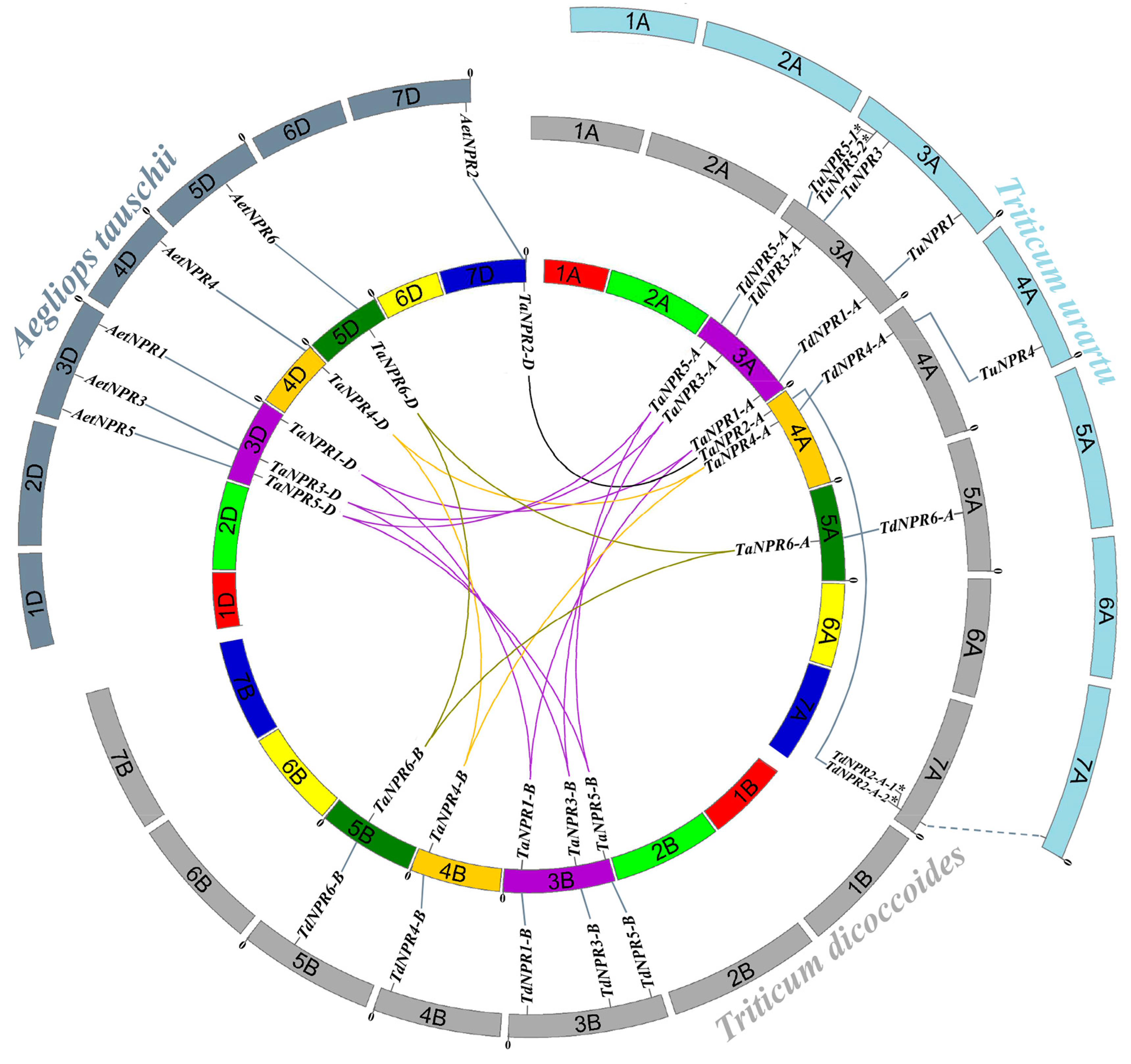
2.4. Chromosomal Distribution and Collinearity Analysis of NPR1-like Genes among Bread Wheat and Its Relatives
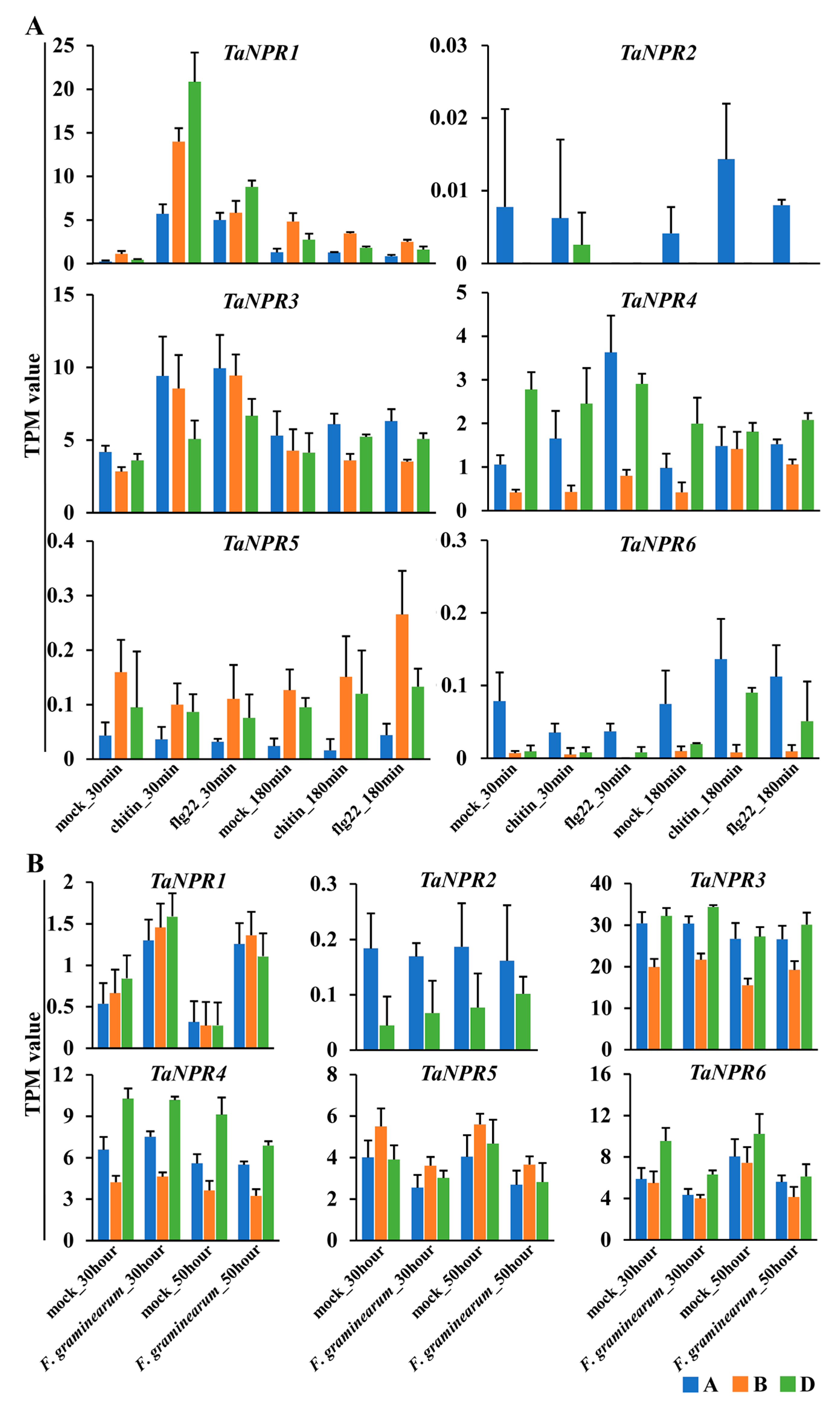
2.5. Expression Analysis of TaNPR1-Like Genes in Various Tissues/Organs
2.6. Expression Analysis of TaNPR1-Like Genes in Response to Biotic Stress
3. Discussion
3.1. Identification and Phylogenic Analysis of NPR1-Like Family
3.2. Sequence and Structural Features of TaNPR1-Like Genes and Proteins
3.3. Evolution and Expansion of NPR1-Like Family among Bread Wheat and Its Relatives
3.4. Functional Divergence of TaNPR1-Like Genes
4. Methods
4.1. Sources of Sequence Data
4.2. Identification of NPR1-Like Genes
4.3. Analysis of NPR1-Like Gene Characteristics
4.4. Phylogenetic Tree Construction, Chromosomal Location, and Homologous Relationships
4.5. Expression Profiles of TaNPR1-Like Genes in RNA-Seq
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SA | salicylic acid |
| JA | jasmonic acid |
| PR | pathogenesis-related |
| NPR1 | NONEXPRESSOR OF PR GENES 1 |
| BOP | BLADE-ON-PETIOLE |
| PTI | pathogen-associated molecular pattern-triggered immunity |
| ETI | effector-triggered immunity |
| PCD | rapid programmed cell death |
| HR | hypersensitive response |
| SAR | systemic acquired resistance |
| ISR | induced systemic resistance |
| FHB | Fusarium head blight |
References
- Marcussen, T.; Sandve, S.R.; Heier, L.; Spannagl, M.; Pfeifer, M.; Jakobsen, K.S.; Wulff, B.B.H.; Steuernagel, B.; Mayer, K.F.X.; Olsen, O.-A. Ancient hybridizations among the ancestral genomes of bread wheat. Science 2014, 345, 1250092. [Google Scholar] [CrossRef]
- Pont, C.; Leroy, T.; Seidel, M.; Tondelli, A.; Duchemin, W.; Armisen, D.; Lang, D.; Bustos-Korts, D.; Goué, N.; Balfourier, F.; et al. Tracing the ancestry of modern bread wheats. Nat. Genet. 2019, 51, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; Luca, V.D.; Despres, C. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012, 1, 639–647. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef]
- Manohar, M.; Tian, M.; Moreau, M.; Park, S.-W.; Choi, H.; Fei, Z.; Friso, G.; Asif, M.; Manosalva, P.; Dahl, C.C.V.; et al. Identification of multiple salicylic acid-binding proteins using two high throughput screens. Front Plant Sci. 2015, 5, 777. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant Immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.G.; Felix, G.; Boller, T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef]
- Livajaa, M.; Zeidlerb, D.; Radc, U.V.; Durner, J. Transcriptional responses of Arabidopsis thaliana to the bacteria-derived PAMPs harpin and lipopolysaccharide. Immunobiology 2008, 213, 161–171. [Google Scholar] [CrossRef]
