Onconase Restores Cytotoxicity in Dabrafenib-Resistant A375 Human Melanoma Cells and Affects Cell Migration, Invasion and Colony Formation Capability
Abstract
1. Introduction
2. Results
2.1. ONC Strongly Affects Cell Viability of both Parental and Dabrafenib Resistant A375 Cells
2.2. ONC Does not Affect Cell Viability of Normal Melanocytes
2.3. ONC Decreases the Proliferation Rate of both A375P and A375DR Cell Subpopulations
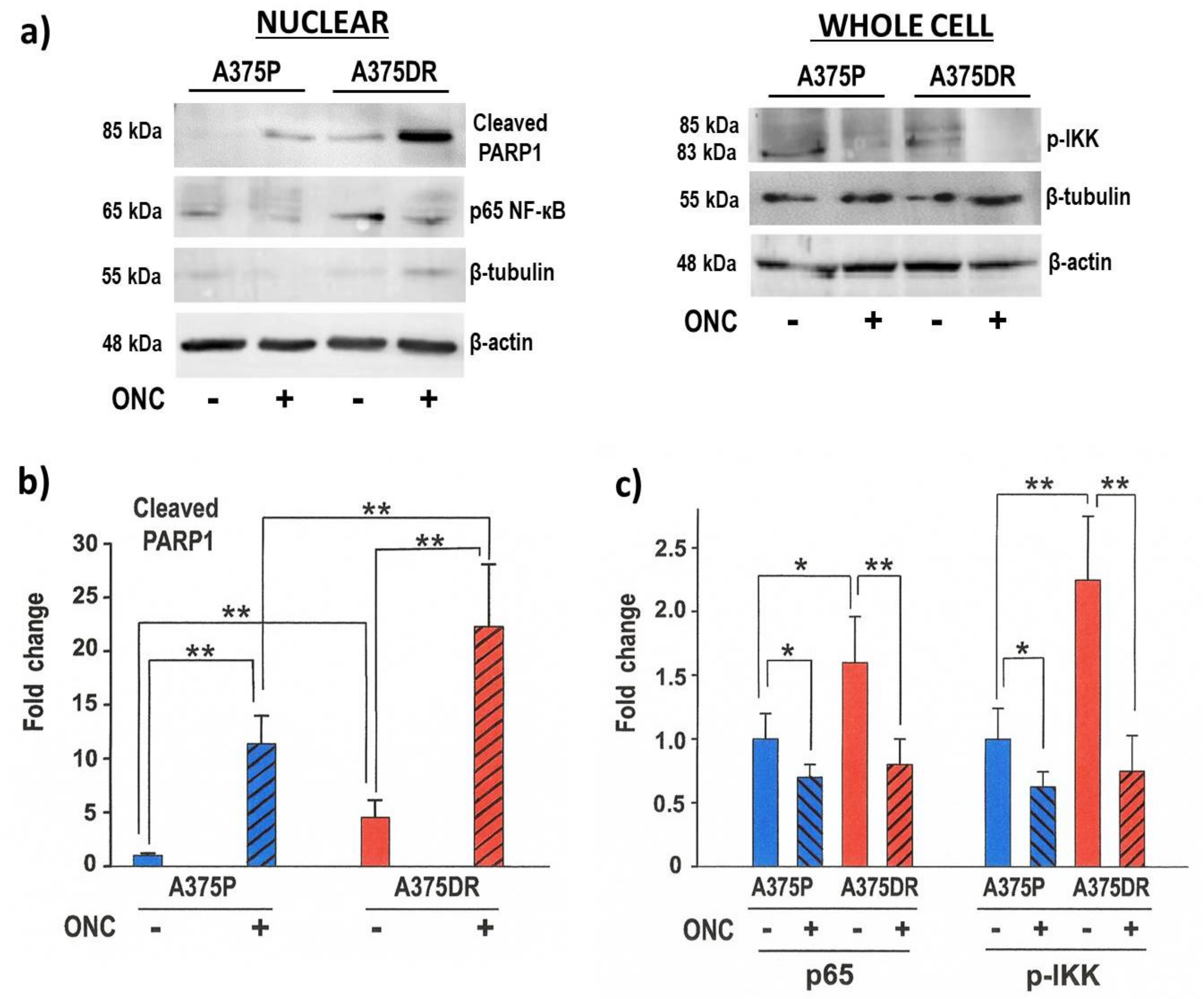
2.4. ONC Induces Apoptotic Cell Death Mainly in A375DR Cells
2.5. ONC Affects NF-κB Activation in both A375 Cell Subpopulations
2.6. The A375DR Cell Subpopulation Displays the Presence of CSC Biochemical Markers
2.7. ONC Reduces the Ability of both A375P and A375DR Cells to Form Soft Agar Colonies
2.8. ONC Reduces Either Cell Migration Rate or Invasion
2.9. ONC Reduces MMP2 Activity in both A375P and A375DR Cells
3. Discussion
4. Materials and Methods
4.1. ONC Expression and Purification
4.2. Cell Cultures
4.3. Cell Viability Assay
4.4. 5-Br-2’-Deoxyuridine Cell Proliferation Assay
4.5. Soft Agar Colony Formation Assay
4.6. Wound Closure Cell Migration Assay
4.7. Transwell Invasion Assay
4.8. Gelatin Zymography
4.9. Total and Nuclear Protein Purification
4.10. Quantitative Real Time-PCR Analysis
| CD133: | (Fw) 5’GCATTGGCATCTTCTATGGTT3’, (Rev) 5’CGCCTTGTCCTTGGTAGTGT-3’ |
| NANOG: | (Fw) 5’AGTCCCAAAGGCAAACAACCCAGTTC3’, (Rev) 5’TGCTGGAGGCTGAGGTATTTCTGTCTC-3’ |
| TBP: | (Fw) 5’TGTATCCACAGTGAATCTTGG-3’, (Rev) 5’-ATGATTACCGCAGCAAACC-3’ |
4.11. WB Assays
4.12. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franklin, C.; Livingstone, E.; Roesch, A.; Schilling, B.; Schadendorf, D. Immunotherapy in melanoma: Recent advances and future directions. Eur. J. Surg. Oncol. 2017, 43, 604–611. [Google Scholar] [CrossRef]
- Luebker, S.A.; Koepsell, S.A. Diverse Mechanisms of BRAF Inhibitor Resistance in Melanoma Identified in Clinical and Preclinical Studies. Front. Oncol. 2019, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Mattia, G.; Puglisi, R.; Ascione, B.; Malorni, W.; Carè, A.; Matarrese, P. Cell death-based treatments of melanoma: Conventional treatments and new therapeutic strategies. Cell Death Dis. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Das Thakur, M.; Salangsang, F.; Landman, A.S.; Pryer, N.K.; Levesque, M.P.; Dummer, R.; McMahon, M.; Stuart, D.D. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013, 494, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, F.G.; De Presbiteris, A.L.; Camerlingo, R.; Mozzillo, N.; Pirozzi, G.; Cavalcanti, E.; Manca, A.; Palmieri, G.; Cossu, A.; Ciliberto, G.; et al. Phenotype characterization of human melanoma cells resistant to dabrafenib. Oncol. Rep. 2017, 38, 2741–2751. [Google Scholar] [CrossRef]