- Boutrot, F.; Zipfel, C. Function, Discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef]
- Macho, A.P.; Schwessinger, B.; Ntoukakis, V.; Brutus, A.; Segonzac, C.; Roy, S.; Kadota, Y.; Oh, M.H.; Sklenar, J.; Derbyshire, P.; et al. A bacterial tyrosine phosphatase inhibits plant pattern recognition receptor activation. Science 2014, 343, 1509–1512. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Li, M.; Chang, M.; Xu, K.; Shang, Z.; Zhao, Y.; Palmer, I.; Zhang, Y.; McGill, J.; et al. A bacterial type III effector targets the master regulator of salicylic acid signaling, NPR1, to subvert plant immunity. Cell Host Microbe. 2017, 22, 777–788. [Google Scholar] [CrossRef]
- Qi, G.; Chen, J.; Chang, M.; Chen, H.; Hall, K.; Korin, J.; Liu, F.; Wang, D.; Fu3, Z.Q. Pandemonium breaks out: Disruption of salicylic acid-mediated defense by plant pathogens. Mol. Plant 2018, 11, 1427–1439. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Curtis, C.; Fu, Z. How plants deploy effector-triggered immunity to combat pathogens. Virulence 2014, 5, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.-Y.; Hunt, M.D. Systemic acquired resistance. Plant Cell 1996, 8, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Cao, H.; Scott, A.; Bowling, A.; Susan Gordon, A.; Dong, X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 1994, 6, 1583–1592. [Google Scholar] [CrossRef]
- Delaney, T.P.; Friedrich, L.; Ryals, J.A. Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc. Natl. Acad Sci. 1995, 92, 6602–6606. [Google Scholar]
- Shah, J.; Tsui, F.; Klessig, D.F. Characterization of a salicylic acid–insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol. Plant-Microbe Interact. 1997, 10, 69–78. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Wees, S.C.M.V.; Pelt, J.A.V.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; Loon, L.C.V. A Novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580. [Google Scholar] [CrossRef]
- Spoel, S.H.; Koornneef, A.; Claessens, S.M.C.; Korzelius, J.P.; Pelt, J.A.V.; Mueller, M.J.; Buchala, A.J.; Métraux, J.-P.; Brown, R.; Kazan, K.; et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 2003, 15, 760–770. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 2008, 3, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Olate, E.; Jiménez-Gómez, J.M.; Holuigue, L.; Salinas, J. NPR1 mediates a novel regulatory pathway in cold acclimation by interacting with HSFA1 factors. Nat. Plants 2018, 4, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Glazebrook, J.; Clarke, J.D.; Volko, S.; Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997, 88, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Aravind, L.; Koonin, E.V. Fold prediction and evolutionary analysis of the POZ domain: Structural and evolutionary relationship with the potassium channel tetramerization domain. J. Mol. Biol. 1999, 285, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Rochon, A.; Boyle, P.; Wignes, T.; Fobert, P.R.; Despres, C. The coactivator function of Arabidopsis NPR1 requires the core of its BTB/POZ domain and the oxidation of C-terminal cysteines. Plant Cell 2006, 18, 3670–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, P.; Su, E.L.; Rochon, A.; Shearer, H.L.; Murmu, J.; Chu, J.Y.; Fobert, P.R.; Despres, C. The BTB/POZ domain of the Arabidopsis disease resistance protein NPR1 interacts with the repression domain of TGA2 to negate its function. Plant Cell 2009, 21, 3700–3713. [Google Scholar] [CrossRef] [Green Version]
- Initiative, T.A.G. Analysis of the genome sequence of the fowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Holub, E.B.; Alonso, J.M.; Ecker, J.R.; Fobert, P.R. An Arabidopsis NPR1-like gene, NPR4, is required for disease resistance. Plant J. 2005, 41, 304–318. [Google Scholar] [CrossRef]
- Norberg, M.; Holmlund, M.; Nilsson, O. The BLADE ON PETIOLE genes act redundantly to control the growth and development of lateral organs. Development 2005, 132, 2203–2213. [Google Scholar] [CrossRef] [Green Version]
- Hepworth, S.R.; Zhang, Y.; McKim, S.; Li, X.; Haughn, G.W. BLADE-ON-PETIOLE-dependent signaling controls leaf and floral patterning in Arabidopsis. Plant Cell 2005, 17, 1434–1448. [Google Scholar] [CrossRef]
- Castello, M.J.; Medina-Puche, L.; Lamilla, J.; Tornero, P. NPR1 paralogs of Arabidopsis and their role in salicylic acid perception. PLoS ONE 2018, 13, e0209835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Cheng, Y.T.; Qu, N.; Zhao, Q.; Bi, D.; Li, X. Negative regulation of defense responses in Arabidopsis by two NPR1 paralogs. Plant J. 2006, 48, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Fan, W.; Dong, X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinkema, M.; Fan, W.; Dong, X. Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 2000, 12, 2339–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Chen, C.; Chen, Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 2001, 13, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Mohan, R.; Zhang, Y.; Li, M.; Chen, H.; Palmer, I.A.; Chang, M.; Qi, G.; Spoel, S.H.; Mengiste, T.; et al. NPR1 promotes its own and target gene expression in plant defense by recruiting CDK8. Plant Physiol. 