- Caporali, S.; Alvino, E.; Lacal, P.M.; Levati, L.; Giurato, G.; Memoli, D.; Caprini, E.; Antonini Cappellini, G.C.; D’Atri, S. Targeting the PI3K/AKT/mTOR pathway overcomes the stimulating effect of dabrafenib on the invasive behavior of melanoma cells with acquired resistance to the BRAF inhibitor. Int. J. Oncol. 2016, 49, 1164–1174. [Google Scholar] [CrossRef]
- Caporali, S.; Alvino, E.; Lacal, P.M.; Ruffini, F.; Levati, L.; Bonmassar, L.; Scoppola, A.; Marchetti, P.; Mastroeni, S.; Antonini Cappellini, G.C.; et al. Targeting the PTTG1 oncogene impairs proliferation and invasiveness of melanoma cells sensitive or with acquired resistance to the BRAF inhibitor dabrafenib. Oncotarget 2017, 8, 113472–113493. [Google Scholar] [CrossRef]
- Fofaria, N.M.; Frederick, D.T.; Sullivan, R.J.; Flaherty, K.T.; Srivastava, S.K. Overexpression of Mcl-1 confers resistance to BRAFV600E inhibitors alone and in combination with MEK1/2 inhibitors in melanoma. Oncotarget 2015, 6, 40535–40556. [Google Scholar] [CrossRef]
- Szász, I.; Koroknai, V.; Kiss, T.; Vízkeleti, L.; Ádány, R.; Balázs, M. Molecular alterations associated with acquired resistance to BRAFV600E targeted therapy in melanoma cells. Melanoma Res. 2019, 29, 390–400. [Google Scholar] [CrossRef]
- Obenauf, A.C.; Zou, Y.; Ji, A.L.; Vanharanta, S.; Shu, W.; Shi, H.; Kong, X.; Bosenberg, M.C.; Wiesner, T.; Rosen, N.; et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 2015, 520, 368–372. [Google Scholar] [CrossRef]
- Marzagalli, M.; Raimondi, M.; Fontana, F.; Montagnani Marelli, M.; Moretti, R.M.; Limonta, P. Cellular and molecular biology of cancer stem cells in melanoma: Possible therapeutic implications. Semin. Cancer Biol. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G.; Lehembre, F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol. Med. 2007, 13, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Nikkola, J.; Vihinen, P.; Vuoristo, M.S.; Kellokumpu-Lehtinen, P.; Kahari, V.M.; Pyrhonen, S. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin. Cancer Res. 2005, 11, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Malaponte, G.; Zacchia, A.; Bevelacqua, Y.; Marconi, A.; Perrotta, R.; Mazzarino, M.C.; Cardile, V.; Stivala, F. Co-regulated expression of matrix metalloproteinase-2 and transforming growth factor-beta in melanoma development and progression. Oncol. Rep. 2010, 24, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Xuea, G.; Romano, E.; Massic, D.; Mandalà, M. Wnt/b-catenin signaling in melanoma: Preclinical rationale and novel therapeutic insights. Cancer Treat. Rev. 2016, 49, 1–12. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, F.; Xing, Q.; Guo, F.; Wang, M.; Li, Y. The biological effect and mechanism of the Wnt/β-catenin signaling pathway on malignant melanoma A375 cells. Exp. Ther. Med. 2018, 16, 2032–2037. [Google Scholar] [CrossRef]
- Sinnberg, T.; Makino, E.; Krueger, M.A.; Velic, A.; Macek, B.; Rothbauer, U.; Groll, N.; Pötz, O.; Czemmel, S.; Niessner, H.; et al. Nexus Consisting of Beta-Catenin and Stat3 Attenuates BRAF Inhibitor Efficacy and Mediates Acquired Resistance to Vemurafenib. EBioMedicine 2016, 8, 132–149. [Google Scholar] [CrossRef]
- Greger, J.G.; Eastman, S.D.; Zhang, V.; Bleam, M.R.; Hughes, A.M.; Smitheman, K.N.; Dickerson, S.H.; Laquerre, S.G.; Liu, L.; Gilmer, T.M. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol. Cancer Ther. 2012, 11, 909–920. [Google Scholar] [CrossRef]
- Hong, S.K.; Starenki, D.; Wu, P.K.; Park, J.I. Suppression of B-RafV600E melanoma cell survival by targeting mitochondria using triphenyl-phosphonium-conjugated nitroxide or ubiquinone. Cancer Biol. Ther. 2017, 18, 106–114. [Google Scholar] [CrossRef]
- Liu, J.F.; Lai, K.C.; Peng, S.F.; Maraming, P.; Huang, Y.P.; Huang, A.C.; Chueh, F.S.; Huang, W.W.; Chung, J.G. Berberine Inhibits Human Melanoma A375.S2 Cell Migration and Invasion via Affecting the FAK, uPA, and NF-κB Signaling Pathways and Inhibits PLX4032 Resistant A375.S2 Cell Migration In Vitro. Molecules 2018, 23, 2019. [Google Scholar] [CrossRef]
- Raineri, M.; Prodomini, S.; Fasoli, S.; Gotte, G.; Menegazzi, M. Influence of onconase in the therapeutic potential of PARP inhibitors in A375 malignant melanoma cells. Biochem. Pharmacol. 2019, 167, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Westekemper, H.; Freistuehler, M.; Bornfeld, N.; Steuhl, K.P.; Scheulen, M.; Hilger, R.A. Chemosensitivity of conjunctival melanoma cell lines to target-specific chemotherapeutic agents. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 251, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ardelt, W.; Mikulski, S.M.; Shogen, K. Amino acid sequence of an anti-tumor protein from Rana pipiens oocytes and early embryos. Homology to pancreatic ribonucleases. J. Biol. Chem. 1991, 266, 245–251. [Google Scholar]
- Ardelt, W.; Shogen, K.; Darzynkiewicz, Z. Onconase and amphinase, the antitumor ribonucleases from Rana pipiens oocytes. Curr. Pharm. Biotechnol. 2008, 9, 215–225. [Google Scholar] [CrossRef]
- Ardelt, W.; Ardelt, B.; Darzynkiewicz, Z. Ribonucleases as potential modalities in anticancer therapy. Eur. J. Pharmcol. 2009, 625, 181–189. [Google Scholar] [CrossRef]
- Rutkoski, T.J.; Raines, R.T. Evasion of ribonuclease inhibitor as a determinant of ribonuclease cytotoxicity. Curr. Pharm. Biotechnol. 2008, 9, 185–189. [Google Scholar] [CrossRef]
- Costanzi, D.; Sidransky, A.; Navon, H.; Goldsweig, H. Ribonucleases as a novel pro-apoptotic anticancer strategy: Review of the preclinical and clinical data for ranpirnase. Cancer Investig. 2005, 23, 643–650. [Google Scholar] [CrossRef]