2019, 181, 289–304. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical elicitors of systemic acquired resistance—Salicylic acid and its functional analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Chern, M.; Fitzgerald, H.A.; Canlas, P.E.; Navarre, D.A.; Ronald, P.C. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol. Plant-Microbe Interact. 2005, 18, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhong, S.; Li, Q.; Zhu, Z.; Lou, Y.; Wang, L.; Wang, J.; Wang, M.; Li, Q.; Yang, D.; et al. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol. J. 2007, 5, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-X.; Cao, L.; Li, J.; Duan, C.-J.; Luo, X.-M.; Le, N.; Wei, H.; Liang, S.; Chu, C.; Pan, Q.; et al. Involvement of OsNPR1/NH1 in rice basal resistance to blast fungus Magnaporthe oryzae. Eur. J. Plant Pathol. 2011, 131, 221–235. [Google Scholar] [CrossRef]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Mehari, H.; Meena, R.P.; Bhat, J.A.; Yadav, P.; Papalou, P.; Rawat, S.; Grover, A. Overexpression of NPR1 in Brassica juncea confers broad spectrum resistance to fungal pathogens. Front. Plant Sci. 2017, 8, 1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, H.; Qin, R.; Fang, L.; Liu, Z.; Yuan, S.; Gai, Y.; Ji, X. Characterization of NPR1 and NPR4 genes from mulberry (Morus multicaulis) and their roles in development and stress resistance. Physiol. Plant. 2018, 167, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Wenig, M.; Langen, G.; Sharma, S.; Kugler, K.G.; Knappe, C.; Hause, B.; Bichlmeier, M.; Babaeizad, V.; Imani, J.; et al. Bacteria-triggered systemic immunity in barley is associated with WRKY and ETHYLENE RESPONSIVE FACTORs but not with salicylic acid. Plant Physiol. 2014, 166, 2133–2151. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, B.; Li, K.; Kang, Z.; Cantu, D.; Dubcovsky, J. A conserved Puccinia striiformis protein interacts with wheat NPR1 and reduces induction of pathogenesis-related genes in response to pathogens. Mol. Plant-Microbe Interact. 2016, 29, 977–989. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Schiff, M.; Marathe, R.; Dinesh-Kumar, S.P. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Chao, T.; Wang, J.; Liu, A.; Ho, F.; Cheng, C. Virus-induced gene silencing reveals the involvement of ethylene-, salicylic acid- and mitogen-activated protein kinase-related defense pathways in the resistance of tomato to bacterial wilt. Physiol. Plant. 2009, 136, 324–335. [Google Scholar] [CrossRef]