- Nasu, M.; Carbone, M.; Gaudino, G.; Ly, B.H.; Bertino, P.; Shimizu, D.; Morris, P.; Pass, H.I.; Yang, H. Ranpirnase Interferes with NF-κB Pathway and MMP9 Activity, Inhibiting Malignant Mesothelioma Cell Invasiveness and Xenograft Growth. Genes Cancer. 2011, 2, 576–584. [Google Scholar] [CrossRef]
- Goparaju, C.M.; Blasberg, J.D.; Volinia, S.; Palatini, J.; Ivanov, S.; Donington, J.S.; Croce, C.; Carbone, M.; Yang, H.; Pass, H.I. Onconase mediated NFKβ downregulation in malignant pleural mesothelioma. Oncogene 2011, 30, 2767–2777. [Google Scholar] [CrossRef]
- Roesch, A.; Fukunaga-Kalabis, M.; Schmidt, E.C.; Zabierowski, S.E.; Brafford, P.A.; Vultur, A.; Basu, D.; Gimotty, P.; Vogt, T.; Herlyn, M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 2010, 141, 583–594. [Google Scholar] [CrossRef]
- Roesch, A. Melanoma stem cells. J. Dtsch. Dermatol. Ges. 2015, 13, 118–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dou, J.; Pan, M.; Wen, P.; Li, Y.; Tang, Q.; Chu, L.; Zhao, F.; Jiang, C.; Hu, W.; Hu, K.; et al. Isolation and Identification of Cancer Stem-Like Cells from Murine Melanoma Cell Lines. Cell. Mol. Immunol. 2007, 4, 467–472. [Google Scholar] [PubMed]
- Wagle, N.; Emery, C.; Berger, M.F.; Davis, M.J.; Sawyer, A.; Pochanard, P.; Kehoe, S.M.; Johannessen, C.M.; Macconaill, L.E.; Hahn, W.C.; et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 2011, 29, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Cvetanova, B.; Shen, Y.C.; Shyur, L.F. Cumingianoside A, a phyto-triterpenoid saponin inhibits acquired BRAF inhibitor resistant melanoma growth via programmed cell death. Front. Pharmcol. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Bianchini, F.; Taddei, M.L.; Becatti, M.; Giannoni, E.; Chiarugi, P.; Calorini, L. Etoposide-Bevacizumab a new strategy against human melanoma cells expressing stem-like traits. Oncotarget 2016, 7, 51138–51149. [Google Scholar] [CrossRef]
- Grichnik, J.M.; Burch, J.A.; Schulteis, R.D.; Shan, S.; Liu, J.; Darrow, T.L.; Vervaert, C.E.; Seigler, H.F. Melanoma, a tumor based on a mutant stem cell? J. Investig. Dermatol. 2006, 126, 142–153. [Google Scholar] [CrossRef]
- Haferkamp, S.; Borst, A.; Adam, C.; Becker, T.M.; Motschenbacher, S.; Windhövel, S.; Hufnagel, A.L.; Houben, R.; Meierjohann, S. Vemurafenib induces senescence features in melanoma cells. J. Investig. Dermatol. 2013, 133, 1601–1609. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, K.; Zhu, X.; Lin, G.; Song, F.; Zhao, Y.; Piao, Y.; Liu, J.; Cheng, W.; Bi, X.; et al. Encorafenib (LGX818), a potent BRAF inhibitor, induces senescence accompanied by autophagy in BRAFV600E melanoma cells. Cancer Lett. 2016, 370, 332–344. [Google Scholar] [CrossRef]
- Zhao, H.; Ardelt, B.; Ardelt, W.; Shogen, K.; Darzynkiewicz, Z. The cytotoxic ribonuclease onconase targets RNA interference (siRNA). Cell Cycle 2008, 7, 3258–3261. [Google Scholar] [CrossRef]