- Bai, W.; Chern, M.; Ruan, D.; Canlas, P.E.; Sze-to, W.H.; Ronald, P.C. Enhanced disease resistance and hypersensitivity to BTH by introduction of an NH1/OsNPR1 paralog. Plant Biotechnol. J. 2011, 9, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Zhang, Y.; Maximova, S.N.; Guiltinan, M.J. TcNPR3 from Theobroma cacao functions as a repressor of the pathogen defense response. BMC Plant Biol. 2013, 13, 204. [Google Scholar] [CrossRef] [Green Version]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Front. Plant Sci. 2018, 9, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, L.; Liao, J.; Lin, N.; Chung, C. Identification of a strawberry NPR-like gene involved in negative regulation of the salicylic acid-mediated defense pathway. PLoS ONE 2018, 13, e0205790. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.; Yang, B.; Ruan, R.; Li, K.; Menzo, V.; Fu, D.; Chern, M.; Ronald, P.C.; Dubcovsky, J. Comparative analysis of protein-protein interactions in the defense response of rice and wheat. BMC Genom. 2013, 14, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Bi, W.; Li, H.; Wu, J.; Yu, X.; Liu, D.; Wang, X. WRKY transcription factors associated with NPR1-mediated acquired resistance in barley are potential resources to improve wheat resistance to Puccinia triticina. Front. Plant Sci. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Makandar, R.; Essig, J.S.; Schapaugh, M.A.; Trick, H.N.; Shah, J. Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol. Plant-Microbe Interact. 2006, 19, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Makandar, R.; Nalam, V.; Lee, H.; N Trick, H.; Dong, Y.; Shah, J. Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol. Plant-Microbe Interact. 2011, 25, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.S.; Kou, X.J.; Li, H.P.; Zhang, J.B.; Saad, A.S.I.; Liao, Y.C. Inverse effects of Arabidopsis NPR1 gene on fusarium seedling blight and fusarium head blight in transgenic wheat. Plant Pathol. 2013, 62, 383–392. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, X.; Yao, J.; Zhou, M.; Ma, H. Resistance against Fusarium head blight in transgenic wheat plants expressing the ScNPR1 gene. J. Phytopathol. 2017, 165, 223–231. [Google Scholar] [CrossRef]
- IWGSC. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [Green Version]
- Ling, H.Q.; Ma, B.; Shi, X.; Liu, H.; Dong, L.; Sun, H.; Cao, Y.; Gao, Q.; Zheng, S.; Li, Y.; et al. Genome sequence of the progenitor of wheat A subgenome Triticum urartu. Nature 2018, 557, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Avni, R.; Nave, M.; Barad, O.; Baruch, K.; Twardziok, S.O.; Gundlach, H.; Hale, I.; Mascher, M.; Spannagl, M.; Wiebe, K.; et al. Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 2017, 357, 1509–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.C.; Gu, Y.Q.; Puiu, D.; Wang, H.; Twardziok, S.O.; Deal, K.R.; Huo, N.; Zhu, T.; Wang, L.; Wang, Y.; et al. Genome sequence of the progenitor of the wheat D genome Aegilops tauschii. Nature 2017, 551, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Maier, F.; Zwicker, S.; Huckelhoven, A.; Meissner, M.; Funk, J.; Pfitzner, A.J.; Pfitzner, U.M. NONEXPRESSOR OF PATHOGENESIS-RELATED PROTEINS1 (NPR1) and some NPR1-related proteins are sensitive to salicylic acid. Mol. Plant Pathol. 2011, 12, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Ryals, J.; Weymann, K.; Lavvton, K.; Friedrich, L.; Ellis, D.; Steiner, H.-Y.; Johnson, J.; Delaney, T.P.; Jesse, T.; VOS, P.; et al. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell 1997, 9, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Priest, H.D.; Filichkin, S.A.; Mockler, T.C. cis-Regulatory elements in plant cell signaling. Curr. Opin. Plant Biol. 2009, 12, 643–649. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Peer, Y.V.D.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Ramirez-Gonzalez, R.H.; Borrill, P.; Lang, D.; Harrington, S.A.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; van Ex, F.; Pasha, A.; et al. The transcriptional landscape of polyploid wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef] [Green Version]
- Shia, Z.; Maximovab, S.; Liua, Y.; Vericab, J.; Guiltinan, M.J. The salicylic acid receptor NPR3 is a negative regulator of the transcriptional defense response during early flower development in Arabidopsis. Mol. Plant 2013, 6, 802–816. [Google Scholar] [CrossRef]
- Kugler, K.G.; Siegwart, G.; Nussbaumer, T.; Ametz, C.; Spannagl, M.; Steiner, B.; Lemmens, M.; Mayer, K.F.; Buerstmayr, H.; Schweiger, W. Quantitative trait loci-dependent analysis of a gene co-expression network associated with Fusarium head blight resistance in bread wheat (Triticum aestivum L.). BMC Genom. 2013, 14, 728. [Google Scholar] [CrossRef] [Green Version]
- Backer, R.; Mahomed, W.; Reeksting, B.J.; Engelbrecht, J.; Ibarra-Laclette, E.; van den Berg, N. Phylogenetic and expression analysis of the NPR1-like gene family from Persea americana (Mill.). Front. Plant Sci. 2015, 6, 300. [Google Scholar] [CrossRef] [Green Version]