- Rybak, S.M.; Pearson, J.W.; Fogler, W.E.; Volker, K.; Spence, S.E.; Newton, D.L.; Mikulski, S.M.; Ardelt, W.; Riggs, C.W.; Kung, H.F.; et al. Enhancement of vincristine cytotoxicity in drug-resistant cells by simultaneous treatment with onconase, an antitumor ribonuclease. J. Natl. Cancer Inst. 1996, 88, 747–753. [Google Scholar] [CrossRef]
- Boix, E.; Wu, Y.; Vasandani, V.M.; Saxena, S.K.; Ardelt, W.; Ladner, J.; Youle, R.J. Role of the N terminus in RNase A homologues: Differences in catalytic activity, ribonuclease inhibitor interaction and cytotoxicity. J. Mol. Biol. 1996, 257, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Iordanov, M.S.; Ryabinina, O.P.; Wong, J.; Dinh, T.H.; Newton, D.L.; Rybak, S.M.; Magun, B.E. Molecular determinants of apoptosis induced by the cytotoxic ribonuclease onconase: Evidence for cytotoxic mechanisms different from inhibition of protein synthesis. Cancer Res. 2000, 60, 1983–1994. [Google Scholar] [PubMed]
- Qiao, M.; Zu, L.D.; He, X.H.; Shen, R.L.; Wang, Q.C.; Liu, M.F. Onconase downregulates microRNA expression through targeting microRNA precursors. Cell Res. 2012, 22, 1199–1202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Altomare, D.A.; Rybak, S.M.; Pei, J.; Maizel, J.V.; Cheung, M.; Testa, J.R.; Shogen, K. Onconase responsive genes in human mesothelioma cells: Implications for an RNA damaging therapeutic agent. BMC Cancer 2010, 10, 34. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Ardelt, B.; Hsieh, T.C.; Darzynkiewicz, Z.; Shogen, K.; Wu, J.M. Treatment of Jurkat acute T-lymphocytic leukemia cells by onconase (Ranpirnase) is accompanied by an altered nucleocytoplasmic distribution and reduced expression of transcription factor NF-kappaB. Int. J. Oncol. 2004, 25, 1745–1752. [Google Scholar] [CrossRef]
- Fratangelo, F.; Camerlingo, R.; Carriero, M.V.; Pirozzi, G.; Palmieri, G.; Gentilcore, G.; Ragone, C.; Minopoli, M.; Ascierto, P.A.; Motti, M.L. Effect of ABT888 on the apoptosis, motility and invasiveness of BRAFi-resistant melanoma cells. Int. J. Oncol. 2018, 53, 1149–1159. [Google Scholar]
- Moro, N.; Mauch, C.; Zigrino, P. Metalloproteinases in melanoma. Eur. J. Cell. Biol. 2014, 93, 23–29. [Google Scholar] [CrossRef]
- Gu, Y.; Ke, G.; Wang, L.; Gu, Q.; Zhou, E.; He, Q.; Wang, S. Silencing Matrix Metalloproteinases 9 and 2 inhibits human retinal microvascular endothelial cell invasion and migration. Ophthalmic Res. 2015, 55, 70–75. [Google Scholar] [CrossRef]
- Peng, B.; Zhu, H.; Klausen, C.; Ma, L.; Wang, Y.; Leung, P.C.K. GnRH regulates trophoblast invasion via RUNX2-mediated MMP2/9 expression. Mol. Hum. Reprod. 2016, 22, 119–129. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Huang, J.; Liu, Y. Matrix metalloproteinase 2 knockdown suppresses the proliferation of HepG2 and Huh7 cells and enhances cisplatin effect. Open Med. 2019, 14, 384–391. [Google Scholar] [CrossRef]