- Lawton-Rauh, A. Evolutionary dynamics of duplicated genes in plants. Mol. Phylogenet. Evol. 2003, 29, 396–409. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Jin, J.; Guo, A.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, H.; He, Y.; Xia, R. TBtools, a Toolkit for Biologists integrating various biological data handling tools with a user-friendly interface. bioRxiv 2018. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koonin, E.V. Orthologs, paralogs, and evolutionary genomics. Annu. Rev. Genet. 2005, 39, 309–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]


| Species | Clade | Gene Name | Sequence ID | Chromosome Location | AA | Mw (kDa) | pI | Splicing |
|---|---|---|---|---|---|---|---|---|
| T.aestivum | I | TaNPR1-A | TraesCS3A02G105400.1 | 3A:69290641-69294697(+) | 577 | 63.57 | 5.37 | - |
| TaNPR1-B | TraesCS3B02G123800.1 | 3B:96812311-96816428(+) | 577 | 63.83 | 5.49 | 2 | ||
| TaNPR1-D | TraesCS3D02G107500.1 | 3D:61072307-61076336(+) | 580 | 63.78 | 5.27 | - | ||
| TaNPR2-A | TraesCS4A02G470500.1 | 4A:731397493-731400686(–) | 489 | 54.91 | 5.18 | - | ||
| TaNPR2-D | TraesCS7D02G019000.1 | 7D:8353564-8356531(+) | 487 | 54.49 | 5.88 | - | ||
| II | TaNPR3-A | TraesCS3A02G298800.2 | 3A:532934212-532938152(–) | 618 | 67.45 | 5.6 | 2 | |
| TaNPR3-B | TraesCS3B02G337700.1 | 3B:544108681-544112656(+) | 609 | 66.45 | 5.59 | 2 | ||
| TaNPR3-D | TraesCS3D02G302900.1 | 3D:417737897-417741772(+) | 607 | 66.31 | 5.45 | - | ||
| TaNPR4-A | TraesCS4A02G294400.1 | 4A:595817163-595821455(+) | 601 | 66.61 | 5.82 | - | ||
| TaNPR4-B | TraesCS4B02G018900.2 | 4B:13944552-13947600(+) | 601 | 66.90 | 5.8 | 2 | ||
| TaNPR4-D | TraesCS4D02G017500.1 | 4D:7769189-7773368(–) | 596 | 66.39 | 5.86 | - | ||
| III | TaNPR5-A | TraesCS3A02G489000.1 | 3A:716756231-716759651(+) | 514 | 54.19 | 6.12 | - | |
| TaNPR5-B | TraesCS3B02G537400.1 | 3B:777578202-777581471(+) | 514 | 54.20 | 6.06 | - | ||
| TaNPR5-D | TraesCS3D02G484100.1 | 3D:581113138-581116563(–) | 511 | 53.78 | 6.03 | - | ||
| TaNPR6-A | TraesCS5A02G134700.2 | 5A:304537866-304540822(–) | 493 | 51.65 | 6.11 | 2 | ||
| TaNPR6-B | TraesCS5B02G133700.1 | 5B:250233025-250236102(–) | 493 | 51.62 | 6.11 | 2 | ||
| TaNPR6-D | TraesCS5D02G139600.1 | 5D:222737713-222740742(+) | 493 | 51.72 | 6.11 | 2 | ||
| T.urartu | I | TuNPR1 | TuG1812G0300001179.01.T01 | 3A:64494723-64498668(+) | 577 | 63.49 | 5.37 | - |
| II | TuNPR3 | TuG1812G0300003503.01.T01 | 3A:544372634-544376515(+) | 609 | 66.35 | 5.52 | 2 | |
| TuNPR4 | TuG1812G0400000243.01.T01 | 4A:12320155-12324208(–) | 600 | 66.54 | 5.82 | 2 | ||
| III | TuNPR5-1 | TuG1812G0300005248.01.T01 | 3A:705429640-705432414(+) | 514 | 54.19 | 6.06 | - | |
| TuNPR5-2 | TuG1812G0300005239.01.T01 | 3A:704992825-704995600(+) | 531 | 58.25 | 9.48 | 2 | ||
| T.dicoccoides | I | TdNPR1-A | TRIDC3AG012960.1 | 3A:64964238-64967906(+) | 580 | 63.81 | 5.37 | - |