- Reck, M.; Krzakoski, M.; Jassem, J.; Eschbach, C.; Kozielski, J.; Costanzi, J.J.; Gatzemeier, U.; Shogen, K.; von Pawel, J. Randomized, multicenter phase III study of ranpirnase plus doxorubicin (DOX) versus DOX in patients with unresectable malignant mesothelioma (MM). J. Clin. Oncol. 2009, 27. [Google Scholar] [CrossRef]
- Notomista, E.; Cafaro, V.; Fusiello, R.; Bracale, A.; D’Alessio, G.; Di Donato, A. Effective expression and purification of recombinant onconase, an antitumor protein. FEBS Lett. 1999, 463, 211–215. [Google Scholar] [CrossRef]
- Fagagnini, A.; Pica, A.; Fasoli, S.; Montioli, R.; Donadelli, M.; Cordani, M.; Butturini, E.; Acquasaliente, L.; Picone, D.; Gotte, G. Onconase dimerization through 3D domain swapping: Structural investigations and increase in the apoptotic effect in cancer cells. Biochem. J. 2017, 474, 3767–3781. [Google Scholar] [CrossRef] [PubMed]
- Kunitz, M. A spectrophotometric method for the measurement of ribonuclease activity. J. Biol. Chem. 1946, 164, 563–568. [Google Scholar] [PubMed]
- Gregorelli, A.; Sgarbossa, A.; Khan, S.; Soriente, A.; De Rosa, M.; Saturnino, C.; Menegazzi, M. Tree arachidonoylamide derivatives inhibit pro-inflammatory genes expression by modulating NF-kB and AP1 activity. Med. Chem. 2016, 12, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raineri, A.; Fasoli, S.; Campagnari, R.; Gotte, G.; Menegazzi, M. Onconase Restores Cytotoxicity in Dabrafenib-Resistant A375 Human Melanoma Cells and Affects Cell Migration, Invasion and Colony Formation Capability. Int. J. Mol. Sci. 2019, 20, 5980. https://doi.org/10.3390/ijms20235980
Raineri A, Fasoli S, Campagnari R, Gotte G, Menegazzi M. Onconase Restores Cytotoxicity in Dabrafenib-Resistant A375 Human Melanoma Cells and Affects Cell Migration, Invasion and Colony Formation Capability. International Journal of Molecular Sciences. 2019; 20(23):5980. https://doi.org/10.3390/ijms20235980
Chicago/Turabian StyleRaineri, Alice, Sabrina Fasoli, Rachele Campagnari, Giovanni Gotte, and Marta Menegazzi. 2019. "Onconase Restores Cytotoxicity in Dabrafenib-Resistant A375 Human Melanoma Cells and Affects Cell Migration, Invasion and Colony Formation Capability" International Journal of Molecular Sciences 20, no. 23: 5980. https://doi.org/10.3390/ijms20235980
APA StyleRaineri, A., Fasoli, S., Campagnari, R., Gotte, G., & Menegazzi, M. (2019). Onconase Restores Cytotoxicity in Dabrafenib-Resistant A375 Human Melanoma Cells and Affects Cell Migration, Invasion and Colony Formation Capability. International Journal of Molecular Sciences, 20(23), 5980. https://doi.org/10.3390/ijms20235980