| TdNPR1-B | TRIDC3BG017420.1 | 3B:105475063-105478776(+) | 580 | 63.94 | 5.43 | 8 | ||
| TdNPR2-A-1 | TRIDC7AG001960.1 | 7A:5808627-5811849(+) | 570 | 63.86 | 5.88 | 5 | ||
| TdNPR2-A-2 | TRIDC7AG002040.2 | 7A:6071183-6076497(–) | 549 | 61.66 | 5.57 | 5 | ||
| II | TdNPR3-A | TRIDC3AG044830.2 | 3A:548025257-548029459(+) | 609 | 66.37 | 5.45 | 5 | |
| TdNPR3-B | TRIDC3BG050750.2 | 3B:556623106-556626770(+) | 609 | 66.45 | 5.59 | 5 | ||
| TdNPR4-A | TRIDC4AG045270.3 | 4A:588643107-588646338(+) | 601 | 66.61 | 5.82 | 3 | ||
| TdNPR4-B | TRIDC4BG003310.1 | 4B:13063896-13067432(+) | 531 | 59.12 | 5.45 | 5 | ||
| III | TdNPR5-A | TRIDC3AG068800.5 | 3A:714043376-714046300(+) | 516 | 54.33 | 6.13 | 9 | |
| TdNPR5-B | TRIDC3BG078000.6 | 3B:789380796-789383751(+) | 516 | 54.33 | 6.04 | 9 | ||
| T.dicoccoides | III | TdNPR6-A | TRIDC5AG022110.4 | 5A:290229998-290232860(+) | 493 | 51.68 | 6.11 | 5 |
| TdNPR6-B | TRIDC5BG023740.4 | 5B:257840960-257843317(–) | 493 | 51.62 | 6.11 | 5 | ||
| Ae. tauschii | I | AetNPR1 | AET3Gv20232400.2 | 3D:64296876-64300961(+) | 608 | 66.59 | 5.41 | 5 |
| AetNPR2 | AET7Gv20038900.2 | 7D:7720290-7726555(+) | 454 | 50.19 | 5.46 | 3 | ||
| II | AetNPR3 | AET3Gv20713100.1 | 3D:425287443-425291551(+) | 607 | 66.31 | 5.45 | 3 | |
| AetNPR4 | AET4Gv20029500.2 | 4D:6713853-6718113(–) | 596 | 66.37 | 5.86 | 7 | ||
| III | AetNPR5 | AET3Gv21117800.1 | 3D:592078399-592081502(–) | 514 | 54.17 | 6.06 | 13 | |
| AetNPR6 | AET5Gv20354800.2 | 5D:229230789-229233557(+) | 515 | 54.18 | 6.63 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, Z.; Niu, X.; Xu, Q.; Yang, L. Genome-Wide Identification and Analysis of the NPR1-Like Gene Family in Bread Wheat and Its Relatives. Int. J. Mol. Sci. 2019, 20, 5974. https://doi.org/10.3390/ijms20235974
Liu X, Liu Z, Niu X, Xu Q, Yang L. Genome-Wide Identification and Analysis of the NPR1-Like Gene Family in Bread Wheat and Its Relatives. International Journal of Molecular Sciences. 2019; 20(23):5974. https://doi.org/10.3390/ijms20235974
Chicago/Turabian StyleLiu, Xian, Zhiguo Liu, Xinhui Niu, Qian Xu, and Long Yang. 2019. "Genome-Wide Identification and Analysis of the NPR1-Like Gene Family in Bread Wheat and Its Relatives" International Journal of Molecular Sciences 20, no. 23: 5974. https://doi.org/10.3390/ijms20235974
APA StyleLiu, X., Liu, Z., Niu, X., Xu, Q., & Yang, L. (2019). Genome-Wide Identification and Analysis of the NPR1-Like Gene Family in Bread Wheat and Its Relatives. International Journal of Molecular Sciences, 20(23), 5974. https://doi.org/10.3390/ijms20235974





